Abstract
Diabetic neuropathic pain (DNP) and depression (DP) are the common complications in patients with diabetes. The purpose of our research was to observe whether brain-derived neurotrophic factor (BDNF) levels and tropomyosin receptor kinase B (TrkB) in the nervous system have effects on rats with comorbid DNP and DP, and to determine whether dihydromyricetin (DHM) may influence BDNF/ TrkB pathway to mitigatethe comorbidity. The study showed that DHM treatment could attenuates pain and depressive behavior in DNP and DP combined rats. Compared with the control group, the expression level of BDNF/TrkB in the hippocampus of DNP + DP group were reduced, while the expression levels in the spinal cord and DRG were increased. However, after treatment with DHM, those changes were reversed. Compared with the control group, the level of IL-1β and TNF-α in the hippocampus, spinal cord and DRG in the DNP + DP group was significantly increased, and DHM treatment could reduce the increase. Thus our study indicated that DHM can relief symptoms of DNP and DP by suppressing the BDNF/TrkB pathway and the proinflammatory factor, and BDNF/TrkB pathway may be an effective target for treatment of comorbid DNP and DP.
Similar content being viewed by others
Introduction
Diabetes is a clinically chronic disease, and by 2045, the estimated worldwide incidence will rise to 693 million people1. There is a rising morbidity of diabetes in the world, and the diabetic population in China is the biggest2. A common symptom of diabetic complications is diabetic neuropathic pain (DNP), which has a great impact on the life of patients3. At present, the mechanism of DNP is not clear, and treatments have not been satisfactory. Therefore, it is especially important to seek more effective treatments.
Depression (DP) is a common public health disease that imposes an extremely serious financial burden on patients4. It has been estimated that the prevalence of depression worldwide is approximately 25%, and women are higher than men5. There is much evidence that there is a correlation between diabetes and depression6,7. Treatment of early depression can improve glycaemic control8. The risk of depression in patients with chronic pain is significantly higher than that in normal people, and the risk of chronic pain in patients with depression is also significantly higher than that in normal people9.
Some patients may have the comorbid conditions of DNP and DP, and the comorbidity brings more serious physical and mental effects to patients, and is more difficult to treat than only one disorder10. Patients with comorbid DNP and depression or anxiety have more healthcare costs than patients who are only suffering from DNP11. Therefore, it is extremely urgent to seek more effective treatments.
Brain-derived neurotrophic factor (BDNF) acts as a survival factor for neurons and is an important member of the neurotrophic family12,13. BDNF is a key signalling molecule in the microglia-neuron signalling pathway, and may be a therapeutic strategy for neuropathic pain treatment14. However, studies have shown that DP can reduce the expression of BDNF in hippocampus12. Some researchers had also confirmed that increased levels of BDNF in the hippocampus mediated the antidepressant-like effects of conventional antidepressants and ketamine, which makes BDNF an important target for depression15. Tropomyosin receptor kinase B (TrkB) is the high affinity receptor of BDNF. In comorbid DNP and DP conditions, the role of BDNF/TrkB pathway in the nervous system remains unclear and requires further research.
Dihydromyricetin (DHM) is extracted from a woody vine with the main active ingredient being flavonoids. DHM has many pharmacological effects such as anti-diabetic, anti-inflammatory, antioxidant and neuroprotective effects16. Our study aimed to observe the effects of BDNF/TrkB pathway in the nervous system on combined DNP and DP rats, and to determine whether DHM may influence BDNF/TrkB pathway to mitigate the comorbidity.
Results
Effect of DHM on thermal withdrawal latency (TWL) and mechanical withdrawal threshold (MWT) in rats with comorbid DNP and DP
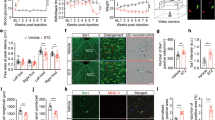
In this study, changes in pain related behaviours in rats were monitored by TWL and MWT. Four weeks after chronic unpredictable stress exposure, the TWL and MWT of rats in the DNP + DP group were significantly lower than in the Control group (p < 0.01). There were no differences in TWL and MWT between the control group and the control + DHM group (p > 0.05). After treatment with DHM for 2 weeks, TWL and MWT were markedly increased (p < 0.01). See Fig. 1.
The above data show that DHM treatment can increase TWL and MWT in DNP + DP rats, indicating that the neuropathic pain behaviours were mitigated by DHM.
Effects of DHM on depressive behavior in rats with comorbid DNP and DP
After the DNP + DP model was successfully established, the levels of sucrose preference test (SPT) and open-field test (OFT) in DNP + DP rats were significantly lower than those in Control rats (p < 0.01), and the immobility time in the forced swimming test (FST) in DNP + DP rats was obviously higher than in the control rats (p < 0.01). After injection of DHM for two weeks, the levels of SPT and OFT in the DNP + DP + DHM group were obviously higher than those in the DNP + DP group (p < 0.01).Furthermore, the immobility time of FST in the DNP + DP + DHM group was lower than that in the DNP + DP group (P < 0.01). See Fig. 2.
Effects of dihydromyricetin on the forced swimming test (FST) (A), sucrose preference test (SPT) (B) and open-field test (OFT) (C) in rats with comorbid DNP and DP. Figure D, E, F and G represented the total distance passed by the rat in each group recorded using a Canon camera model Powershot A610. Values are the mean ± SEM of 6 observations. *p < 0.05 and **p < 0.01 vs. control group; #p < 0.05 and ##p < 0.01 vs. DNP + DP group.
The above data showed that the depression-related behaviours were relieved by DHM.
Effects of DHM on the expression levels of BDNF and TrkB in rats with comorbid DNP and DP
We used quantitative real-time PCR and Western blotting to detect mRNA and protein expression levels of BDNF and TrkB in the rats. The results showed that in DRG and spinal cord tissue, the mRNA and protein expression levels of BDNF and TrkB were higher in the DNP + DP group than in the Control group, but in thehippocampus, the expression levels of BDNF and TrkB in the DNP + DP group were lower than in the Control group (p < 0.01). DHM treatment can significantly reverse these changes (p < 0.01). See Figs 3 and 4.
Effects of dihydromyricetin on the expression of BDNF (A,B) and TrkB (A,C) protein in the DRGs, spinal cord and hippocampus of DNP + DP rats as detected by Western blotting. Values are the mean ± SEM of 6 observations. *p < 0.05 and **p < 0.01 vs. control group; #p < 0.05 and ##p < 0.01 vs. DNP + DP group.
We also used immunofluorescence double-labelling to detect the immunoreactivity of BDNF and TrkB in the hippocampus, spinal cord and DRG. NeuN is a marker of neurons. The results confirmed that the co-expression of BDNF-NeuN and TrkB-NeuN in these tissues, and BDNF and TrkB were mainly expressed in neurons in DRG. The results also showed that the co-expression of BDNF-NeuN and TrkB-NeuN in the spinal cord and DRG were higher inthe DNP + DP groupthan in the control group, and treatment with DHM could block this increase (p < 0.01). In the hippocampus, the co-expression of the BDNF-NeuN and TrkB-NeuN was lower in the DNP + DP group than that in the control group, and DHM treatment increased the change (p < 0.01). See Figs 5, 6 and 7.
The above data indicated that DHM may reverse the expression of BDNF and TrkB receptors in the hippocampus, spinal cord and DRG of DNP and DP comorbid rats.
Effects of DHM on the levels of IL-1β and TNF-α in rats with comorbid DNP and DP
The mRNA expression levels of IL-1β and TNF-α in the hippocampus, spinal cord and DRG were detected by qPCR. The results showed that the mRNA expression levels of IL-1β and TNF-αwere higher in the DNP + DP group than those in the Control group. However, the expressions of IL-1β and TNF-α mRNA were lower in the DNP + DP + DHM group than those in the DNP + DP group (p < 0.01). See Fig. 8.
In addition, we also detected the IL-1β and TNF-α level in each group using Western blotting. The IL-1β and TNF-α protein levels were higher in the DNP + DP group than those in the Control group. But the DHM treatment could down-regulate the increased change to normal level (p < 0.01). See Fig. 9.
Effects of DHM on the expression levels of IL-1β (A,B) and TNF-α (A,C) protein in the DRG, spinal cord and hippocampus of DNP + DP rats as detected by Western blotting. Values are the mean ± SEM of 6 observations. *p < 0.05 and **p < 0.01 vs. control group; #p < 0.05 and ##p < 0.01 vs. DNP + DP group.
Discussion
Clinically, diabetes is a high-risk chronic disease. Diabetes patients often have multiple complications. DNP and DP are twoof themost common complications in diabetes patients. The two diseases can sometimes co-occur, which greatly impacts the quality of life of patients17,18. At present the mechanism is still unclear and traditional pharmacotherapies for chronic pain and depression cannot meet the needs of patients with comorbid DNP + DP17,19. Therefore, it is urgent to elucidate the mechanism underlying this comorbidity and develop new drugs to treat patients with the DNP + DP comorbidity more effectively.
In our study, a rat model of DNP and DP was established by a high-sugar and high-fat diet, injecting STZ, and exposing them to chronic unpredictable stress20. Meanwhile, we measured responses in several ways per week to verify that rats had both symptoms. The significantly reduced values for the TWL, MWT, SP, and OFT distance, as well as the significantly increased FST values, confirmed the successful generation of the DNP + DP model.DHM is a natural flavonoid in Ampelopsis grossedentata, such as vine tea, which has various functions such as anti-inflammatory and anti-tumor effects16,21,22. The results of these behavioural tests showed that DHM treatment can reverse these changes, indicating that DHM therapy can mitigate the symptoms of the comorbidity.
BDNF is widely present in the peripheral and central nervous systems and plays an important role in maintaining pain, depression, anxiety and memory-related functions23. BDNF has a protective effect in Parkinson’s disease24, Alzheimer’s disease25, metabolic syndrome26 and depression27, however,BDNF has facilitated effects on the transmission of neuropathic pain28. The regulation of BDNF concentrations is directly related todepressionaetiology;for example, the upregulation of BDNF causes an antidepressant-like effect and the downregulation of BDNF accounts for depression29,30. Meanwhile many studies have confirmed that BDNF/TrkB is involved in neuropathic pain progression, reducing BDNF/TrkB signalling in the spinal dorsal horn produced anti-neuropathic pain effects31,32,33. During the comorbidity of DNP and DP, our previous study found a decrease in the expression of BDNF in the hippocampus20. However, at present the effects of BDNF/TrkB signaling in the nervous system are not completely understandin the context of comorbid DNP and DP. Thus, we assessed the expression of BDNF/TrkB in the hippocampus, spinal cord and DRG of L4/L5. The results of qPCR and Western blotting tests indicated that the level of BDNF/TrkB in the hippocampus was lower in comorbid DNP and DP rats than in the control group, but the results in the DRG and spinal cord were opposite to those in the hippocampus. DHM treatment can block these changes. We also used immunofluorescence double-labelling to detect the immunoreactivity of BDNF and TrkB in the hippocampus, spinal cord and DRG, respectively. Our study found there were co-expressions of BDNF-NeuN and TrkB-NeuN in these tissues. The co-expression of the BDNF-NeuN and TrkB-NeuN in the spinal cord and DRG were higher in the DNP + DP group than in the control group, but it was lower in the hippocampus. DHM treatment can also reverse these changes. Thus we hypothesized that there are different mechanisms in the hippocampus, spinal cord and DRG for BDNF/TrkBsignalling during comorbid DNP and DP, and DHM can relieve the comorbidity by normalizing the changes of BDNF/TrkBsignalling in the nervous system.
Studies have confirmed that chronic pain, depression behavior and neuroinflammation are closely related34,35. Inhibiting TNF-α can prevent stress-induced memory disorder by maintaining BDNF levels of hippocampus in the CUS rat model of depression36. Lithium may be a neuroprotective agent against the induced toxicity of methylphenidate by suppressing hippocampal BDNF, IL-1β and TNF-α37. Our previous study had founded that the hippocampal IL-1β and TNF-α levels of rats with DNP and DP were significantly higher than control rats20. In this study the results were similar to the previous research. DHM treatment can block the increased inflammatory factors. We speculate that the high inflammatory factor levels in the hippocampus will decrease BDNF/TrkB expression to develop the depression, but in the spinal cord and DRG, the high inflammatory factor levels will increase BDNF/TrkB expression to facilitate the transmission of pain. However, the exact underlying mechanism still needsadditional exploration. We provide support that DHM has antiinflammation effects, can decrease thelevels of IL-1β and TNF-αin the hippocampus,spinal cord and DRGof model rats, and then influence BDNF/TrkB expression to mitigate the comorbidity of DNP and DP.
Conclusion
In conclusion, DHM can alleviate the symptoms of diabetic neuropathic pain and depression by regulating the level of BDNF expression in the nervous system, and its mechanism may involve the inhibition of the release of the inflammatory factors IL-1β and TNF-α.DHM is expected to become a new drug for the treatment of the comorbidity of DNP and DP.
Materials and Methods
Animals and treatments
Male Sprague Dawley (SD) rats(180–220 g) were supplied by the Centre of Laboratory Animal Science of Jiangxi University of Traditional Chinese Medicine. The Animal Care and Ethics Committee of Nanchang University approved the procedures of this research. The IASP’s ethical guidelines for the study of pain in animals were followed. Five SD rats were housed in each cage at a temperature of 25 °C and a humidity of 60%, and provided freely accessible food and water.
The first week before the experiment started,SD rats were fed aregular diet. Then, for the next 4 weeks, rats were fed a high-glucose-high-fat diet. After a 12-h fast on the weekend of week 4, they were given an intraperitoneal injection of streptozotocin(STZ) (35 mg/kg)20,38,39.
Rats were randomly tested and those with blood glucose concentrations above 16.7 mmol/L were selected as type 2 diabetes mellitus rats. Then within four weeks after STZ injection, rats were exposed to a chronic unpredictable stress (CUS) procedure.During this period, the thermal withdrawal latency (TWL), mechanical withdrawal threshold (MWT), the sucrose preference test (SPT), the immobility time of forced swimming test (FST) and the open-field test (OFT) were monitored once a week to select the rats with comorbid diabetic neuropathic pain and depression. The CUS procedureis a classic animal model of depression40,41,42, incorporating reversed light/dark cycle, food deprivation (24 h), water deprivation (24 h), tail pinch (1 min), swimming in cold water (4 °C, 5 min), swimming in hot water (45 °C, 5 min), and no stressor. These seven kinds of conditions were administered daily at random for five weeks. Sixty male SD rats were stochastically divided into 4 groups as follows: (1) control group (Control); (2) control + dihydromyricetin group (Control + DHM); (3) diabetic neuropathic pain and depression group (DNP + DP); (4) dihydromyricetin treatment group (DNP + DP + DHM). Rats in the Control + DHM and DNP + DP + DHM groups were injected intraperitoneally with DHM for 2 weeks at a dose of 30 mg/kg43. Figure 10 shows the timeline for the establishment of an animal model. STZ was purchased from Solarbio. DHM ((2 R,3 R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl) benzoin-4-one; CAS No. 27200-12-0; HPLC purity ≥ 98%) was provided by Zelang Co., Nanjing, China.
Mechanical withdrawal threshold (MWT)
The MWT was evaluated by a paw withdrawal response to mechanical stimulation by BME-404 electromechanical stimulator (Institute of Biomedical Engineering, Chinese Academy of Medical Sciences)44,45. The test needle’s face diameter was 0.6 mm, the pressure measurement range was from 0.1 g to 50 g, and the pressure measurement resolution was 0.05 g. Rats rested on the wire mesh for at least 30 min as an adaptation time prior to the test. The same observer used the test needle to touch the left hind paw of the rat (between the third and fourth metatarsal) and applied force until the paw tried to lift. The MWT was recorded via the computer and expressed in grams. The stimulation interval was 2 min. The average of the three consecutivestable measurementswas obtained.
Thermal withdrawal latency (TWL)
The TWL was evaluated by radiant heat and the BME-410C Thermal Paw Stimulation System (Institute of Biomedical Engineering, Chinese Academy of Medical Sciences) to obtain the retraction latency after thermal stimulation in the rats44,45. Prior to testing, each rat was placed in a transparent box on the glass surface for 30 min.The test began with a beam of radiant heat stimulating the underside of the midfoot surface of the hind paw and activation of the timer to begin the trial. The cutoff time for thermal stimulation was 30 s. When the rat lifts the paw and disconnects the light source, the timer ends. The time displayed on the timer was recorded as TWL and expressed in seconds.Finally, the average of the three stable data points was taken and the test was initiated at 2 min intervals.
Sucrose preference test (SPT)
Before the test, rats were fasted for 24 h and then placed individually in separate cages. Two identical water bottles, one containing 100 ml of 1% sucrose in water and another containing 100 ml pure water, were placed in all cages at the same time. SP was evaluated by measuring the levels of sugar-water and pure-water consumption in one hour20,46. The SP rate was calculated as the ratio of sugar water/total liquid consumption x 100%.
Forced swimming test (FST)
Rats were placed in an 80-cm-high glass cylinder with a 40-cm inner diameter. The water temperature was approximately 20 °C, and the water depth was 30 cm. The immobility time of FST of rats in the water, i.e., when rats stopped struggling and floated in a fixed shape, was recorded for 5 min and expressed in seconds20,47.
Open-field test (OFT)
The rats were placed in a dark environment for 30 minutes before the test. Then, they were placed in a black box that measured 40 × 60 × 50 cm. Each rat was placed gently in the middle of the box, and the animal’s behavior was recorded using a Canon Powershot A610 camera (Canon Co. local distributer, Tehran, Iran) during a 5-min session. The recorded videos were analysed and processed using MATLAB (Mathworks Co., Massachusetts, USA) to determine the total distance travelled, expressed in centimetres20,48. The apparatus was cleaned with a 10% ethanol solution before the next animal was introduced into the box.
Quantitative real-time PCR (qPCR)
Rats were anaesthetized by i.p. injection of 10% chloral hydrate (Batch No: 050101; Shanghai Xingya Medical Company, China). DRGs, the spinal cord at the level of L4-L5 vertebrae, and hippocampus were isolated immediately after sacrifice from rats in the different groups, flushed with ice-cold PBS, and stored in RNA Store solution at −20 °C until use. All instruments were treated with DEPC before use.
Total RNA was separated from tissue sample using the TRIzol Total RNA Reagent(Beijing TransGen Biotech Co.). Total RNA (2 µg) was used to synthesise complementary DNA (cDNA) by the RevertAid™ HMinus First Strand cDNA Synthesis Kit. Applied Primer Express 3.0 Software to designed the primer sequences, as follows:
β-actin forward 5′-TAAAGACCTCTATGCCAACA -3′ and reverse 3′-CACGATGGAGGGGCCGGACTCATC -5′;
TrkB forward 5′-TGGAGGAAGGGAAGTCTGTG -3′ and reverse 3′-AGTGGTGGTCTGAGGTTGGA -5′;
BDNF forward 5′-CCTCTGCTCTTTCTGCTGGA -3′ and reverse 3′-GCTGTGACCCACTCGCTAAT -5′;
IL-1β forward 5′-CCTATGTCTTGCCCGTGGAG-3′ and reverse 3′-CACACACTAGCAGGTCGTCA-5′;
TNF-α forward5′-CACGTCGTAGCAAACCACCAA-3′ and reverse 3′-GTTGGTTGTCTTTGAGATCCAT-5′.
QPCR was performed using the SYBR® Green Master Mix in the ABI PRISM® 7500 Sequence Detection System (Applied Biosystems Inc., Foster City, CA). The expression of each gene was quantified using the ΔΔCT method, with CT as the threshold cycle20. The relative levels of target genes were obtained by the software within the ABI7500 PCR instrument.
Western blotting experiment
After rats were anaesthetized, the hippocampus, DRGs and spinal cord at L4-L5 were separated and flushed with ice-cold PBS and stored at −20 °C. Tissue was positioned in a spherical portion of a 2 ml homogenizer, and moderate RIPA lysis buffer(Beyotime Biotechnology, Shanghai, China) was added to homogenize the sample. Tissue was ground for 30 min on ice and centrifuged at 4 °C at 12000 rpm for 10 min. The supernatants were collected, diluted with 6x loading buffer, and heated to 95 °C for 10 min. The protein concentration was calculated with the BCA Protein Assay Kit, and samples were kept at −20 °C until use. Proteins in samples from each group (20 μg) were separated by 12% SDS-polyacrylamide gel electrophoresis, using a Bio-Rad electrophoresis device, and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were incubated with 5% non-fat dry milk prepared with 1X TBST for 2 h at normal temperature. Next, PVDF membranes were incubated with rabbit antibodies against TNF-α (1:800 concentration, Abcam, USA), IL-1β (1:800 concentration, Abcam, USA), BDNF (1:500 concentration, Abcam, USA), β-actin (1:1000 concentration, Beijing Zhongshan Biotech Co., China), and TrkB (1:1000 concentration, Abcam, USA) at 4 °C overnight. The membranes were washed 3 times with 1x TBST every 10 min. The secondary antibody (goat anti-rabbit IgG, 1:2000 concentration, Beijing Zhongshan Biotech Co., China) was diluted with blocking buffer and incubated at room temperature for 2 h. The PVDF membranes were washed every 10 min 3 times. The labelled proteins were then visualized with enhanced chemiluminescence on a Bio-Rad system. Band intensities were quantified using Image-Pro Plus software, and the intensities of target proteins were normalized against the respective β-actin internal control20.
Double-labelled immunofluorescence
Rats were anaesthetized with 10% chloral hydrate and were transcardially perfused with 4% paraformaldehyde (PFA). The hippocampus,spinal cord and DRG were removed and placed in 4% PFA solution at 4 °C. Dehydration was carried out by immersing tissue for 24 h at 4 °C in 30% sucrose solutions (in 4% PFA), and the solutions were renewed every 8 h. DRG were cut into 8-µm-thick slices by a cryostat microtome (Leica). The hippocampus tissuewascut into 10µmslices and the spinal cord tissue was cut into 12 µm slices. Tissue sections were dried at 37 °C for 2 h, and then stored at −20 °C. Before staining, sections were balanced at room temperature for 30 min, rinsed with 0.01 M PBS for 5 min x 3 times, incubated with 0.3% Triton X-100, and washed again with PBS for 5 min x 3 times. Then, slices were incubated in 10% goat serum for 1 h at 37 °C, followed by incubation with the diluted antibodies (rabbit anti-BDNF, 1:500, Abcam, USA; rabbit anti-TrkB, 1:500, Abcam, USA; mouse anti-GFAP, 1:200, Millipore) overnight at 4 °C. Secondary antibodies (Goat anti mouse 1:800, Abcam, USA; Goat anti rabbit 1:800,Abcam, USA) were added for incubation at 37 °C for 1 h. Sections were washed for 5 min x 3 times with PBS, sealed with antifade solution, and imaged using a fluorescence microscope (Olympus, Tokyo, Japan). The immunofluorescence intensity was analysed using Image-Pro Plus6.0 software20.
Statistical analysis
Statistical analyses were performed using SPSS21 software. Data were analysed with a one-way analysis of variance (ANOVA) followed by LSD post-hoc test for multiple comparisons. The experimental results are expressed as the mean ± standard error of the mean and were considered significant at p < 0.05.
Ethical approval
The Animal Care and Ethics Committee of Nanchang University approved the procedures of this research. The IASP’s ethical guidelines for the study of pain in animals were followed.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Cho, N. H. et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes research and clinical practice 138, 271–281, https://doi.org/10.1016/j.diabres.2018.02.023 (2018).
Wang, L. et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA: the journal of the American Medical Association 317, 2515–2523, https://doi.org/10.1001/jama.2017.7596 (2017).
Van Acker, K. et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes & metabolism 35, 206–213, https://doi.org/10.1016/j.diabet.2008.11.004 (2009).
Peytremann-Bridevaux, I. & Chevrou-Severac, H. Economic grand rounds: Financial burden of medical care and risk of forgoing care among Europeans with depressive symptoms. Psychiatric services 59, 840–842, https://doi.org/10.1176/ps.2008.59.8.840 (2008).
Mushtaque, A., Gulati, R., Hossain, M. M. & Azmi, S. A. Prevalence of depression in patients of type 2 diabetes mellitus: A cross sectional study in a tertiary care centre. Diabetes & metabolic syndrome 10, 238–241, https://doi.org/10.1016/j.dsx.2016.06.016 (2016).
Egede, L. E. & Hernandez-Tejada, M. A. Effect of comorbid depression on quality of life in adults with Type 2 diabetes. Expert review of pharmacoeconomics & outcomes research 13, 83–91, https://doi.org/10.1586/erp.12.86 (2013).
Tabak, A. G., Akbaraly, T. N., Batty, G. D. & Kivimaki, M. Depression and type 2 diabetes: a causal association? The lancet. Diabetes & endocrinology 2, 236–245, https://doi.org/10.1016/S2213-8587(13)70139-6 (2014).
Lustman, P. J. et al. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes care 23, 934–942, https://doi.org/10.2337/diacare.23.7.934 (2000).
Hirsh, A. T., Hollingshead, N. A., Bair, M. J., Matthias, M. S. & Kroenke, K. Preferences, experience, and attitudes in the management of chronic pain and depression: a comparison of physicians and medical students. The Clinical journal of pain 30, 766–774, https://doi.org/10.1097/AJP.0000000000000035 (2014).
Jain, R., Jain, S., Raison, C. L. & Maletic, V. Painful diabetic neuropathy is more than pain alone: examining the role of anxiety and depression as mediators and complicators. Current diabetes reports 11, 275–284, https://doi.org/10.1007/s11892-011-0202-2 (2011).
Boulanger, L. et al. Impact of comorbid depression or anxiety on patterns of treatment and economic outcomes among patients with diabetic peripheral neuropathic pain. Current medical research and opinion 25, 1763–1773, https://doi.org/10.1185/03007990902997309 (2009).
Kalueff, A. V., Avgustinovich, D. F., Kudryavtseva, N. N. & Murphy, D. L. BDNF in anxiety and depression. Science 312, 1598–1599; author reply 1598–1599, https://doi.org/10.1126/science.312.5780.1598 (2006).
Guillin, O. et al. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411, 86–89, https://doi.org/10.1038/35075076 (2001).
Coull, J. A. et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021, https://doi.org/10.1038/nature04223 (2005).
Bjorkholm, C. & Monteggia, L. M. BDNF - a key transducer of antidepressant effects. Neuropharmacology 102, 72–79, https://doi.org/10.1016/j.neuropharm.2015.10.034 (2016).
Zhang, J. et al. Recent Update on the Pharmacological Effects and Mechanisms of Dihydromyricetin. Frontiers in pharmacology 9, 1204, https://doi.org/10.3389/fphar.2018.01204 (2018).
Bair, M. J. et al. Prevalence of pain and association with quality of life, depression and glycaemic control in patients with diabetes. Diabetic medicine: a journal of the British Diabetic Association 27, 578–584, https://doi.org/10.1111/j.1464-5491.2010.02971.x (2010).
Duan-Porter, W. et al. Reporting of Sex Effects by Systematic Reviews on Interventions for Depression, Diabetes, and Chronic Pain. Annals of internal medicine 165, 184–193, https://doi.org/10.7326/M15-2877 (2016).
Sacco, W. P., Bykowski, C. A. & Mayhew, L. L. Pain and functional impairment as mediators of the link between medical symptoms and depression in type 2 diabetes. International journal of behavioral medicine 20, 22–29, https://doi.org/10.1007/s12529-011-9210-5 (2013).
Shen, Y. et al. Effects of palmatine on rats with comorbidity of diabetic neuropathic pain and depression. Brain research bulletin 139, 56–66, https://doi.org/10.1016/j.brainresbull.2018.02.005 (2018).
Hou, X. L. et al. Dihydromyricetin protects endothelial cells from hydrogen peroxide-induced oxidative stress damage by regulating mitochondrial pathways. Life sciences 130, 38–46, https://doi.org/10.1016/j.lfs.2015.03.007 (2015).
Hou, X. L. et al. Suppression of Inflammatory Responses by Dihydromyricetin, a Flavonoid from Ampelopsis grossedentata, via Inhibiting the Activation of NF-kappaB and MAPK Signaling Pathways. Journal of natural products 78, 1689–1696, https://doi.org/10.1021/acs.jnatprod.5b00275 (2015).
Fukuhara, K. et al. Intracerebroventricular 4-methylcatechol (4-MC) ameliorates chronic pain associated with depression-like behavior via induction of brain-derived neurotrophic factor (BDNF). Cellular and molecular neurobiology 32, 971–977, https://doi.org/10.1007/s10571-011-9782-2 (2012).
Rahmani, F. et al. Plasma levels of brain-derived neurotrophic factor in patients with Parkinson disease: A systematic review and meta-analysis. Brain research 1704, 127–136, https://doi.org/10.1016/j.brainres.2018.10.006 (2019).
Beeri, M. S. & Sonnen, J. Brain BDNF expression as a biomarker for cognitive reserve against Alzheimer disease progression. Neurology 86, 702–703, https://doi.org/10.1212/WNL.0000000000002389 (2016).
Motamedi, S., Karimi, I. & Jafari, F. The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): Kill two birds with one stone. Metabolic brain disease 32, 651–665, https://doi.org/10.1007/s11011-017-9997-0 (2017).
Koike, H., Fukumoto, K., Iijima, M. & Chaki, S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behavioural brain research 238, 48–52, https://doi.org/10.1016/j.bbr.2012.10.023 (2013).
Sanna, M. D., Ghelardini, C. & Galeotti, N. Blockade of the spinal BDNF-activated JNK pathway prevents the development of antiretroviral-induced neuropathic pain. Neuropharmacology 105, 543–552, https://doi.org/10.1016/j.neuropharm.2016.02.016 (2016).
Khan, H., Amin, S. & Patel, S. Targeting BDNF modulation by plant glycosides as a novel therapeutic strategy in the treatment of depression. Life sciences 196, 18–27, https://doi.org/10.1016/j.lfs.2018.01.013 (2018).
Hurley, L. L. et al. Antidepressant-like effects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF. Behavioural brain research 239, 27–30, https://doi.org/10.1016/j.bbr.2012.10.049 (2013).
Ulmann, L. et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. The Journal of neuroscience: the official journal of the Society for Neuroscience 28, 11263–11268, https://doi.org/10.1523/JNEUROSCI.2308-08.2008 (2008).
Khan, N., Gordon, R., Woodruff, T. M. & Smith, M. T. Antiallodynic effects of alpha lipoic acid in an optimized RR-EAE mouse model of MS-neuropathic pain are accompanied by attenuation of upregulated BDNF-TrkB-ERK signaling in the dorsal horn of the spinal cord. Pharmacology research & perspectives 3, e00137, https://doi.org/10.1002/prp2.137 (2015).
Chen, J. et al. The impact of glutamine supplementation on the symptoms of ataxia-telangiectasia: a preclinical assessment. Molecular neurodegeneration 11, 60, https://doi.org/10.1186/s13024-016-0127-y (2016).
Benatti, C. et al. Disease-Induced Neuroinflammation and Depression. CNS & neurological disorders drug targets 15, 414–433, https://doi.org/10.2174/1871527315666160321104749 (2016).
Lees, J. G. et al. Cytokines in Neuropathic Pain and Associated Depression. Modern trends in pharmacopsychiatry 30, 51–66, https://doi.org/10.1159/000435932 (2015).
Sahin, T. D. et al. TNF-alpha inhibition prevents cognitive decline and maintains hippocampal BDNF levels in the unpredictable chronic mild stress rat model of depression. Behavioural brain research 292, 233–240, https://doi.org/10.1016/j.bbr.2015.05.062 (2015).
Motaghinejad, M., Seyedjavadein, Z., Motevalian, M. & Asadi, M. The neuroprotective effect of lithium against high dose methylphenidate: Possible role of BDNF. Neurotoxicology 56, 40–54, https://doi.org/10.1016/j.neuro.2016.06.010 (2016).
Srinivasan, K., Viswanad, B., Asrat, L., Kaul, C. L. & Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacological Research 52, 313–320, https://doi.org/10.1016/j.phrs.2005.05.004 (2005).
Chao, P. C. et al. Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomedicine & Pharmacotherapy 101, 155–161, https://doi.org/10.1016/j.biopha.2018.02.084 (2018).
Huang, P. et al. Voluntary wheel running ameliorates depression-like behaviors and brain blood oxygen level-dependent signals in chronic unpredictable mild stress mice. Behavioural Brain Research 330, 17–24, https://doi.org/10.1016/j.bbr.2017.05.032 (2017).
Liang, X. et al. Exercise improves depressive symptoms by increasing the number of excitatory synapses in the hippocampus of CUS-Induced depression model rats. Behavioural Brain Research 374, 112115, https://doi.org/10.1016/j.bbr.2019.112115 (2019).
Wang, S. et al. LncRNA uc.48+ is involved in diabetic neuropathic pain mediated by the P2X receptor in the dorsal root ganglia. Purinergic signalling 12, 139–148, https://doi.org/10.1007/s11302-015-9488-x (2015).
Liu, L. et al. Dihydromyricetin delays the onset of hyperglycemia and ameliorates insulin resistance without excessive weight gain in Zucker diabetic fatty rats. Molecular and cellular endocrinology 439, 105–115, https://doi.org/10.1016/j.mce.2016.10.028 (2017).
Islam, M. S. J. Jo. D. R. Animal models of diabetic neuropathy: progress since 1960s. Journal of Diabetes Research 2013, 149452, https://doi.org/10.1155/2013/149452 (2013).
Rao, S. et al. The effect of sinomenine in diabetic neuropathic pain mediated by the P2X3 receptor in dorsal root ganglia. Purinergic Signal 13, 227–235, https://doi.org/10.1007/s11302-016-9554-z (2017).
Willner, P., Towell, A., Sampson, D., Sophokleous, S. & Muscat, R. J. P. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93, 358–364, https://doi.org/10.1007/bf00187257 (1987).
Lucki, I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behavioural Pharmacology 8, 523–532, https://doi.org/10.1097/00008877-199711000-00010 (1997).
Borsini, F., Lecci, A., Sessarego, A., Frassine, R. & Meli, A. Discovery of Antidepressant Activity by Forced Swimming Test May Depend on Pre-Exposure of Rats to a Stressful Situation. Psychopharmacology 97, 183–188, https://doi.org/10.1007/Bf00442247 (1989).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (Nos 81760152, 81460106 and 81860199), Major Disciplines of Academic and Technical Leaders Project of Jiangxi Province (No. 20142BCB22001). We thank Prof. Shangdong Liang and Prof.Guodong Li for assisting us in the experimental design.
Author information
Authors and Affiliations
Contributions
Y.G. designed the study; H.G. and S.G. carried out the experiments; X.W. analyzed the data; Y.S., M.S., Y.H., L.H., L.L., C.Y. and R.H. assisted H.G. to carry out the fndings; H.G. drafed the manuscript and all authors reviewed the manuscript and approved the final version for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ge, H., Guan, S., Shen, Y. et al. Dihydromyricetin affects BDNF levels in the nervous system in rats with comorbid diabetic neuropathic pain and depression. Sci Rep 9, 14619 (2019). https://doi.org/10.1038/s41598-019-51124-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51124-w
This article is cited by
-
Dihydromyricetin Protects Against Salsolinol-Induced Toxicity in Dopaminergic Cell Line: Implication for Parkinson’s Disease
Neurotoxicity Research (2023)
-
Pinocembrin Inhibits P2X4 Receptor–Mediated Pyroptosis in Hippocampus to Alleviate the Behaviours of Chronic Pain and Depression Comorbidity in Rats
Molecular Neurobiology (2022)
-
BDNF Participates in Chronic Constriction Injury-Induced Neuropathic Pain via Transcriptionally Activating P2X7 in Primary Sensory Neurons
Molecular Neurobiology (2021)
-
Effects of palmatine on BDNF/TrkB-mediated trigeminal neuralgia
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.