Abstract
The residual cancer burden index is an important quantitative measure used for assessing treatment response following neoadjuvant therapy for breast cancer. It has shown to be predictive of overall survival and is composed of two key metrics: qualitative assessment of lymph nodes and the percentage of invasive or in situ tumour cellularity (TC) in the tumour bed (TB). Currently, TC is assessed through eye-balling of routine histopathology slides estimating the proportion of tumour cells within the TB. With the advances in production of digitized slides and increasing availability of slide scanners in pathology laboratories, there is potential to measure TC using automated algorithms with greater precision and accuracy. We describe two methods for automated TC scoring: 1) a traditional approach to image analysis development whereby we mimic the pathologists’ workflow, and 2) a recent development in artificial intelligence in which features are learned automatically in deep neural networks using image data alone. We show strong agreements between automated and manual analysis of digital slides. Agreements between our trained deep neural networks and experts in this study (0.82) approach the inter-rater agreements between pathologists (0.89). We also reveal properties that are captured when we apply deep neural network to whole slide images, and discuss the potential of using such visualisations to improve upon TC assessment in the future.
Similar content being viewed by others
Introduction
Neoadjuvant systemic therapy (NAT) for breast cancer (BC) is used to treat locally advanced and operable BC to allow breast-conserving surgery1. NAT provides an opportunity to monitor clinical, radiological and ultimately pathologic response. Pathologic complete response (pCR) to NAT has been shown to provide accurate surrogate endpoint for patient survival2 and in some studies has been prognostic for rate of local recurrence3. As such, accurate assessment of pathologic response to NAT provides important prognostic information.
As residual disease can be subtle and/or scattered, the assessment of pCR to neoadjuvant therapy relies upon a stable and standardized protocol applicable across multiple institutions. Symmans et al.4 proposed a method for quantifying residual disease by calculating the residual cancer burden index (RCB). RCB is recognized as long-term prognostic utility4 and has shown to be more predictive of overall survival compared to other measurements5. The RCB index accounts for two key metrics: qualitative assessment of residual disease in the breast (via tumour cellularity (TC) in the tumour bed (TB) and proportion of in situ component) and assessment of lymph nodes. Here, the TB area comprises of morphologic changes in the stroma suggestive of tumor regression with or without residual invasive or in situ carcinoma. While RCB calculator produces a continuous score, scores are further categorized in four RCB classes from pCR (RCB-0) to extensive residual disease (RCB-III) that are easily reproducible6. Accurate quantification of TC is a laborious, time consuming task that most practicing pathologists are not trained to perform. Yet, TC is crucial for computing the RCB index.
Currently TC is estimated by manually “eyeballing” the TB area at multiple microscopic fields through several slides that represent the largest linear dimension, and comparing the involved area with graphic standard sketches7. Such illustrations although helpful are semi-quantitative, subjective measurements. A single case-level score is then obtained by taking an average of TC scores from different fields and rounded to the nearest 10th percent. However, these scores can also be reformulated on a continuous scale. As manual analysis is limited to predefined discrete values, we are yet to discover the potential benefits of continuous TC scores for prognosis. In theory such measurements can be more precise, however, acquiring them manually is infeasible given there is a greater chance of error and reproducibility is not possible.
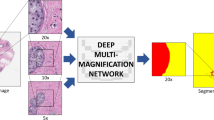
With latest technological advancement in digital pathology, including tissue scanners capable of scanning whole slides at high resolutions, there is potential to leverage image analysis techniques to gain more accurate metrics than is achievable by the human eye, and reduce pathology workload by eliminating time-consuming tasks. TB region can be captured digitally on scanned slides, using annotation tools (Fig. 1). Towards an ultimate goal to automate RCB assessment, we explored methods to evaluate TC as the first step. In this paper, we report the use of automation to compute TC scores using two different image analysis approaches (Fig. 2):
-
1.
An image analysis pipeline which encompasses hand-engineered features designed to mimic the pathologist’s eye.
-
2.
A deep learning approach which learns features directly from raw image data of digital slides using deep convolutional neural networks (DCNNs).
We performed detailed comparisons between the above approaches to validate the feasibility of automation in the pathology workflow and measure the progression of image analysis techniques over the last few years to perform RCB assessment, which currently relies heavily on expert opinion. In this study we define TC as the percentage of total area within predefined areas of interest or patches, occupied by malignant tumour cells. The tumour area defined in Symmans et al.4 excludes tumour infiltrating lymphocytes (TILS). Pathologists, however, will also include areas of cytoplasm surrounding malignant nuclei in their cellularity assessment and this is a somewhat subjective process. Whilst automation can also be used to locate the TB, in this paper we specifically report computation of TC with predefined boundaries, with the intention of implementing this as part of a larger pipeline in future work.
In this paper, we report the benefits and limitations of latest advancements in artificial intelligence, compared to a more traditional hand-engineered approach for designing algorithms. To evaluate the clinical relevance of automation on whole slide images, we also applied our trained models to high resolution digital slides scanned at 20X, achievable in a matter of minutes. We show that a localised analysis of TC on a patch-by-patch basis can be used to give a more descriptive representation of the heterogeneity of the TB area and distribution of TC scores.
Results
Agreements between manual and automated scores
A quantitative comparison between two pathologists: a pathology fellow (Pathologist A) and an experienced breast pathologist (Pathologist B) and our two proposed image analysis pipelines described in Section 4 are given in Table 1 in the form of intra-class correlation coefficient (ICC) values. ICC values are reported between patches i.e. regions of interest measuring 258 μm × 258 μm, extracted from digital slides. The reported intra-rater agreement between the study pathologists was 0.89 and this value indicates the level of variability between our readers in the independent test set. Both automated methods fell short of reported intra-rater agreements however automatically-generated scores were more consistent as shown by reported confidence intervals (shown in square brackets). Such outcomes suggests that automated TC scores are consistently stable with scores retrieved from both annotators, demonstrating the advantage of reproducibility with such systems. Out of the two automated methods, DCNNs were superior, with an average agreement of 0.82, close to agreements between our experts and substantially higher than hand-engineered features. Given the upper and lower bounds of reported scores, DCNNs were on par with inter-rater agreements, and furthermore are reproducible.
To further evaluate DCNN, we also combined both automated systems by identifying cancerous patches from the first InceptionNet model in DCNN (Fig. 2) and then using hand-engineered features to determine an automated TC score. Results are shown on the right in Table 1. Whilst ICC values are better than hand-engineered features alone, DCNN is still superior suggesting both InceptionNets are needed for optimal performance.
Comparison between hand-engineered features and deep learning
A breakdown of prediction accuracies between both automated systems revealed that the DCNN trained to solely distinguish between health and cancerous tissue performed exceptionally well, giving accuracy rates of 93% when compared against both experts. Our hand-engineered approach fell short at 81% due to mis-identified malignant cell nuclei during the cell classification stage.
When comparing TC scores for those patches which contained few cancerous structures i.e. TC scores just above 0%, we found the hand-engineered approach produced cellularity scores with strong concordance with expert pathologists (Fig. 3 (left)). Whilst DCNN generated cellularity scores with good agreement with our study experts, as shown by the line of best fit, generally scores were not as precise as the hand-engineered approach. However the lack of outliers, particularly in the 0–30% range, meant that DCNN performed the best overall. The hand-engineered approach particularly suffered in the >70% range as shown in Fig. 4, suggesting further work is needed to represent regions containing high proportions of tumour cells.
Discussion
In this paper, we evaluated three methods for generating TC scores on digital slides of breast tissue for the purposes of tumour burden assessment. The standard method for computing TC scores is by visual interpretation of the TB which is a time consuming process and is limited to a rough estimate of the proportion of cancerous structures in an irregular region of interest performed by an expert. Furthermore, manual analysis is subject to inter- and intra-rater variability therefore reproducibility of TC scores is a limitation in current practice.
To increase throughput, we designed two alternative methods for generating TC scores which leverage advancements in technology to automatically analyse large whole slide images. One approach was to mimic the way in which a pathologist would compute a score, by first identifying cells in a given region of interest and then measuring the proportion of malignant to benign cells and stroma. This approach has been well adopted in the digital pathology community which has led to a large literature on cell classification and segmentation8,9,10 and feature extraction methods11.
In the last five years there has been a shift in medical image analysis to automatically extract features from image data alone using deep architectures12. The advantages of this approach is that there is no hand-engineering of features involved, and instead appropriate image properties are captured in a model containing several layers. There has been previous work using deep neural networks in digital pathology13,14, and comparisons have shown we can achieve superior performance compared to traditional feature extraction methods15,16,17. In this study, we also found that by using deep neural networks, we could achieve strong agreements with scores produced by two study pathologists; achieving ICC agreements of 0.82, approaching the intra-rater agreement of 0.89 and with tighter upper and lower bounds, suggesting more stable measurements than can be achieved manually. Our hand-engineered approach fell short at 0.75 ICC agreement. Given these outcomes, there is potential to use automation to alleviate the burden of manually estimating TC scores which would allow assessments such as the RCB index more manageable on a routine basis. Furthermore, our method can be used across multiple tumour types for TC assessment with some fine-tuning.
Upon closer inspection of our results, we also found under certain conditions the use of latest automated techniques produced TC scores more similar to our experts. A subset of the scores produced by both automated systems are shown in Fig. 5. The deep neural networks (D) performed better when identifying healthy tissue (top row) and patches containing almost all cancerous tissue (bottom row). The cascade approach we adopted of training a separate cancer detector, proved to be ideal for removing healthy tissue first, giving accuracy rates of 93% when identifying patches containing only healthy structures. The advantage of the deep learning approach comes in distinguishing between >70% TC, and this is demonstrated in Fig. 3, whereby automated scores are tighter around those scores assigned manually.
For the purposes of determining RCB, accurate quantification of TC in the lower range leading to RCB-0 and RCB-1 classes, may enhance clinical prognostication by adopting automation. Symmans et al.4 reported RCB-1 was a good predictor of survival outcome with 89% of triple negative breast cancer patients with RCB-1 relapse-free after 5 years; RCB-2 and RCB-3 were not prognostic. Given that the deep neural networks excelled at low TC range, the use of automation for measuring TC as a RCB component could potentially be improved by adding further training examples containing low TC scores. As it is particularly difficult to quantify TC manually, automation offers an easier method for achieving precise scores which can further contribute to use of the RCB index as opposed to RCB categorical readings.
It should be noted that whilst both automated methods reported here output scores on a continuous scale, the scores provided to the systems during training were not. Manual assessment was performed by providing an estimate of the proportion of carcinoma in each patch, often to the nearest 5% in our experiments; some variations between automation and pathologists’ scores can be explained by the scoring protocol.
Whilst here we specifically evaluated TC, the RCB index also encompasses a measure of the TB area7. Assessment of TB size relies upon consideration of preNAT imaging, gross examination and expert interpretation of the TB. In the current study, TC was assessed in patches derived from predefined TB regions, determined by Pathologist A. As such, this work is only an initial step in automating the entire RCB calculation pipeline. Further work is needed to identify “TB” and to distinguish between invasive and in situ carcinoma in a fully automated pipeline. This may require assessment of multiple digital slides per patient. Furthermore, we were only able to obtain scores from two raters; this is an expensive and time consuming process and it was not possible to recruit more pathologists to carry out this task. Another limitation is that all slides were prepared and scanned at the same centre; a secondary test set from another institution would allow generalizability to be assessed.
One of the main advantages of using automation is the ability to perform detailed analysis across entire whole slide images to give further contextual information. An example of our trained deep neural networks applied to whole slide images is shown in Fig. 6, as heatmaps overlaid on original digital slides. We have appended a higher resolution image as Supplementary Fig. S2. Blue overlays denotes low TC and red denotes patches with high TC scores. In its simplest form, this tool can be used to navigate the reader to the most interesting parts of the tissue thus eliminating around 90% of the slide. This is a desirable property in digital pathology as the substantial portion of a pathologists’ time is performed sifting through benign tissue18 and any method for increasing throughput has significant advantage in the pathology workflow. It is important to note that the deep neural network designed to distinguish between healthy and cancerous patches suffered when applied to whole slide images compared to our patch-based test set. During training, the model was only exposed to a small subset of healthy structures and as such fatty tissue, folding tissue, red blood cells etc. were therefore unrecognizable during testing. We anticipate that with further training with more healthy patches, such errors can be avoided. In the long term, a preprocessing phase to first identify the TB region is recommended.
In Fig. 6, we can also see a distinct distribution of TC scores across the TB, suggesting that a global score of the entire TB may not reflect the characteristics of the TB accurately. There are clearly “pockets” of high cellularity regions and most of the TB consists of healthy or low cellularity regions. The RCB index recommends recording the average TC, however our results suggests an alternative metric which takes into account spatial distribution of TC in the TB may offer new features possibly advantageous for assessing tumour burden. Further work is also needed to investigate using continuous scores for clinical assessment, specifically the relationship between heterogeneity of the TB and prognosis.
To summarise, we performed a comparison between manual and automated assessment of TC and showed that we can gain reproducible scores with automation, and superior performance with deep neural networks. We showed that leveraging such tools on whole slide images can give us richer representations of tumour heterogeneity across the TB, and can potentially be used as an alternative metric to approximated TC scores currently used in practice.
Methods
Data
To validate different methods for computing TC, representative sections from 62 patients with residual invasive BC on resection specimens following NAT were acquired. After de-identification, representative routine Hematoxylin and Eosin glass slides were scanned at 20X magnification (0.5 lm/pixel) in the Department of Anatomic Pathology at Sunnybrook Health Sciences Centre (SBHSC), Toronto, Canada. After initial assessment and quality control, nine patients in our study were excluded. Table 2 shows a summary of patients’ clinical characteristics in this study.
This study was approved by the Research Ethics Board (REB) of Sunnybrook Health Sciences Centre (project ID: 312 2014) and all methods were carried out in accordance with relevant guidelines and regulations. Informed consent was waived by the REB of SBHSC. The distribution of our training and test sets were such that patient data used for training was excluded from testing. Patches, each with dimensions 512 × 512 pixels, were hand-selected from 96 whole slide images, 25 of which were reserved for testing purposes. Patches were selected to represent a wide representation of range of TC scores. In total we extracted 3,700 patches (training: 2579, test: 1121) which were then labelled manually.
Manual interpretation
For this study, we recruited two pathology experts: a breast pathology fellow (Pathologist A) and an experienced pathologist with focused practice in breast pathology (Pathology B). Each pathologist independently annotated identified patches on a digital pathology viewing platform, Sedeen Viewer (Pathcore)19. Initially, Pathologist A was asked to select representative patches spanning a full range of TC scores (i.e. from low to high TC) as well as patches containing healthy tissue. For each patch, a TC score, ranging from 0% to 100% for assessment of RCB7, was provided. Patches which did not contain any tumour cells were assigned a TC score of 0%. The training set was only annotated by Pathology A. Both Pathologist A (the annotator for the training set) and Pathologist B annotated the test set, and we compared the variability between both experts in reported results. In addition to continuous scores, both pathologists also classified each patch to low, medium, high TC and no tumour cells. Annotations were performed independently therefore each expert was unaware of scores assigned by the other.
The distribution of TC scores provided by each pathologist is given in Table 3. Note that the distribution varies considerably at higher cellularity scores (i.e. >70%) within our test sets, with 18% of Pathologist A’s scores within this range, and 31% in Pathologist B’s scores. Any automated system must be able to adjust for these differences between our experts.
Hand-engineered features
To mimic scores provided by pathologists in an automated manner, we first designed a cell nuclei segmentation algorithm to identify boundaries of individual cells of the following types: lymphocytes, epithelial cells, malignant cells. Cells boundaries were identified by removing stain variations through a series of color stretch and color space conversions. A support vector machine (SVM) classifier was trained from several appearance, textural and spatial features extracted from identified cell nuclei, producing a cell map during testing (Fig. 2 (top)).
To compute a cellularity score, we segmented all malignant nuclei in each patch, dilated the resulting binary mask to account for the presence of cytoplasm, and then computed the fraction of area covered by malignant tissue. Finally, a calibration step was performed to bring computed TC scores closer to scores manually assigned by the pathologists in our study. A full description of this algorithm is given by Peikari et al.20.
Deep convolutional neural network
Deep convolutional neural networks (DCNNs) are a family of architectures in artificial intelligence which are derived from a conceptual model of the human brain12. Typically, a DCNN consists of multiple layers, each of which contain several artificial neurons. By learning connections between hundreds or even millions of these neurons through simple linear activation functions, we can capture representations of complex data inputs. In a DCNN, groups of neurons are stacked in a series of specialised layers which can model further abstract representations of the data without manual intervention and this is where the power of DCNNs lie. Whilst there are many approaches to building DCNNs, here we opted to finetune a prebuilt network called InceptionNet21 which has been well-adopted in digital pathology. To compute TC scores, we trained two separate InceptionNets: one that distinguished between healthy and cancerous tissue, and the other to output regression scores on a continuous scale between 0% and 100%. Details of the implementation of the InceptionNet models is provided in supplementary material (see Supplementary Data S1).
Software and tools
Algorithms to produce hand-engineered features were developed in Matlab and the built-in “fitcsvm” library was used to produce SVM predictions. The DCNN system was implemented in Python and Keras with a TensorFlow backend, a well-adopted library developed by Google for building deep neural networks. In each platform, we developed functions to load manual annotations generated by the Sedeen Viewer (see Section 4.2).
R was used to perform statistical analysis in this study.
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. The Lancet Oncol. 19, 27–39 (2018).
Nahleh, Z., Sivasubramaniam, D., Dhaliwal, S., Sundarajan, V. & Komrokji, R. Residual cancer burden in locally advanced breast cancer: a superior tool. Curr. Oncol. 15, 271–278 (2008).
Mamounas, E. P. et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: Results from combined analysis of national surgical adjuvant breast and bowel project B-18 and B-27. J. Clin. Oncol. 30, 3960–3966 (2012).
Symmans, W. F. et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 25, 4414–22 (2007).
Pientinger, F. et al. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod Pathol. 28, 913–920 (2015).
Romero, A. et al. Correlation between response to neoadjuvant chemotherapy and survival in locally advanced breast cancer patients. Annals of Oncology 24, 655–661 (2013).
Detailed pathology methods for using residual cancer burden, https://www.mdanderson.org/education-and-research/resources-for-professionals/clinical-tools-and-resources/clinical-calculators/calculators-rcb-pathology-protocol2.pdf.
Yang, X., Li, H. & Zhou, X. Nuclei segmentation using marker-controlled watershed, tracking using mean-shift, and kalman filter in time-lapse microscopy. IEEE Transactions on Circuits Syst. 53, 2405–2414 (2006).
Wienert, S. et al. Detection and segmentation of cell nuclei in virtual microscopy images: A minimum-model approach. Sci. Reports 2, https://doi.org/10.1038/srep00503 (2012).
Sirinukunwattana, K. et al. Locality sensitive deep learning for detection and classification of nuclei in routine colon cancer histology images. IEEE Transactions on Med. Imaging 35, 1196–1206 (2016).
Liu, C., Shang, F., Ozolek, J. A. & Rohde, J. K. Detecting and segmenting cell nuclei in two-dimensional microscopy images. J. Pathol. Informatics 7, 42 (2016).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2016).
Cruz-Roa, A. et al. High-throughput adaptive sampling for whole-slide histopathology image analysis (HASHI) via convolutional neural networks: Application to invasive breast cancer detection. PLoS One, https://doi.org/10.1371/journal.pone.0196828 (2018).
Wang, D., Khosla, A., Gergeya, R., Irshad, H. & Beck, A. H. Deep learning for identifying metastatic breast cancer. Preprint at https://arxiv.org/abs/1606.05718 (2016).
Janowczyk, A. & Madabhushi, A. Deep learning for digital pathology image analysis: A comprehensive tutorial with selected use cases. J. Pathol. Informatics 7 (2016).
Kothari, S., Chaudry, Q. & Wang, M. D. Extraction of informative cell features by segmentation of densely clustered tissue images. Conf Proc IEEE Eng Med Biol Soc. 6706–6709, https://doi.org/10.1109/IEMBS.2009.5333810 (2009)
Doyle, S., Agner, S., Madabhushi, A., Feldman, M & Tomaszewski, J. Automated grading of breast cancer histopathology using spectral clustering with textural and architectural image features. 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, https://doi.org/10.1109/ISBI.2008.4541041 (2008).
Gurcan, M. N. et al. Histopathological image analysis: A review. IEEE Rev Biomed Eng. 2, 147–171 (2009).
Martel, A. L. et al. An image analysis resource for cancer research: PIIP-pathology image informatics platform for visualization, analysis, and management. Cancer Res. 77, e83-e86, http://cancerres.aacrjournals.org/content/77/21/e83 (2017).
Peikari, M., Salama, S., Nofech-Mozes, S. & Martel, A. L. Automatic cellularity assessment from post-treated breast surgical specimens. Cytometry A. 91, 1078–1087, https://doi.org/10.1002/cyto.a.23244 (2017).
Szegedy, C., Vanhoucke, V., Ioffe, S., Shlens, J. & Wojna, Z. Rethinking the inception architecture for computer vision. Preprint at https://arxiv.org/abs/1512.00567 (2015).
Acknowledgements
This work has been supported by grants from the Canadian Breast Cancer Foundation, Canadian Cancer Society [grant number 703006] and the National Cancer Institute of the National Institutes of Health [grant number U24CA199374-01]. We also thank NVidia Corporation for their GPU donations for this research.
Author information
Authors and Affiliations
Contributions
A.L.M. and S.N.-M. designed and organised the research study. S.N.-M. and S.S. annotated the dataset. A.Y.P. developed software to gather and store annotations for this study. S.A. implemented the deep learning models and M.P. implemented the hand-engineered image analysis pipeline. S.A. performed the analysis. All authors equally contributed towards the writing of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akbar, S., Peikari, M., Salama, S. et al. Automated and Manual Quantification of Tumour Cellularity in Digital Slides for Tumour Burden Assessment. Sci Rep 9, 14099 (2019). https://doi.org/10.1038/s41598-019-50568-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-50568-4
This article is cited by
-
Standardized Pre-clinical Surgical Animal Model Protocol to Investigate the Cellular and Molecular Mechanisms of Ischemic Flap Healing
Biological Procedures Online (2024)
-
Algorithm-assisted diagnosis of Hirschsprung’s disease – evaluation of robustness and comparative image analysis on data from various labs and slide scanners
Diagnostic Pathology (2024)
-
Biological insights and novel biomarker discovery through deep learning approaches in breast cancer histopathology
npj Breast Cancer (2023)
-
Deep learning in histopathology: the path to the clinic
Nature Medicine (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.