Abstract
Earthquake/tsunamis can have profound impacts on species and their genetic patterns. It is expected that the magnitude of this impact might depend on the species and the time since the disturbance occurs, nevertheless these assumptions remain mostly unexplored. Here we studied the genetic responses of the crustacean species Emerita analoga, Excirolana hirsuticauda, and Orchestoidea tuberculata to the 27F mega-earthquake/tsunami that occurred in Chile in February 2010. mtDNA sequence analyses revealed a lower haplotype diversity for E. analoga and E. hirsuticauda in impacted areas one month after the 27F, and the opposite for O. tuberculata. Three years after the 27F we observed a recovery in the genetic diversity of E. analoga and E. hirsuticauda and decrease in the genetic diversity in O. tuberculata in 2/3 of sampled areas. Emerita analoga displayed decrease of genetic differentiation and increase in gene flow explained by long-range population expansion. The other two species revealed slight increase in the number of genetic groups, little change in gene flow and no signal of population expansion associated to adult survival, rapid colonization, and capacity to burrow in the sand. Our results reveal that species response to a same disturbance event could be extremely diverse and depending on life-history traits and the magnitude of the effect.
Similar content being viewed by others
Introduction
Large-scale natural disturbances can have profound impacts on ecosystems, affecting several ecological and fitness traits of key species through the reduction in population sizes and habitat destruction (e.g.1,2,3,4). For example, mega natural disturbances, such as tsunamis, earthquakes, fires, and hurricanes can drive long-term variations in the abundance and distribution of species, community dynamics and ecosystem processes in terrestrial and marine ecosystems1,2,3,4,5,6,7,8,9,10,11,12,13,14. While the extension of these disturbances is short, they can have deep effects on species. Nevertheless, extreme disturbances not only affect species on an ecological level, but also a genetic level. Provided that genetic diversity is linked to the evolutionary potential of a species to respond to a changing environment, the genetic composition of a population plays an important role for its persistence, since populations with more genetic diversity are less prone to extinction15,16,17. Thus, evaluating the impact of disturbances on species’ genetic diversity and their responses on short and long temporal scales is fundamental to understanding the factors and mechanisms that determine population and ecosystem resilience.
Empirical evidence evaluating populations’ genetic responses to disturbances are scarce and mostly focused on the effects of rapid anthropogenic destruction of forest ecosystems (see18). The existing evidence has shown that impacts on genetic diversity appear to be idiosyncratic, depending not only on the magnitude of the disturbance, but also on life history traits associated with species’ breeding systems and their dispersal capacities18. For example, genetic studies have demonstrated a loss in genetic diversity in the tropical tree Pithecellobium elegans after the destruction of its habitat19. On the contrary, no effects were observed in any of the genetic diversity indices of the tree Swietenia macrophylla as a consequence of a century of logging20. This patterns in S. macrophylla was explained by the increase in pollen flow and range expansion as a result of logging, which reduced the loss of diversity due to this species’ dispersal capacity. In one of the few studies available on marine species, authors reported that the genetic diversity of populations of the kelp Lessonia nigrescens in northern Chile was reduced by up to 50% after the El Niño–Southern Oscillation event of 1982/8321. Since then, this species has shown a slow genetic recovery that could be explained by its low dispersal potential. On the other hand, authors studying the intertidal goby Chaenogobius annularisthe and the mud snail Batillaria attramentaria found no evidence of loss in genetic diversity as a consequence of the 2011 Tohoku earthquake and tsunami22,23. Provided that the scale, magnitude and duration of each disturbance varies widely depending upon the study, generalisations about species’ responses to mega perturbations are difficult to draw. To overcome this limitation, it has been proposed that, when possible, studies should examine the genetic responses of multiple species with contrasting ecological and life history traits to the same ecological disturbance18.
On February 27th, 2010 (27F), the southern-central coast of Chile was struck by a mega-earthquake (8.8 Mw) and tsunami. The earthquake’s rupture zone covered mainly a region localized between 35° and 37°S24, generating changes in the elevation of the coastline of up to 3 m. As a consequence of the earthquake, massive mortality of intertidal flora and fauna occurred in localities with extreme coastal uplift3,4. In addition, the tsunami produced by the earthquake significantly affected nearshore marine life, devastating hundreds of kilometres of coast3,4,12. The magnitude of the tsunami was variable along the Chilean coast, according to the distance from the epicentre and orientation of the coastline25. In a study of the ecological consequences of the 27F earthquake authors found that the mortality of intertidal invertebrates on sandy beaches was positively correlated with tsunami height4. This study suggested that even in the absence of changes in coastal height, the ecological effects of the 27F tsunami on Chilean marine ecosystems could be significant; similar observations have been reported for subtidal macrofauna in other coastal areas of the world26,27,28,29.
Here we evaluated the population genetic response to the 27F mega-earthquake and tsunami in three common crustacean species of Chilean sandy beach ecosystems: Emerita analoga, Excirolana hirsuticauda, and Orchestoidea tuberculata. These species have diverse life history traits, which have differential consequences on their dispersal ability (Fig. 1). The sand mole E. analoga is a widely distributed decapod crustacean (58°N to 55°S) inhabiting the lower intertidal zone with a meroplanktonic life cycle and high dispersal ability whose larvae can be found in Antarctica and Polynesia, although adults are only found along the coasts of Chile and Peru30,31. The cirolanid isopod E. hirsuticauda is a conspicuous endemic component of the middle shore intertidal level of Chilean sandy beaches between 22°S and 42°S32,33 with juveniles and adult individuals being active swimmers34,35. The talitrid amphipod O. tuberculata inhabits the upper level of the intertidal zone36,37,38,39,40 of Chile (35°S–40°S), with females incubating their offspring to the juvenile stage and low dispersal potential. These three species were strongly affected by the 27F mega-earthquake, but their responses varied intraspecific and interspecifically depending upon the particular impacts experienced by the diverse populations (variation of uplift level and tsunami height depending on the beach) and the species studied4,41. For instance, ecological studies have shown that E. analoga was highly affected during the 27F, but presented a low colonization following the 27F; a contrasting pattern was observed in E. hirsuticauda and O. tuberculata, where a rapid post-disturbance colonization was recorded4,41,42.
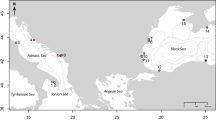
In this study, we assessed the effects of the 27F mega disturbance on genetic diversity and population structure of E. analoga, E. hirsuticauda, and O. tuberculata along the Chilean coast. Comparisons among sampled localities were made through time and among highly impacted areas (37°09′–37°42′S, Fig. 2) and non-impacted control areas located south and north of the disturbed area. We hypothesised that higher impacts on population genetic traits would be observed in E. analoga given its lower adult mobility in comparison to the other two species. However, a fast recovery of population genetic diversity was also expected in this species given the presence of a free larval stage with a high dispersion potential.
Results
The sequence alignment length and concatenation of both genes was 875 bp for T1 and T2, respectively, in E. analoga, 1070 bp (T1 and T2) in E. hirsuticauda, and 1078 bp (T1 and T2) in O. tuberculata (Genbank Accession number MK914652 - MK917441). No evidence of stop codons were detected in the COI gene, no substitution saturation was found for any species or sampling time, indicating that data sets were suitable for all of the subsequent genetic analyses.
Genetic structure
The Bayesian approach of spatial clustering based on Geneland analyses showed changes in the genetic structure of all of the studied species through time (Figs 3–5). The number of genetic clusters decreased throughout time in E. analoga (from K = 5 to K = 2) and slightly increased in E. hirsuticauda (from K = 4 to K = 6) and O. tuberculata (from K = 7 to K = 8; Figs 3a,b–5a,b). Probabilities for each genetic cluster were greater than 0.5 in most cases, except for E. hirsuticauda in T2, where the probabilities ranged between 0.2–0.4. Emerita analoga populations in the impacted area were well differentiated in T1, while they were part of the largest cluster in T2 (Fig. 3a,b). Excirolana hirsuticauda and O. tuberculata in the impacted area maintained the same genetic differentiation in southern and northern non-impacted areas between T1 and T2 (Figs 4a,b and 5a,b). The AMOVA comparing the zones with different levels of impact showed that E. analoga and E. hirsuticauda have different spatiotemporal patterns of haplotype structure than O. tuberculata. In both periods of analysis, the genetic-population structure of the first two species was mainly explained by the haplotype intrapopulation variation, followed by the haplotype variations between populations within each zone. In contrast, for O. tuberculata, the greatest contribution to the haplotype structure in both periods was explained by the variation between populations within zones, followed by intrapopulation variation; although this last level did not constitute a significant determinant of haplotype structuring (Table S1).
Genetic clusters identified with GENELAND (each color represents a genetic cluster) at Time 1 (T1; a) and Time 2 (T2; b), mean haplotype diversity (c) and nucleotide diversity + SD (d) including genetic differences and results of AMOVA analyses among sites, times (T1 and T2), and sites-times, mismatch distribution (e), and gene flow estimated with MIGRATE-n for E. analoga at T1 (f) and T2 (g). Arrows’ width represents the amount of immigrant numbers, circles’ size represents the value of Ne. NNI: northern non-impacted area, IA: impacted area and SNI: southern non-impacted area.
Genetic clusters identified with GENELAND (each color represents a genetic cluster) at Time 1 (T1; a) and Time 2 (T2; b), mean haplotype diversity (c) and nucleotide diversity + SD (d) including genetic differences and results of AMOVA analyses among sites, times (T1 and T2), and sites-times, mismatch distribution (e), and gene flow estimated with MIGRATE-n for E. hirsuticauda at T1 (f) and T2 (g). Arrows’ width represents the amount of immigrant numbers, circles’ size represents the value of Ne. NNI: northern non-impacted area, IA: impacted area and SNI: southern non-impacted area.
Genetic clusters identified with GENELAND (each color represents a genetic cluster) at Time 1 (T1; a) and Time 2 (T2; b), mean haplotype diversity (c) and nucleotide diversity + SD (d) including genetic differences and results of AMOVA analyses among sites, times (T1 and T2), and sites-times, mismatch distribution (e), and gene flow estimated with MIGRATE-n for O. tuberculata at T1 (f) and T2 (g). Arrows’ width represents the amount of immigrant numbers, circles’ size represents the value of Ne. NNI: northern non-impacted area, IA: impacted area and SNI: southern non-impacted area.
Genetic variation
Mean haplotype diversity (Hd) in E. analoga populations from the impacted area was lower than Hd from northern and southern non-impacted areas at T1 (Table S2). At T2, Hd from impacted areas became very similar to both non-impacted areas at T2. Hd in E. analoga was significantly different between sites (p < 0.001), time (p < 0.002), and site*time (p < 0.002; Fig. 3c). For E. hirsuticauda, no significant differences were found in the mean value of Hd for either of the two factors (site and sampling time) nor for their interaction site*time (p < 0.330, p < 0.230, p < 0.480; Fig. 4c; Table S2). Mean Hd in O. tuberculata in the impacted area was higher than Hd in northern and southern non-impacted areas at T1 (p < 0.02; Fig. 5c; Table S2). At T2, the mean Hd in the impacted area was still higher than in both northern and southern non-impacted areas, but northern non-impacted areas showed less Hd than southern non-impacted areas. The lowest mean value of nucleotide diversity (π) for E. analoga was observed in the impacted area at T1, and increased significantly between T1 and T2 (Fig. 3d). At T2, π of this species in impacted and northern non-impacted areas was similar, while it was higher in southern non-impacted areas. Nucleotide diversity (π) in E. analoga showed significant differences for both the factor area and sampling time (p < 0.001 and p = 0.004 respectively), but not for their interaction (Fig. 3d). For E. hirsuticauda, π did not differ for either of the two factors or for the interaction between them (Fig. 4d). Nucleotide diversity of O. tuberculata was lower than in the other two species (Fig. 4d). At T1, π was higher among populations in impacted than in non-impacted areas. At T2, a decrease in π in populations in impacted were observed while values of π were maintained in northern non-impacted areas. Contrastingly, an increase in π among southern non-impacted areas between T1 and T2 was observed. In O. tuberculata, differences in π values were non-significant among sites, times and sites*times (Fig. 5d). Differences between pairs of sequences estimated using the mismatch analyses showed a unimodal distribution for E. analoga at T2, a signal of population expansion (Fig. 3e). Contrastingly, a bimodal distribution for E. hirsuticauda and O. tuberculata in T1 as well as in T2 was observed (Fig. 4e,e).
Gene flow analyses
In all cases, the migration analysis supported the panmictic model (BF(Panmictic-Full Model) >5.0) and showed temporal changes in effective population size (Ɵ) and migration rate among clusters (M). In E. analoga the cluster composed of populations in the impacted area had the lowest value of effective population size in T1 (Ɵ: 0.43E-3 ± 0.37E-3; Fig. 3f). This magnitude was approximately 8 and 6 times lower than the maximum values of Ɵ at T1, which corresponded to the Cocholgüe (Ɵ: 3.42E-3 ± 3.39E-3) and Boca (Ɵ: 3.42E-3 ± 3.39E-3) clusters, both located in the northern non-impacted area. At T2, the effective population size of the cluster formed by the population of Colcura (Ɵ: 9.49E-3 ± 3.28E-3) was approximately 9.1 times greater than the Ɵ value of the cluster combining the remaining populations (Ɵ: 1.04E-3 ± 1.09E-3; Fig. 3g). Gene flow was moderate to high among all populations at T1, with the highest number of immigrants occurring in non-impacted areas in the southernmost localities. In T2, gene flow was high and mostly symmetric for the two identified clusters. Despite the difference in Ɵ, the migration rate from the largest cluster to Colcura (M: 3901 ± 1539 migrants*generation−1) was 12% greater than the gene flow from Colcura to the largest cluster (M: 2731 ± 1421 migrants*generation−1; Fig. 4g).
In E. hirsuticauda, the two clusters that contained the populations of the impacted area, the Lebu-Rumena and Lobería-Nigue-Morhuilla clusters, presented the lowest values of effective population size in T1 (Ɵ: 2.94E-3 ± 3.27E-3 and Ɵ: 2.54E-3 ± 4.08E-3 respectively; Fig. 4f). These Ɵvalues were about 75% lower than the effective population size of the cluster formed by the extreme populations from both the northern and southern non-impacted areas (Cocholgüe-Boca-Cheuque-Pichicuyín cluster), and approximately 24% lower than Ɵ of the Chivilingo-Colcura-Lota cluster. At T2, the two northernmost locations (Cocholgüe and Boca) constituted the cluster with the largest population size (Ɵ = 4.92E-3 ± 5.35E-3), followed by three clusters combining localities from the affected area and the southern non-affected area. Of these three clusters, the individuals of the affected populations in Lebu and Rumena were genetically grouped with Nigue, forming the cluster with the second largest population size Ɵ: 2.96E-3 ± 2.50E. At T1, gene flow among all populations was moderate to low and mostly symmetric (Fig. 4f), while it was moderate and symmetric at T2 (Fig. 4g).
In O. tuberculata at T1, individuals from localities belonging to the impacted area formed two clusters showing the highest values of population size (Rumena cluster Ɵ: 5.19E-3 ± 7.57E-3 and Lebu-Morgüilla cluster Ɵ: 4.00E-3 ± 8.91E-3; Fig. 5f). Values of Ɵ for these clusters were more than twice those estimated for the third largest cluster (Ɵ: 1.94E-3 ± 2.87E-3) composed of populations from the four localities of the southern non-impacted area. Of the eight genetic clusters observed at T2, two were located within the impacted area (Fig. 5g), with the Rumena cluster showing a larger population size (Ɵ: 2.97E-3 ± 7.02E-3) than the Lebu-Morgüilla cluster (Ɵ: 1.45E-3 ± 5.12E-3). However, Ɵ values of both of these clusters were smaller than those estimated in the Boca and Lobería clusters, which presented the largest population sizes (Ɵ: 4.83E-3 ± 8.55E-3 and Ɵ: 3.58E-3 ± 8.10E-3, respectively). Gene flow among all populations was symmetric and moderate to low at T1 (Fig. 5f) and moderate and symmetric at T2 (Fig. 5g).
Discussion
Disturbance processes, such as earthquakes associated with coastal uplift and tsunamis, can shape the genetic patterns of coastal species (i.e. population structure, gene flow and diversity)43. When populations are suddenly impacted by a mega disturbance, their genetic diversity tends to decline and the rate of that decline depends mostly on the effective population size, the number of mutations occurring after the disruptive event, and the amount of new genetic variants in a population as a result of gene flow44,45,46. Nevertheless, not only the genetic attributes of populations can help to understand the genetic responses of species to a disruptive event; ecological traits also play an important role in explaining the direction and magnitude of changes observed in the genetic composition of species under perturbation. As a result of regional and local variations in habitat, magnitude of the disruptive event and ecological attributes characterizing affected communities, each population is expected to react uniquely to perturbations, revealing a unique history of bottleneck and recovery18. Our results, comparing the genetic consequences of the 27F earthquake/tsunami in three species with different history traits suggest that species’ responses to disruptive events can be extremely diverse, even when exposed to the same disturbance. These responses are highly depending on the combined effects of the main species traits and modalities such as movement type, mobility degree, and intertidal zone habitat occupied (Fig. 1).
The negative impacts of the combined effects of earthquake and tsunami on impacted areas through time is evident in the genetic parameters of E. analoga. The genetic diversity of E. analoga was lower among populations from impacted areas at T1, showing a significant increase by T2, reaching values similar to those found in non-impacted areas. After the earthquake, E. analoga showed a significant reduction in the number of populations; nonetheless, throughout time, as a consequence of gene flow and population expansion, E. analoga showed a higher genetic diversity at T2 when compared to T1. At T2 the largest cluster, including individuals from 13 of the 14 sampled localities, presented the lowest effective population size (Ne) compared to the populations comprised of individuals from the single locality of Colcura. This lower Ne in the largest cluster could be explained by the observed population expansion signal and increase in the gene flow, leading to the homogenization of the 13 populations at T2, a pattern that has been well documented in theoretical and empirical studies47. Ecological studies analysing the effects of the 27F earthquake in E. analoga have suggested that this species was one of the most affected by the coastal uplift and tsunami during the 27 earthquake4,41. Consequently, the reduction of the genetic diversity of E. analoga through time could be the result of the combined effect of the removal of recruits and adults during the 27F tsunami as well as a source-sink dynamic. In addition, it has been noticed that the higher recruitment of E. analoga throughout the sampled area occurs near February41, thus the disturbance events from the 27F might have reduced the available habitat for new recruits. Provided that the massive adult mortality was a consequence of the coastal uplift, which may have wiped out most of the rare genetic variants in adults, a lower genetic diversity and higher population differentiation could be expected after the earthquake. However, our results suggest that three years after the disruptive event of the 27F, populations might have recovered, with new recruits settled, reducing the genetic differentiation, increasing the genetic diversity and revealing signals of population expansion in the mismatch analyses. This last finding could be the result of a recolonization of individuals from distant non-impacted areas, which can be explained by the high dispersal potential of E. analoga given its intermediate larval dispersal stage in areas like Polynesia and Antarctic waters30,31.
The genetic patterns observed in E. hirsuticauda are in line with the ones of other intertidal species23. This species showed no significant differences in either genetic diversity index at the two sampling times, in impacted and non-impacted areas. However, the analysis of population differentiation throughout time revealed a slight increase in population differentiation, no signal of population expansion, and an increase in gene flow along with a reduction of Ne in the southern non-impacted area at T2. A low or nil impact of mega disturbances has also been reported in other marine species. Though this is the first study assessing the effects of earthquakes/tsunamis on crustacean’s species, studies in other species can also be a matter of interest for contrasting results. For instance, along the coast of Japan, authors have noticed that the intertidal mud snail Batillaria attramentaria experienced a reduction in its effective population size (Ne) after the 2011 Tohoko tsunami23. Nevertheless, the effect of this mass mortality produced no significant reduction in the allelic richness of this mud snail. These authors suggested that the lack of impact on this genetic diversity descriptor may have been the result of a combination of very large populations before the tsunami and a high recruitment of juveniles after the disturbance.
Although the general patterns revealed in our study found that at T2 E. analoga and E. hirsuticauda species decreased their Hd in impacted areas and increased their Hd in non-impacted areas, in O. tuberculata the highest Hd was recorded in the impacted area at T1. The same pattern was observed at T1 for π in O. tuberculata, but the increase in π in northern non-impacted areas was so high that it turned out to be the area with the highest Hd at T2. Similar to that observed in E. hirsuticauda, an increase in the number of populations was detected in O. tuberculata at T2, with the differentiated populations located within the southern non-impacted area. Although there are only a few of studies available linking ecology and genetics in sandy beach organisms exposed to disturbances, our results are on the line of previous results with talitrids48,49,50. Ecological studies have found a similar trend, with the species E. hirsuticauda and O. tuberculata showing similar abundances before and after the 27F earthquake4. Other authors have found that both species quickly recovered their abundance after the tsunami51, suggesting that the particular life history traits of these species may help to buffer the impacts of the mega-perturbation on their local populations. For instance, a high recovery of Orchestoidea tuberculata has been suggested to be related with its feeding strategy as scavengers and detritivorous (Fig. 1)51. For E. hirsuticauda, the lack of significant reductions in its density and genetic diversity could be explained by the high mobility of adults, and high dispersal potential of planktonic larvae. The swimming capacity of adults may allow them to resist the tsunami wave and then, relocate to a new site. In addition, the free larval stage has the capacity to rapidly colonize impacted areas as was suggested previously42. Contrary to E. hirsuticauda, O. tuberculata does not present an intermediate dispersal stage given that females incubate their offspring until they reach the juvenile stage (Fig. 1). However, adults have the capacity to jump and burrow up to 30 cm in the sand for shelter (Fig. 1)34, which together with their high resistance to desiccation could help this species to survive after disrupting events. Thus, it appear that O. tuberculata shows a high genetic and ecological resilience, possibly providing this species with advantageous adaptations to evade the direct effects of the tsunami and survive a coastal uplift and tsunami such as the 27F mega-earthquake.
Our study clearly reveal that species’ response to a major disturbance, in this case an earthquake and tsunami, depends on the ecology of each species; and that these distinct responses can influence the genetic diversity of species. The use of highly variable DNA sequences allowed to observe these changes in genetic diversity over a short period of time, only a few generations within each species. Whether DNA sequences can be used for recent processes has been the debate of multiple studies throughout the last decade (e.g.52,53,54), with no decisive answer. Here we have shown that recent massive disruptive events can actually be detected with DNA sequences, most likely due to the magnitude of the event. Nevertheless, it will be necessary to compare these results with other markers characterized by faster mutation rates, such as microsatellite or SNPs53, in order to confirm our conclusion. In a broader perspective, no hypotheses has been made yet on the ecologically meaningful units at which genetic patterns are revealed for sandy beach resident organisms. Our results suggest that we can detect changes in the genetic patterns of sandy beach organisms at population level after a disruptive event, even between relatively close populations. Although more observations are needed, this study can help future researchers to evaluate what major drivers explain the genetic diversity of species (with different life history traits) inhabiting areas frequently exposed to disruptive events.
Although areas suffering constant disturbances present important challenges for human populations, they also present interesting opportunities, since they act as natural laboratories to study changes in the ecological and genetic patterns of populations during recovery process after strong perturbations. It could be crucial to submit these areas to continuous sampling and monitoring in order to understand how species respond to changes in a landscape under recurrent perturbation regime. This information could then be used to predict what may happen to species facing natural or human-induced habitat modifications and thus provide better information for the conservation of species undergoing environmental disturbances.
Methodology
Individuals’ collection and laboratory protocols
Given that no samples were available before the mega-earthquake that occurred in February 2010, we used an impact-control approach. For this, we sampled 14 localities (sandy beach localities between 36°S and 39°S) with different levels of impacts, according to the uplift level and tsunami strength4,14. The magnitude of the impact was categorized in 3 areas: northern non-impacted area (NNI), impacted area (IA) and southern non-impacted area (SNI) (Fig. 2; Table S2). Samplings were carried out in March 2010 (T1), one month after the mega-earthquake, and repeated three years later in 2013 (T2), between February and June. All sampled localities were inside non-protected areas. Whole DNA was extracted from collected individuals using an already published protocol55 and its quality was evaluated in a ND 1000 spectrophotometer (NanoDrop Technologies). DNA templates were amplified for the mitochondrial genes COI and 16S using the PCR protocols described by other authors56,57,58. Amplified products were then sequenced in Macrogen Inc. (Seoul, Korea). Sequences of both genes were concatenated with Mesquite 3.4 software59 and translated to determine whether stop codons were present in the COI gene.
Characterization of the sequence matrices
The potential existence of saturation60 and adjustment to a neutral model of mutation-drift equilibrium61 were assessed with the software DAMBE 6.4.10062 and DnaSP 6.063, respectively. DnaSP was also used to calculate both haplotype and nucleotide diversity (Hd and π). For each species, mean Hd and π values were compared using a two-way Analysis of Variance (ANOVA), with two fixed factors: area with three levels (northern non-impacted area, impacted area and southern non-impacted area), and time with two levels (T1 and T2). When significant effects were detected, Tukey a posteriori tests were performed. All two-way ANOVAs were run in the STATISTICA 7.0 program64.
Analysis of spatial genetic structure
GENELAND 4.08 was used to detect genetic discontinuities under a spatially explicit genotypic context65 for each species and sampling time (T1 and T2). Short analyses were carried out with K = 12 for all species, with 5,000,000 MCMC iterations, thinning each 1,000 iterations and burn-in of 10%, using the Akaike Information Criteria in MCMC context (AICM)66. Final simulations were carried out by fixing the number of most probable clusters (K) obtained in the first simulations for each species and sampling time, using 30,000,000 iterations of MCMC, thinning each 1,000 iterations and burn-in of 10%. In each analysis, we computed the Poisson-Voronoi tessellation maximizing the value for the rate of the Poisson process (λ)67. The number of genetic clusters was assessed through probability density distribution graphs, for each species in T1 and T2.
Genetic comparison and population expansion
From the variations in the observed haplotype frequency, the genetic differentiation for each species and sampling time was compared applying an Analysis of Molecular Variance (AMOVA). The design of these analyses was carried out by grouping localities depending on the level of impact of the tsunami/earthquake (i.e., impacted area vs. northern and southern non-impacted areas). The genetic differentiation was assessed through AMOVAs based on matrices of estimated genetic distances according to the better-fitted sequence evolution model68 using the software Arlequin 3.5.2.2 69. Evolution models of the sequences (estimated for each gene independently and for the concatenated data set), and the underlying parameters, were estimated with the program jModelTetst 2.1.1070,71. To investigate signals of population expansion in the three species at each sampling time (T1 and T2) we computed the distribution of pairwise differences from the segregating sites of the concatenated data set by calculating the mismatch distribution analyses implemented in DnaSP.
Gene flow
It has been recently noted that Migrate-n can provide accurate results of recent migration rates, despite the fact that it has most frequently been used to explain long-term historical patterns72. Therefore, we used this software to calculate the genetic diversity and migration patterns of populations with the parameters effective population size (Θ) and migration rate among clusters (M) scaled by the mutation rate (μ) in Migrate-n 3.6.1173,74,75,76. The analyses were performed using 10 replicates of short chains with 50,000 iterations and one long chain with 1,000,000 iterations and 10% burn-in. The obtained values were used to parameterize the priors of Bayesian runs using six independent replicates for each species and sampling time. Each Bayesian run was performed with the transition/transversion ratio value of each gene obtained from the previously inferred evolution models. In each replicate we computed 5,000,000 iterations with samples taken every 1,000 steps and 10% burning. We compared the parameters obtained based on: (1) better adjustment considering constant or variable mutation rates for both genes, and (2) better adjustment to an exponential or uniform model of a posteriori distribution. For each type of mutation rate and distribution model, the six replicates were merged, and the associated marginal likelihood was compared under the Bayes Factor criterion for the selection of models77 implemented in Tracer 1.6.0 software78 with 10,000 Bootstrap replicates. The mean and standard deviation of the obtained Θ and M distributions were represented in gene flow diagrams using the Excel add-ins PopTools 3.279. Additionally, the inferred population genetic structure was contrasted with a panmictic model, where it was assumed that all of the clusters form part of the same genetically interconnected unit. The adjustment was determined using Bayes Factor criterion estimated from the marginal likelihoods of both models using Tracer 1.6.0 software with 10,000 Bootstrap replicates.
Data Availability
DNA sequences were uploaded to Genbank with Accession numbers MK914652 - MK917441.
References
Gardner, T. A., Cote, I. M., Gill, J. A., Grant, A. & Watkinson, A. R. Hurricanes and Caribbean coral reefs: impacts, recovery patterns, and role in long-term decline. Ecology 86, 174–184 (2005).
Lugo, A. E. Visible and invisible effects of hurricanes on forest ecosystems: an international review. Austral Ecology 33, 368–398 (2008).
Castilla, J. C., Manríquez, P. H. & Camaño, A. Effects of rocky shore coseismic uplift and the 2010 Chilean mega earthquake on intertidal biomarker species. Mar. Ecol. Prog. Ser. 418, 17–23 (2010).
Jaramillo, E. et al. Ecological Implications of Extreme Events: Footprints of the 2010 Earthquake along the Chilean Coast. PLoS ONE 7(5), e35348, https://doi.org/10.1371/journal.pone.0035348 (2012).
Castilla, J. C. Earthquake-Caused Coastal Uplift and Its Effects on Rocky Intertidal Kelp Communities. Science 242, 440–443 (1988).
Putz, F. E. & Sharitz, R. R. Hurricane damage to old-growth forest in Congaree Swamp National Monument, South Carolina, USA. Can J For Res 21, 1765–70 (1991).
Foster, D. R. & Boose, E. R. Patterns of forest damage resulting from catastrophic wind in central New England, USA. J Ecol 80, 79–98 (1992).
Merrens, E. J. & Peart, D. R. Effects of hurricane damage on individual growth and stand structure in a hardwood forest in New Hampshire, USA. J Ecol 80, 787–95 (1992).
Allen, B. P., Pauley, E. F. & Sharitz, R. R. Hurricane impacts on liana populations in an old-growth southeastern bottomland forest. J Torrey Bot Soc 124, 34–42 (1997).
Platt, W. J., Doren, R. F. & Armentano, T. V. Effects of hurricane Andrew on stands of slash pine (Pinus elliottii var. densa) in the everglades region of south Florida (USA). Plant Ecol 146, 43–60 (2000).
Boose, E. R., Chamberlin, K. E. & Foster, D. R. Landscape and regional impacts of hurricanes in New England. Ecol Monogr 71, 27–48 (2001).
Crabbe, M. J. C. et al. Growth modelling indicates hurricanes and severe storms are linked to low coral recruitment in the Caribbean. Mar. Env. Res. 65, 364–368 (2008).
Xi, W., Peet, R. K. & Urban, D. L. Changes in forest structure, species diversity and spatial pattern following hurricane disturbance in a Piedmont North Carolina forest, USA. J Plant Ecol 1, 49–57 (2008).
Vargas, G. et al. Coastal uplift and tsunami effects associated to the 2010 Mw8.8 Maule earthquake in Central Chile. Andean. Geology 38, 219–238 (2010).
Burger, R. & Lynch, M. Evolution and extinction in a changing environment: A quantitative-genetic analysis. Evolution 49, 151–163 (1995).
Lande, R. & Shannon, S. The role of genetic variation in adaptation and population persistence in a changing environment. Evolution 50, 434–437 (1996).
Hughes, A. R. et al. Ecological consequences of genetic diversity. Ecology letters 11(6), 609–623 (2008).
Lowe, A. J., Boshier, D., Ward, M., Bacles, C. F. E. & Navarro, C. Genetic resource impacts of habitat loss and degradation; reconciling empirical evidence and predicted theory for neotropical trees. Heredity 95, 255–273 (2005).
Hall, P., Walker, S. & Bawa, K. Effects of forest fragmentation on genetic diversity and mating system in a tropical tree, Pithecellobium elegans. Conserv Biol. 10, 757–768 (1996).
Céspedes, M., Gutierrez, M. V., Holbrook, N. M. & Rocha, O. J. Restoration of genetic diversity in the dry forest tree Swietenia macrophylla (Meliaceae) after pasture abandonment in Costa Rica. Mol Ecol 12, 3201–3212 (2003).
Martínez, E. A., Cárdenas, L. & Pinto, R. Recovery and genetic diversity of the intertidal kelp Lessonia nigrescens (Phaeophyceae) 20 years after El Niño 1982/83. J. Phycol. 39, 504–508 (2003).
Hirase, S., Ikeda, M., Hayasaka, S., Iwasaki, W. & Kijima, A. Stability of genetic diversity in an intertidal goby population after exposure to tsunami disturbance. Mar Ecol. 37, 1161–1167 (2016).
Miura, O. et al. Ecological and genetic impact of the 2011 Tohoku Earthquake Tsunami on intertidal mud snails. Scientific reports 7, 44375 (2017).
Ruegg, J. C. et al. Interseismic strain accumulation measured by GPS in the seismic gap between Constitución and Concepción in Chile. Physics of the Earth and Planetary Interiors 175, 78–85 (2009).
Fritz, H. M. et al. Field survey of the 27 February 2010 Chile Tsunami. Pure Appl Geophys 168, 1989–2010 (2011).
Chavanich, S., Siripong, A., Sojisuporn, P. & Menasveta, P. Impact of Tsunami on the seafloor and corals in Thailand. Coral Reefs 24, 535 (2005).
Kumaraguru, A. K., Jayakumar, K., Jerald Wilson, J. & Ramakritinan, C. M. Impact of the Tsunami of 26 December 2004 on the coral reef environment of Gulf of Mannar and Palk Bay in the southeast coast of India. Curr. Sci. 89, 1729–1741 (2005).
Whanpetch, N. et al. Temporal changes in benthic communities of seagrass beds impacted by a tsunami in the Andaman Sea, Thailand. Estuar. Coast. Shelf Sci. 87, 246–252 (2010).
Lomovaskya, B. J., Firstater, F. N., Gamarra Salazar, A., Mendo, J. & Iribarne, O. Macro benthic community assemblage before and after the 2007 tsunami and earthquake at Paracas Bay, Peru. J. Sea Research 65, 205–212 (2011).
Mujica, A. Zooplancton en las aguas que circundan a Isla de Pascua. Ciencia y Tecnología del Mar. 16, 55–61 (1993).
Thatje, S. & Fuentes, V. First record of anomuran and brachyuran larvae (Crustacea: Decapoda) from antartic waters. Polar Biology. 26, 279–282 (2003).
Jaramillo, E. Patterns of species richness in sandy beaches of South America. South African Journal of Zoology. 29, 227–234 (1994).
Contreras, H., Duarte, C., Jaramillo, E. & Fuentes, N. Morphometric variability in sandy beach crustaceans of Isla Grande de Chiloé, Southern Chile. Revista de Biología Marina y Oceanografía. 48(3), 487–496 (2013).
Jaramillo, E. & Fuentealba, S. Down-shore zonation of two cirolanid isopods during two spirng-neap tidal cycles in a sandy beach of south central Chile. Revista Chilena de Historia Natural. 66, 439–454 (1993).
Contreras, H. & Jaramillo, E. Geographical variation in natural history of the sandy beach isopod Excirolana hirsuticauda Menzies (Cirolanidae) on the Chilean coast. Estuarine, Coastal and Shelf Science. 58S, 117–126 (2003).
Jaramillo, E., Carrasco, F., Quijón, P., Pino, M. & Contreras, H. Distribución y estructura comunitaria de la macroinfauna bentónica en la costa del norte de Chile. Revista Chilena de Historia Natural. 71, 459–478 (1998).
Naylor, E. & Kennedy, F. Ontogeny of behavioural adaptations in beach crustaceans: some temporal considerations for integrated coastal zone. Estuarine, Coastal and Shelf Science. 58S, 169–175 (2003).
Baessolo, L., Pérez-Schultheiss, J., Aguilera, A., Suazo, C. & Castro, M. Nuevos registros de Orchestoidea tuberculata Nicolet 1849 (Amphipoda, Talñitridae) en la costa de Chile. Hidrobiológica. 20(2), 191–193 (2010).
Jaramillo, E., Contreras, H., Duarte, C. & Avellanal, M. H. Locomotor activity and zonation of upper shore arthropods in a sandy beach of north central Chile. Estuarine, Coastal and Shelf Science. 58, 177–197 (2003).
Scapini, F. Behaviour of mobile macrofauna is a key factor in beach ecology as response to rapid environmental changes. Estuarine, coastal and Shelf Science. 150(A), 36–44 (2014).
Veas, R. et al. The influence of environmental factors in the abundance and recruitment of the sand crab Emerita analoga (Stimp- son 1857): a source-sink dynamics? Mar Environ Res 89, 9–20 (2013).
Sepúlveda, R. D. & Valdivia, N. Macrobenthic Community Changes of Intertidal Sandy Shores after a Mega-Disturbance. Estuaries and coasts 40(2), 493–501 (2017).
Banks, S. C. et al. How does ecological disturbance influence genetic diversity? Trends in ecology &. evolution 28(11), 670–679 (2013).
Wright, S. Evolution in Mendelian populations. Genetics 16(2), 97 (1931).
Nei, M. et al. The bottleneck effect and genetic variability in populations. Evolution 29(1), 1–10 (1975).
Allendorf, F. W. Genetic drift and the loss of alleles versus heterozygosity. Zoo biology 5(2), 181–190 (1986).
Fraser, D. J. et al. Comparative estimation of effective population sizes and temporal gene flow in two contrasting population systems. Molecular Ecology 16(18), 3866–3889 (2007).
Baldanzi, S., McQuaid, C. D., Cannicci, S. & Porri, F. Environmental domains and range-limiting mechanisms: testing the Abundant Centre Hypothesis using Southern African sandhoppers. PLoS One 8(1), e54598 (2013).
Ketmaier, V., Matthaeis, E. D., Fanini, L., Rossano, C. & Scapini, F. Variation of genetic and behavioural traits in the sandhopper Talitrus saltator (Crustacea Amphipoda) along a dynamic sand beach. Ethology Ecology &. Evolution 22(1), 17–35 (2010).
Fanini, L. et al. Life-history, substrate choice and Cytochrome Oxidase I variations in sandy beach peracaridans along the Rio de la Plata estuary. Estuarine, Coastal and Shelf Science 187, 152–159 (2017).
Sepúlveda, R. D. & Valdivia, N. Localised effects of a mega-disturbance: spatiotemporal responses of intertidal sandy shore communities to the 2010 Chilean earthquake. PloS one 11(7), e0157910 (2016).
Wang, I. J. Recognizing the temporal distinctions between landscape genetics and phylogeography. Molecular Ecology 19(13), 2605–2608 (2010).
Wang, I. J. Choosing appropriate genetic markers and analytical methods for testing landscape genetic hypotheses. Molecular Ecology 20(12), 2480–2482 (2011).
Bohonak, A. J. & Vandergast, A. G. The value of DNA sequence data for studying landscape genetics. Molecular Ecology 20(12), 2477–2479 (2011).
Sambrook, J., Fritschi, E. F. & Maniatis, T. Molecular cloning: a laboratory manual (Cold Spring Harbor Laboratory Press, 1989).
Pavesi, L. et al. Genetic connectivity between land and sea: the case of the beachflea Orchestia montagui (Crustacea, Amphipoda, Talitridae) in the Mediterranean Sea. Frontiers in Zoology 10(1), 21 (2013).
Yang, L., Hou, Z. & Li, S. Marine incursion into East Asia: a forgotten driving force of biodiversity. Proc R Soc B 280(1757), 20122892 (2013).
Giribet, G. et al. The position of crustaceans within the Arthropoda - evidence from nine molecular loci and morphology. Crustacean Issues 16: Crustacea and Arthropod Relationships. Festschrift for Frederick R. Schram. S. Koenemann and R. A. Jenner. Boca Raton (Taylor & Francis, 2005).
Maddison, W. P. & Maddison, D. R. Mesquite: a modular system for evolutionary analysis. Versión 3.4. http://mesquiteproject.org (2018).
Xia, X., Xie, Z., Salemi, M., Chen, L. & Wang, Y. An index of substitution saturation and its application. Molecular Phylogenetics and Evolution. 26, 1–7 (2003).
Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 123, 585–595 (1989).
Xia, X. DAMBE6: New tools fpr microbial genomics, phylogenetics and molecular evolution. Journal of Heredity. 108, 431–437 (2017).
Rozas, J. et al. DnaSP v6: DNA sequence polymorphism analysis of large datasets. Molecular Biology ans Evolution. 34, 3299–3302 (2017).
StatSoft. Inc. STATISTICA (data analysis software system). Version 7, www.statsoft.com. (2004).
Guillot, G., Estoup, A., Mortier, F. & Cosson, J. F. A spatial model for landscape genetics. Genetics. 170, 1261–1280 (2005).
Baele, G., Sibon, W. L., Drummond, A. J., Suchard, M. A. & Lerney, P. Accurate model selection of relaxed molecular clocks in Bayesian phylogenetics. Molecular Biology and Evolution. 30(2), 239–243 (2012).
Lantuéjoul, C. Geostatistical simulations. Models and algorithms. Springer, Berlin. 256 pp (2013).
Excoffier, L., Smouse, P. E. & Quattro, J. M. Analysis of molecular variance inferred from metric distances among dna haplotypes: Application to human mitochondrial DNA restriction data. Genetics. 131, 479–491 (1992).
Excoffier, L. & Lischer, H. E. L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources. 10, 564–567 (2010).
Guindon, S. & Gascuel, O. A simple, fast and accurate methods to estimate large phylogenies by maximum-likelihood. Systematic Biology. 52, 669–704 (2003).
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 9(8), 772 (2012).
Samarasin, P. et al. The problem of estimating recent genetic connectivity in a changing world. Conservation Biology 31(1), 126–135 (2017).
Kuhner, M. K., Yamato, J. & Felsenstein, J. Estimating effective population size and mutation rate from sequence data using Metropolis-Hastings sampling. Genetics 140, 1421–1430 (1995).
Beerli, P. & Felsenstein, J. Maximum-likelihood estimation of migration rates and effective population numbers in two populations using a coalescent approach. Genetics. 152, 763–773 (1999).
Kingman, J. F. C. Origins of the Coalescent: 1974–1982. Genetics 156, 1461–1463 (2000).
Beerli, P. How to use migrate or why are Markov Chain Monte Carlo programs difficult to use? In: Population Genetics for Animal Conservation, volume 17 of Conservation Biology. (Bertorelle, G; M.W. Bruford, H.C. Hauffe, A. Rizzoli & C. Vernesi, eds) (Cambridge University Press, 2009).
Gelman, A., Carlin, J. B., Stern, H. S. & Rubin, D. B. Bayesian data analysis (Chapman & Hall, London, 1995).
Rambaut, A. & Drummond, A. J. Tracer version 1.6. Available in, http://beast.bio.ed.ac.uk/Tracer (2007).
Hood, G. M. PopTools version 3.2.5, http://www.poptools.org (2010).
Acknowledgements
We thank the anonymous reviewers for their comments, S. Faugeron for field support and sample collection and Benjamín Guzmán, P. Antileo, K. Contreras, O. Huanel, J. Khan and P. Valenzuela for their help during sampling. Antonio Brante was funded by FONDECYT 1130868 and FONDECYT 1170598, Cristian Hernández was funded by FONDECYT 1170815, Garen Guzmán-Rendón was funded by CONICYT doctoral fellowship 21161759, Erwin M. Barría and Garen Guzmán-Rendón were funded by project EDPG LPR-161 of the Dirección de Postgrado, Universidad de Concepción, Erwin M. Barría was funded by CONICYT doctoral fellowship 21161588.
Author information
Authors and Affiliations
Contributions
A.B. and M.L.G. conceived the study. E.M.B., G.G.-R. and I.V.-E. conducted the analyses. All authors contributed with the interpretation of the results, discussion, and writing of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that this project was funded by FONDECYT (Fondo Nacional de Desarrollo Científico y Tecnológico).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brante, A., Guzmán-Rendón, G., Barría, E.M. et al. Post-Disturbance Genetic Changes: The Impact of the 2010 Mega-Earthquake and Tsunami on Chilean Sandy Beach Fauna. Sci Rep 9, 14239 (2019). https://doi.org/10.1038/s41598-019-50525-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-50525-1
This article is cited by
-
Natural mega disturbances drive spatial and temporal changes in diversity and genetic structure on the toadfish Aphos porosus
Scientific Reports (2023)
-
Hurricane-induced disturbance increases genetic diversity and population admixture of the direct-brooding isopod, Gnathia marleyi
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.