Abstract
Extracorporeal life support (ECLS) is a world-famous life-saving method. Until now, changes in arterial wave properties due to ECLS have remained unexamined. In this study, we determined the effects of ECLS on arterial wave properties and ventricular/arterial coupling in male Wistar rats with the measured aortic pressure alone. Ascending aortic pressure signals were measured before ECLS and at 30, 60, and 90 min after weaned off. The aortic pressure signal then calculated by fourth-order derivative to obtain an assumed triangular flow wave. The ratio of mean systolic pressure to mean diastolic pressure (Pms/Pmd), a parameter for evaluating the matching condition between myocardial oxygen demand and supply, was significantly higher after ECLS. The magnitude of forward pressure (|Pf|) augmented by ECLS prevailed over the backward pressure (|Pb|), leading to a decline in wave reflection factor. Pms/Pmd was positively linearly correlated with |Pf| (Pms/Pmd = 0.9177 + 0.0078 × |Pf|, r = 0.8677; P < 0.0001). These findings suggest that |Pf| was a predominant factor responsible for the mismatch between the myocardial oxygen demand and supply in rats after ECLS phase of experiment.
Similar content being viewed by others
Introduction
Extracorporeal life support (ECLS) has become a life-saving method for adults1,2 and children3,4 worldwide since its first successful use in 19715. The implementation usually involves femoral cannulation due to its simplicity, efficiency, and ease of use outside the operating room6. Although the success rates of ECLS have improved, it still causes some severe side effects. Reports from clinical investigations have indicated that management with ECLS might induce significant complications, such as acute kidney injury7, bleeding8, and inflammatory insults8. Moreover, femoral cannulation causes massive retrograde aortic ECLS flow6,9, causing a watershed of the cardiac and ECLS flows in the aortic arch during ECLS9. Whether the massive retrograde aortic ECLS flow might exert deteriorative effects on arterial wave properties and matching condition of oxygen demand and supply in myocardium has not been investigated until now.
The accurate measurement of the pulsatile nature of the arterial system, including arterial wave transit time (τw) and the forward (Pf) and backward (Pb) pressure waves, requires simultaneous recording of aortic pressure and flow signals10,11,12. However, it is difficult to steadily measure the ascending aortic flow signal in rats and humans before, during, and after ECLS. In 2006, Westerhof et al.13 described and validated that the aortic flow can be approximated by a triangle (Qtri), which is constructed from the measured aortic pressure waveform. Based on aortic pressure and Qtri, Pf and Pb, components of aortic pressure wave, were successfully obtained by the wave separation method. Moreover, Chang et al.14 discovered that the aortic impulse response is effective for estimating arterial τw by a single pressure pulse with its corresponding Qtri. The novelty of the concept proposed by Westerhof et al. is that the constructed Qtri, approximately to its paired flow signal, is based on the measured aortic pressure wave, and the calibration is not required in the analysis13.
Ventricular/arterial coupling concerns two matters, action of the heart as a pump connected to a hydraulic load and perfusion of the heart as an organ. In 1972, Buckberg et al.15 showed that an index based on LV and aortic pressures could predict subendocardial ischemia: the area between the diastolic aortic and LV pressures (DPTI) represented the oxygen supply to the myocardium, and the area under the systolic LV pressure curve (SPTI) represented the oxygen demand by the myocardium. As pressure in the ventricle during systole is approximated by aortic pressure during systole, for practical purpose, O’Rourke10 and O’Rourke et al.16 suggested that ventricular function as pumping action could be described using mean pressure generated in the ascending aorta during systole. Thus, the ratio of mean systolic aortic pressure (Pms) to the mean diastolic aortic pressure (Pmd) could be an indicator for evaluating the matching condition between myocardial oxygen demand and supply.
In this study, we used the measured aortic pressure and an assumed Qtri to determine the effects of ECLS on arterial wave properties and ventricular/arterial coupling in male Wistar rats. The construction of Qtri was derived from the fourth-order derivative of the measured aortic pressure wave13,14,17. The aortic input impedance (Zi) was calculated from the ratio of the ascending aortic pressure harmonics to the corresponding Qtri harmonics. The arterial τw was determined by the aortic impulse response, which is the time-domain equivalent of its Zi in the frequency-domain. The Pf and Pb components of the pressure pulse were separated using the wave decomposition technique. Thus, the arterial τw, and magnitudes of the forward (|Pf|) and backward (|Pb|) pressure waves were derived from the only measured aortic pressure to delineate the changes caused by ECLS in the pulsatile nature of the LV afterload.
Results
Hemodynamic parameters compared between baseline and post-ECLS 30, 60, 90 min
The mean body weight of the rats was 500 ± 35 g. Table 1 shows the basal heart rate (HR), cardiac cycle length (CL), LV ejection time (LVET), and aortic pressure profiles before and after ECLS treatment. After weaning from ECLS, the rats had increased HR, decreased CL, and shortened LVET. By contrast, no significant difference was observed in these parameters between post-ECLS 30, 60, and 90 min. The systolic (Ps), diastolic (Pd), and mean (Pm) aortic blood pressures did not change significantly in these rats after ECLS. By contrast, the PP values were markedly higher at post-ECLS 30, 60, and 90 min than at baseline.
Table 2 shows the mean systolic (Pms), and mean diastolic (Pmd) aortic blood pressures and the fold changes compared with its baseline. They did not change significantly after ECLS. However, the Pms/Pmd ratio significantly increased following ECLS and markedly decreased at post-ECLS 60 and 90 min compared with 30 min.
Pulse wave reflection changed by ECLS
Table 3 shows the pulse wave reflection obtained from the aortic pulsatile pressure signal in rats before and after ECLS. After ECLS, the rats had significantly higher |Pf| and |Pb| than before ECLS. The fold changes of |Pf| and |Pb| also significantly increased after ECLS, with the fold change level of |Pf| being higher than that of |Pb|. Although there was a trend toward decreasing arterial τw in rats following ECLS, the τw/CL ratio remained unaltered.
The constructed Q tri derived from the measure pressure wave in one rat
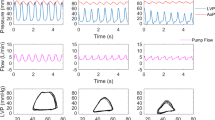
Figure 1 shows the construction of an uncalibrated Qtri obtained from the measured pressure wave in a rat at baseline (A) and at 30 (B), 60 (C), and 90 (D) min post-ECLS. The aortic pressure wave was measured from the ascending aorta under anesthesia and in the closed-chest condition. Aortic impulse response function curve (Fig. 1E–H) obtained from the measured aortic pressure with its paired Qtri. In I-L, the amplitudes (peak – trough) of the Pf and Pb are represented by |Pf| and |Pb|, respectively.
Construction of an uncalibrated Qtri derived from the measured pressure waveform in a rat at baseline (A) and at 30 (B), 60 (C), and 90 (D) min post-ECLS. In (E–H), the aortic impulse response function curve derived from the measured aortic pressure and its assumed Qtri. In (I–L), the amplitudes (peak – trough) of the Pf and Pb are described by |Pf| and |Pb|, respectively. CL = cardiac cycle length; HR = basal heart rate; LVET = left ventricular ejection time; Pb = backward pressure wave; Pd = diastolic pressure; Pf = forward pressure wave; Pm = mean aortic pressure; Ps = systolic pressure; PP = pulse pressure; Qtri = triangular flow; RM = wave reflection magnitude, given by |Pb|/|Pf|; RI = wave reflection index, given by |Pb|/(|Pf| + |Pb|); τw = wave transit time.
P f dominated the increase in P ms/P md ratio, which can also be easily detected by PP, after ECLS
Figure 2 illustrates the performance of ECLS on the arterial wave properties including RM, RI, and τw/LVET ratio. The RM (Fig. 2A) and RI (Fig. 2B) values at baseline were significantly higher than at post-ECLS 30, 60, and 90 min. By contrast, the τw/LVET ratio did not significantly change before and after ECLS (Fig. 2C).
Effects of ECLS on the changes of RM (A), RI (B), and τw/LVET (C). Each line represents a different rat in this study. RM and RI show downward trends, which decreased markedly after ECLS treatment. The τw/LVET ratio tended to slightly increase after ECLS treatment. RI = wave reflection index; RM = wave reflection magnitude; τw/LVET = the ratio of wave transit time to left ventricular ejection period.
Figure 3 shows the prediction of Pms/Pmd ratio from the τw/LVET ratio and RI before and after ECLS. Taking Pms/Pmd as the dependent variable and arterial τw/LVET and RI as independent variables, multiple linear regression was employed to fit the data. Only the correlation between Pms/Pmd and RI reached significance. The influences of |Pf| and |Pb| on Pms/Pmd ratio are depicted in Fig. 4A. The correlation between Pms/Pmd and |Pf| (but not |Pb|) achieved significance. Figure 4B shows the positive linear relationship between the Pms/Pmd and |Pf| in rats before and after ECLS (Pms/Pmd = 0.9177 + 0.0078 × |Pf| (r = 0.8677; P < 0.0001).
Implication of τw/LVET and RI in Pms/Pmd ratio in rats before and after ECLS. As shown by multiple linear regression analysis, only the correlation between Pms/Pmd and RI reached significance, suggesting that the Pms/Pmd impaired by ECLS could be affected by the magnitude of RI rather than the wave transmission time (τw/LVET). Pmd = mean diastolic pressure; Pms = mean systolic pressure; RI = wave reflection index; τw/LVET = the ratio of wave transit time to left ventricular ejection period.
|Pf| but not |Pb| strongly correlated with Pms/Pmd in rats before and after ECLS. As shown by multiple linear regression analysis, the correlation between Pms/Pmd and |Pf| and |Pb| reached significance. (A) However, only |Pf| was significantly positively related to Pms/Pmd before and after ECLS (B), indicating that the Pms/Pmd impaired by ECLS might be influenced by |Pf| rather than |Pb|. Pb = backward pressure wave; Pf = forward pressure wave; Pmd = mean diastolic pressure; Pms = mean systolic pressure.
Figure 5 displays the relationship of Pms/Pmd ratio and PP in rats before and after ECLS. PP had a significantly positive regression with Pms/Pmd: Pms/Pmd = 0.8716 + 0.0074 × PP (r = 0.9059; P < 0.0001).
Discussion
Although ECLS can be used as a bridge to recovery for patients with severe heart failure18 or heart transplantation19, this technology is associated with several side effects, such as blood cell consumption and activation of the cytokine network and the complement cascade systems20,21. Theoretically, retrograde aortic ECLS flow can increase cardiac afterload, thus leading to LV distension and endangering ventricular recovery9. However, little attention has been given to the pulsatile hemodynamic response to ECLS, which disrupts the ventricular/arterial coupling. To the best of our knowledge, this is the first study suggesting that ECLS can enhance |Pf|, the predominant factor responsible for the mismatch between the myocardial oxygen demand and supply.
In this study, the rats exhibited increased HR and shortened LVET after ECLS. We had previously observed high levels of inflammatory cytokines in rats at 120 min after reperfusion22, demonstrating persistent inflammatory insults, even after the rats had been weaned from ECLS. These inflammatory responses can be induced by the adrenergic pro-inflammatory pathway23, which might cause accelerated heartbeat after ECLS22. However, ECLS exerted no significant alterations in aortic pressure profiles such as Ps, Pd, Pm, Pms, and Pmd except PP and Pms/Pmd.
The arterial τw can be determined by the pulse wave velocity (PWV) and traveling distance of pressure waves to the reflecting site24. In this study, we calculated the arterial τw using the aortic impulse response, which presented the two discrete reflection peaks respectively linked to the effective reflection sites (Fig. 1E–H)25. Since CL was a factor influencing the calculation of arterial τw, the τw/CL ratio was derived as an index for the description of aortic stiffness. Neither arterial τw nor τw/CL changed significantly after the rats had been weaned from ECLS, indicating that ECLS did not alter vasculature distensibility. Moreover, the return time of |Pb| occurred during diastole in rats before and after ECLS (Fig. 1I–L). Both |Pf| and |Pb| were increased by ECLS (Table 3). However, the predominant role in increasing |Pf| rather than |Pb| contributed to a decline in RM (Fig. 2A) and RI (Fig. 2B). Although controversial, the prevalent view considers that the |Pf| value primarily reveals the interaction between peak aortic flow and proximal aortic diameter/stiffness while minimal/delayed reflections occur26,27,28. Thus, the increased |Pf| in the absence of changes in both τw and τw/CL indicated that ECLS may cause a functional mismatch between peak flow and physical properties of the proximal aortas.
The LV-arterial system interaction is an essential determinant of cardiovascular function and plays a crucial role in various cardiovascular disease states. Scholars have expressed great interest in characterizing the LV-arterial coupling by only measuring the ascending aortic pressure. Since coronary flow to the LV wall can occur only during diastole, the coronary perfusion pressure is the pressure gradient between the aorta and the LV during diastole, when the myocardium receives oxygen. However, the pressure generated by the left ventricle during systole is the pressure that opposes ejection of blood from the ventricle, and that determines myocardial oxygen requirements29. As mentioned previously, O’Rourke et al.16 suggested that the ascending aortic pressure wave comprises two components: Pms, describing the LV performance as a pump, and Pmd, relating to LV perfusion. Depending on this, the “ideal” ventricular/arterial coupling for adequate organ flow needs to meet as low a Pms and as high a Pmd as possible. Low Pms allows adequate ventricular ejection with low oxygen demands by myocardium and high Pmd allows adequate coronary perfusion. Therefore, the increased Pms/Pmd ratio may cause a mismatch of the oxygen demand and supply in myocardium. In the present study, the rats after ECLS had higher Pms/Pmd ratio than before (Table 2). Because the pulsatile nature of LV afterload could influence the LV function, we investigated the association of Pms/Pmd with τw/LVET and RI by using multiple linear regression analysis. We found that Pms/Pmd was significantly affected by the magnitude of RI rather than the wave transmission time (τw/LVET) (Fig. 3). Because |Pf| and |Pb| are the determinants of RI, the multiple linear regression analysis was performed to determine the influences of |Pf| and |Pb| on the Pms/Pmd ratio. We identified a positive linear correlation between the Pms/Pmd and |Pf| (Fig. 4), indicating that the increased |Pf| by ECLS impaired the matching condition of oxygen demand and supply in myocardium.
In the present study, the PP value was markedly larger after ECLS than before (Table 1). It has been considered that increased PWV, associated with vascular stiffening, and the consequent earlier return of reflected waves, prominently contribute to elevated PP24,30,31. However, we found that ECLS caused a decline in RM and RI, with no alteration in arterial τw and τw/CL. Recent reports from Framingham investigators have demonstrated that increased |Pf| is the predominant contributor to elevated PP with aging, with only modest contributions from wave reflections26,27,28. Thus, increased |Pf| may be the predominant factor responsible for the elevated PP in rats after ECLS, with little alteration in wave transit time and a decline in wave reflection factor. The Pms/Pmd ratio surged by ECLS was positively relevant to aortic PP (Fig. 5), indicating that the increased PP by ECLS impaired the matching condition of oxygen demand and supply in myocardium.
This study has some limitations. Because a rat’s blood volume is approximately 7% of that rat’s body weight32, the priming volume of our extracorporeal membrane oxygenator (ECMO) circuit was about 19–20 ml, which accounted for 61% of blood volume in the studied rats22. The priming volume used in this study was the minimum volume that we could attain in our model. As Zi cannot be measured in conscious animals, it is difficult to evaluate the effects of anesthesia on the pulsatile hemodynamics in rats. The results reported here, therefore, pertain only to the measurements made in anesthetized rats. This condition might have induced changes in the aortic pressure profiles and introduced reflex effects not observed under ordinary conditions. The degree to which anesthesia influences the pulsatile hemodynamics in rats remains unclear. However, studies with other animal models suggest that the biological and experimental variability amid animals were small enough to neglect33. Moreover, the Qtri was constructed by the measured aortic pressure wave to its assumed pairing flow signal. Although the approximated Qtri may differ from the actual flow waveform, the use of this concept describing the arterial wave properties has been validated by Westerhof et al.13 and Chang et al.17.
Conclusions
We demonstrated the mechanical defects of the vasculature in rats receiving ECLS based on the measured ascending aortic pressure and an assumed triangular flow. Although RI was diminished by ECLS, there existed a negative linear correlation between Pms/Pmd and RI. In advance, we identified a positive linear correlation between Pms/Pmd and |Pf|, indicating that the increased |Pf| by ECLS impaired the LV-arterial coupling. The Pms/Pmd value was also positively associated with aortic PP, indicating that the increased PP by ECLS caused a mismatch of oxygen demand and supply in myocardium. All these findings suggest that |Pf| was a predominant factor responsible for the impaired oxygen demand/supply ratio in the rats after ECLS. The advantage of this study is that an unknown Qtri can obtain from the measured aortic pressure and the calibration of the flow is not necessary. Thus, it would provide a path to evaluate the arterial wave properties in the future clinical settings.
Materials and Methods
Animals and surgical procedure
The effects of ECLS on the pulsatile nature of the arterial system were evaluated in normal Wistar–Kyoto male rats weighing 450–550 g (NC + ECLS; n = 14). All rats were free access to Purina rat chow and water and housed two per cage in a 12-h light/dark cycle animal room. Periodic checks of the cages and body weights ensured that the food was appropriately administered. The experiment was conducted according to the Guide for the Care and Use of Laboratory Animals, and our study protocol was approved by the Animal Care and Use Committee of National Taiwan University.
The procedure for the implementation of ECMO in rats was conducted as described previously22. In brief, rats were anesthetized with 5% isoflurane/100% oxygen in an induction chamber (Serial number 4468, NorVap, Skipton, UK; Panion & BF Biotech Inc., Nangang Dist., Taipei, Taiwan), and then mechanically ventilated (tidal volume 7 mL kg−1, rate 40 breaths min−1, positive end-expiratory pressure 2 cmH2O; Model 131, New England Medical Instruments, Medway, MA, USA) by orotracheal intubation with 1.75%–2% isoflurane/0.7–0.8 L min−1 oxygen for maintenance. The rats were placed on a circulating warm water blanket (B401H, Firstek Scientific Co. Ltd., Xinzhuang Dist, New Taipei City, Taiwan; TP22G, Gaymar Industries, Inc., Orchard Park, NY, 14127 USA) with a heating lamp above for maintaining body temperature22.
The left femoral artery and vein were separately cannulated for arterial pressure monitoring (BIOPAC systems, Inc., Goleta, CA, USA) and drug administration22. The right femoral artery and the right external jugular vein were cannulated for the arterial inflow and venous outflow ports. Afterwards, heparin (500 UI) was administered to prevent blood clotting22. A high-fidelity pressure catheter (model SPC 320, size 2 French; Millar Instruments, Houston, TX, USA) was used to measure the pulsatile ascending aortic pressure from the isolated carotid artery of the right side. The electrocardiogram (ECG) of lead II was recorded using a Gould ECG/Biotech amplifier (Gould Electronics, Cleveland, OH, USA)34. Selective aortic pressure signals from 5 to 10 beats were averaged in the time domain, using the peak R wave of the ECG as a fiducial point17. Ascending aortic pressure signals were recorded in the anesthetized rats as a baseline before ECLS.
Initiation and discontinuation of ECMO and intensive care unit phase
The ECMO device designed for rats consisted of an open venous reservoir (TERUMO\(R\), Tokyo, Japan; 5-mL syringe), a membrane oxygenator (Micro-1 Rat Oxygenator; Dongguan Kewei Medical Instrument Co., Ltd, Guangdong, China), a heat exchanger (Radnoti Glass Technology Inc., Monrovia, CA, USA), silicone tubing (ID 1.6 mm), and a roller pump (Masterflex, Barrington, IL, USA)22. ECLS lasted for 30 min. The ECMO rate was initially quickly increased to the peak rate of 70 mL min−1—approximately the normal cardiac output in rats—and gradually decreased to approximately 20 mL min−1 to meet the venous outflow. In total, 12–15 mL complement fluid made from Plasma-Lyte A (Baxter, Deerfield, IL, USA) and 6% hydroxyethyl starch 130/0.4 (Voluven\(R\); Fresenius Kabi, Bad Homburg, Germany) at the ratio of 1:1 was injected during ECLS to maintain the output of the circuit system.
The rats were weaned off the system after 30 min of ECLS, and the aortic pressure waves were measured at 30, 60, and 90 min after weaning (post-ECLS 30, 60, and 90 min). Blood transfusion or drugs such as bicarbonate, inotropes, or vasopressors did not perform on rats, and the rats were all humanely euthanized at the end of the experiment.
Construction of the unknown flow wave by using a triangle
The unknown Qtri was derived from the pressure waveform measured in the ascending aorta13,14,17. The start and end time points of LV ejection were identified as the intersection of two vertical lines near the foot of the pressure wave (the first vertical blue line in each of Fig. 1A–D) and near the incisura (the third vertical blue line in each of Fig. 1A–D), respectively35. The time at the peak of the triangle was derived from the fourth-order derivative of the aortic pressure wave13,14,17 (the pink curve in each of Fig. 1A–D). After ejection commenced, the first zero-crossing curve from above to below (the second vertical blue line in each of Fig. 1A–D) determined the peak of the triangle of blood flow, which was the inflection point of the pressure wave13,14,36. Thus, the uncalibrated Qtri was approximated by a triangular shape (the green curves in each of Fig. 1A–D) and represented the corresponding flow wave of the aortic pressure signal.
Impulse response function curve
Zi was obtained from the ratio of the ascending aortic pressure harmonics to the corresponding harmonics from Qtri by using a standard Fourier series expansion technique11,14,35,37. The aortic characteristic impedance (Zc) was calculated by averaging the high-frequency moduli of the Zi data points from 4 to 10 harmonics. Arterial τw was computed using the impulse response function curve (the pink line in each of Fig. 1E–H)38,39, which was generated using an inverse Fourier transformation of the Zi after multiplying the first 12 harmonics by a Dolph–Chebyshev weighting function with order 2435,40. One-half of the time difference between the appearance of the second reflected peak (long arrow) and the initial peak (short arrow) in the impulse response curve approximates the arterialτw in the lower body circulation34,38,40,41.
Arterial wave separation analysis
The equations used to resolve the measured aortic pressure wave (Pao) into its Pf and Pb components were described as follows35,42:
The calculations of the Pf and Pb using Qtri(t) are depicted in Fig. 1I–L. The amplitudes (peak − trough) of the Pb and Pf are represented by |Pb| and |Pf|, respectively. The aortic reflection magnitude (RM) was then defined as the ratio of |Pb| and |Pf|: RM = |Pb|/|Pf|13, and the reflection index (RI) was calculated as RI = |Pb|/(|Pf| + |Pb|).
Statistical analysis
The results are expressed as median ± interquartile range (IQR). SigmaPlot 12.5 were used for statistical analysis. Wilcoxon signed-rank testing was performed to determine the statistical significance of the results between different time points for multiple comparisons of the effects of ECLS on arterial wave properties. Statistical significance was set as P < 0.05.
References
Chen, Y. S. et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 372, 554–561, https://doi.org/10.1016/s0140-6736(08)60958-7 (2008).
Shin, T. G. et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: A comparison with conventional cardiopulmonary resuscitation. Crit Care Med 39, 1–7, https://doi.org/10.1097/CCM.0b013e3181feb339 (2011).
Huang, S. C. et al. Extracorporeal membrane oxygenation rescue for cardiopulmonary resuscitation in pediatric patients. Critical care medicine 36, 1607–1613, https://doi.org/10.1097/CCM.0b013e318170b82b (2008).
Topjian, A. A., Nadkarni, V. M. & Berg, R. A. Cardiopulmonary resuscitation in children. Curr Opin Crit Care 15, 203–208 (2009).
Chauhan, S. & Subin, S. Extracorporeal membrane oxygenation, an anesthesiologist’s perspective: physiology and principles. Part 1. Ann Card Anaesth 14, 218–229, https://doi.org/10.4103/0971-9784.84030 (2011).
Haneya, A. et al. A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation 83, 1331–1337, https://doi.org/10.1016/j.resuscitation.2012.07.009 (2012).
Hsu, P. S. et al. Extracorporeal membrane oxygenation for refractory cardiogenic shock after cardiac surgery: predictors of early mortality and outcome from 51 adult patients. Eur J Cardiothorac Surg 37, 328–333, https://doi.org/10.1016/j.ejcts.2009.07.033 (2010).
Loforte, A. et al. Peripheral extracorporeal membrane oxygenation system as salvage treatment of patients with refractory cardiogenic shock: preliminary outcome evaluation. Artif Organs 36, E53–61, https://doi.org/10.1111/j.1525-1594.2011.01423.x (2012).
Rupprecht, L. et al. Cardiac decompression on extracorporeal life support: a review and discussion of the literature. Asaio j 59, 547–553, https://doi.org/10.1097/MAT.0b013e3182a4b2f6 (2013).
O’Rourke, M. F. Vascular impedance in studies of arterial and cardiac function. Physiol Rev 62, 570–623 (1982).
Milnor, W. R. Hemodynamics 2nd Edition edn, (Williams & Wilkins, 1989).
McDonald, D. A. Blood flow in arteries. 2nd Edn. edn, (Williams & Wilkins, 1974).
Westerhof, B. E., Guelen, I., Westerhof, N., Karemaker, J. M. & Avolio, A. Quantification of wave reflection in the human aorta from pressure alone: a proof of principle. Hypertension 48, 595–601, https://doi.org/10.1161/01.hyp.0000238330.08894.17 (2006).
Chang, C. Y. et al. Defects in Vascular Mechanics Due to Aging in Rats: Studies on Arterial Wave Properties from a Single Aortic Pressure Pulse. Front Physiol 8, 503, https://doi.org/10.3389/fphys.2017.00503 (2017).
Buckberg, G. D., Fixler, D. E., Archie, J. P. & Hoffman, J. I. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res 30, 67–81 (1972).
O’Rourke, M. F., Yaginuma, T. & Avolio, A. P. Physiological and pathophysiological implications of ventricular/vascular coupling. Ann Biomed Eng 12, 119–134 (1984).
Chang, R. W. et al. Determining arterial wave transit time from a single aortic pressure pulse in rats: vascular impulse response analysis. Sci Rep 7, 40998, https://doi.org/10.1038/srep40998 (2017).
Vardi, A. et al. ECMO–extra corporeal membrane oxygenation as a bridge to recovery, following surgery for complex congenital cardiac anomalies, in children and neonates. Harefuah 142, 583–587, 648, 647 (2003).
Marasco, S. F. et al. Extracorporeal membrane oxygenation in primary graft failure after heart transplantation. Ann Thorac Surg 90, 1541–1546, https://doi.org/10.1016/j.athoracsur.2010.05.066 (2010).
Hirthler, M., Simoni, J. & Dickson, M. Elevated levels of endotoxin, oxygen-derived free radicals, and cytokines during extracorporeal membrane oxygenation. J Pediatr Surg 27, 1199–1202 (1992).
Underwood, M. J. et al. Changes in “inflammatory” mediators and total body water during extra-corporeal membrane oxygenation (ECMO). A preliminary study. Int J Artif Organs 18, 627–632 (1995).
Chang, R. W., Luo, C. M., Yu, H. Y., Chen, Y. S. & Wang, C. H. Investigation of the pathophysiology of cardiopulmonary bypass using rodent extracorporeal life support model. BMC Cardiovasc Disord 17, 123, https://doi.org/10.1186/s12872-017-0558-6 (2017).
Rittirsch, D., Flierl, M. A. & Ward, P. A. Harmful molecular mechanisms in sepsis. Nat Rev Immunol 8, 776–787, https://doi.org/10.1038/nri2402 (2008).
Nichols, W. W. et al. Effects of arterial stiffness, pulse wave velocity, and wave reflections on the central aortic pressure waveform. J Clin Hypertens (Greenwich) 10, 295–303 (2008).
O’Rourke, M. F. Pressure and flow waves in systemic arteries and the anatomical design of the arterial system. J Appl Physiol 23, 139–149, https://doi.org/10.1152/jappl.1967.23.2.139 (1967).
Torjesen, A. A. et al. Forward and backward wave morphology and central pressure augmentation in men and women in the Framingham Heart Study. Hypertension 64, 259–265, https://doi.org/10.1161/hypertensionaha.114.03371 (2014).
Cooper, L. L. et al. Components of hemodynamic load and cardiovascular events: the Framingham Heart Study. Circulation 131, 354–361, https://doi.org/10.1161/circulationaha.114.011357 (2015).
Mitchell, G. F. et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation 122, 1379–1386, https://doi.org/10.1161/circulationaha.109.914507 (2010).
Sarnoff, S. J. et al. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol 192, 148–156 (1958).
O’Rourke, M. F. & Nichols, W. W. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 45, 652–658, https://doi.org/10.1161/01.HYP.0000153793.84859.b8 (2005).
Laurent, S. & Boutouyrie, P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension 49, 1202–1206, https://doi.org/10.1161/hypertensionaha.106.076166 (2007).
Lee, H. B. & Blaufox, M. D. Blood volume in the rat. J Nucl Med 26, 72–76 (1985).
Cox, R. H. Three-dimensional mechanics of arterial segments in vitro: methods. J Appl Physiol 36, 381–384 (1974).
Wang, C. H. et al. Prevention of arterial stiffening by using low-dose atorvastatin in diabetes is associated with decreased malondialdehyde. PloS one 9, e90471, https://doi.org/10.1371/journal.pone.0090471 (2014).
Chang, R. W. et al. Systolic aortic pressure-time area is a useful index describing arterial wave properties in rats with diabetes. Sci Rep 5, 17293, https://doi.org/10.1038/srep17293 (2015).
Kelly, R., Hayward, C., Avolio, A. & O’Rourke, M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation 80, 1652–1659 (1989).
Nichols, W. W. & O’Rourke, M. F. McDonald’s blood flow in arteries 6th Edition edn, (Edward Arnold, 2011).
Sipkema, P., Westerhof, N. & Randall, O. S. The arterial system characterised in the time domain. Cardiovasc Res 14, 270–279 (1980).
Latson, T. W., Yin, F. C. & Hunter, W. C. In Ventricular/vascular coupling (ed Frank CP Yin) 334–383 (Springer-Verlag, 1987).
Laxminarayan, S., Sipkema, P. & Westerhof, N. Characterization of the arterial system in the time domain. IEEE Trans Biomed Eng 25, 177–184, https://doi.org/10.1109/tbme.1978.326244 (1978).
Wu, M. S., Chang, C. Y., Chang, R. W. & Chang, K. C. Early return of augmented wave reflection impairs left ventricular relaxation in aged Fisher 344 rats. Exp Gerontol 47, 680–686, https://doi.org/10.1016/j.exger.2012.06.006 (2012).
Murgo, J. P., Westerhof, N., Giolma, J. P. & Altobelli, S. A. Manipulation of ascending aortic pressure and flow wave reflections with the Valsalva maneuver: relationship to input impedance. Circulation 63, 122–132 (1981).
Acknowledgements
This study was supported by grants from the National Science Council of Taiwan (MOST 106-2314-B-002-155-MY3) and National Taiwan University Hospital (NTUH 106-16 and NTUH 107-s3897). This manuscript was edited by Wallace Academic Editing and supported by the NGS and Microarray Core Facility of the National Taiwan University Center of Genomic and Precision Medicine.
Author information
Authors and Affiliations
Contributions
R.W.C. and C.H.W. developed the concept, designed study, and wrote the manuscript. R.W.C. performed the animal experiment, collected data, and performed statistical analysis. Y.J.H., M.S.W., Y.S.C., H.Y.Y., and S.L.Y. supported funding and provided advice on surgical procedure. M.S.W., Y.S.C., E.T.W., and L.C.L. participated in data interpretation. E.T.W. and C.H.W. supervised this work and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, CH., Chang, RW., Wu, E.T. et al. Extracorporeal Life Support Enhances the Forward Pressure Wave to Cause a Mismatch between Cardiac Oxygen Demand and Supply. Sci Rep 9, 13882 (2019). https://doi.org/10.1038/s41598-019-50428-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-50428-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.