Abstract
Better knowledge of the face of the current dengue virus (DENV) epidemiology in Africa can help to implement efficient strategies to curb the burden of dengue fever. We conducted this systematic review and meta-analysis to determine the prevalence of DENV infection in Africa. We searched PubMed, EMBASE, African Journals Online, and Africa Index Medicus from January 1st, 2000 to June 10th, 2019 without any language restriction. We used a random-effects model to pool studies. A total of 76 studies (80,977 participants; 24 countries) were included. No study had high risk of bias. Twenty-two (29%) had moderate and 54 (71%) had low risk of bias. In apparently healthy individuals, the pooled prevalence of DENV was 15.6% (95% confidence interval 9.9–22.2), 3.5% (0.8–7.8), and 0.0% (0.0–0.5) respectively for immunoglobulins (Ig) G, IgM, and for ribonucleic acid (RNA) in apparently healthy populations. In populations presenting with fever, the prevalence was 24.8% (13.8–37.8), 10.8% (3.8–20.6k) and 8.4% (3.7–14.4) for IgG, IgM, and for RNA respectively. There was heterogeneity in the distribution between different regions of Africa. The prevalence of DENV infection is high in the African continent. Dengue fever therefore deserves more attention from healthcare workers, researchers, and health policy makers.
Similar content being viewed by others
Introduction
The incidence of dengue fever has grown in the last decade worldwide. According to the World Health Organization (WHO), 3.9 billion people in 128 countries are at risk of infection and about 390 million of dengue infection occur each year in people worldwide among which 96 million (25%) clinically manifest the disease1. DENV is one of the most common arboviruses. It is a tropical and subtropical mosquito bone-viral disease2,3. DENV is transmitted by two main epidemic vectors: Aedes aegypti and Aedes albopictus. These vectors have become widely distributed across tropical and subtropical region and spread globally with the advent of global phenomena such as urbanization, high rate of population growth, inadequate water supply, sewer, poor waste management system, and international travel2,3. DENV infection is asymptomatic in more than 50% of cases or can be presented as a flu-like illness including headache, myalgia, and rash like other endemic fever diseases in Africa (example: malaria and chikungunya fever)2,4. Currently, there is no treatment for dengue infection. The main method of controlling DENV transmission is through the active monitoring and surveillance of vectors5,6.
Before 1970, only 9 countries had experienced severe dengue epidemics. Dengue fever is now endemic in more than 100 countries in the regions of Africa, the Eastern Mediterranean, South-East Asia, the Western Pacific, the Americas. The three last ones are the most seriously affected regions7. In Africa, the first reported dengue fever outbreaks occurred in Zanzibar in 1823 and 1870. Several other African countries including Burkina-Faso, Egypt, South Africa and Senegal, reported unconfirmed outbreaks of dengue fever in early 1900s. Although many outbreaks aren’t ever officially reported, between 1960 and 2017, more than 20 laboratory-confirmed dengue epidemics were reported in more than 20 African countries2,8,9. A systematic review without meta-analysis by Humphrey and colleagues conducted for Middle East and North Africa have shown a median seroprevalence of dengue infection in general population of 25% (range from 0 to 62%) with most of the seroprevalence studies reported from Red Sea region of Pakistan9. Although a total of 22 countries in Africa reported sporadic cases or outbreaks of dengue fever2, to date there is no study accurately investigating the epidemiology of DENV infection among febrile and apparently healthy populations in this continent. In order to fill this gap, we conducted this systematic review and meta-analysis with the aim to determine the prevalence of DENV infection in Africa. This review would help better characterize the epidemiology in the continent and build effective interventions to curb the burden of dengue fever in the continent.
Methods
Design
The Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines served as the template for reporting the present review (Supplementary Table 1, Appendix). This review is registered in the PROSPERO International Prospective Register of Systematic Reviews, registration number CRD42018104829. For this systematic review and meta-analysis, we used the same methodology as in already published meta-analysis of prevalence studies for chikungunya fever10.
Criteria for considering studies for the review
We considered observational studies (cross-sectional, case-control, and cohort). Studies in which DENV infection were imported were excluded. Studies had to be conducted out of outbreak periods. DENV infection had to be diagnosed by the detection of the three biological markers of DENV including RNA, Immunoglobulins (Ig) M, or G; among people residing in Africa. Studies lacking or with no extractable primary data, and/or explicit methods description, were excluded. In the case of missing data, we contacted authors.
Strategy to identify relevant studies
A comprehensive search of MEDLINE through PubMed, Excerpta Medica database (EMBASE), African Journals Online, and Africa Index Medicus was conducted to identify all relevant articles published on DENV infection among people living in Africa from January 1st, 2000 to June 10th, 2019, without any language restriction. We considered recent studies to have current and updated overview on the epidemiology in the continent. A search strategy based on the combination of relevant terms was designed and applied. The main search strategy in EMBASE is shown in Supplementary Table 2 (Appendix). This search strategy has been adapted for search in other databases. A manual search that consists of scanning reference lists of eligible studies and relevant reviews was performed.
Selection of studies to include in the review
Two investigators independently screened records for eligibility based on titles and abstracts. Full-texts of articles deemed potentially eligible were retrieved. Further, these investigators independently assessed the full-text of each study for eligibility, and consensually retained studies to be included. Disagreements were solved through a discussion or by an arbitration of a third investigator.
Data extraction and management
The data have been extracted using a preconceived, piloted and standardized data abstraction form. Two investigators independently extracted data including: name of first author, year of publication, title of the study; recruitment site (country, city), setting (rural, urban), sampling method; number of persons tested for DENV, number of persons infected with DENVs, mean and median age, proportion male, and clinical presentation. In the case of multinational studies, data have been separated to present the estimate in each country when possible. We assigned a United Nations Statistics Division (UNSD) African region (Central, Eastern, Northern, Southern, and Western) to each study according to the country of recruitment11. We have also identified studies where plaque resorption neutralization test (PRNT) where used to measure specific neutralizing antibodies titers helping to rule out false-positive ELISA results12.
Methodological quality assessment
We used an adapted version of the tool developed by Hoy and colleagues to assess the methodological quality of included studies13. Two investigators independently ran the assessment. Discrepancies were discussed and resolved by these investigators. Inter-rater agreements between investigators for study inclusion and methodological quality assessment were assessed using Cohen’s κ.
Data synthesis and analysis
Data have been analyzed using the ‘meta’ packages of the R statistical software (version 3.5.1, The R Foundation for statistical computing, Vienna, Austria). We used the reference method to synthetize prevalence data as recommended by Barendregt and colleagues14. The prevalence of DENV infection has been recalculated on the basis of crude numerators and denominators provided by individual studies. To minimize the effect of studies with extremely small or extremely large prevalence estimates on the overall estimate, the variance of study-specific prevalence was stabilized with the Freeman-Tukey double arcsine transformation before pooling the data with the random effects meta-analysis model14. Heterogeneity was assessed by the chi-square test on Cochrane’s Q statistic15, which was quantified by I-squares values, assuming I-squares values of 25, 50 and 75% respectively representing low, medium and high heterogeneity16. When substantial heterogeneity was detected (I² > 50%), we performed subgroup analyzes to investigate possible sources of heterogeneity using the following grouping variables: clinical presentation (febrile vs apparently healthy) and UNSD regions. The symmetry of the funnel plots and the Egger test have been used to assess the presence of publication bias17. A p value < 0.10 was considered indicative of a statistically significant publication bias for studies reporting the prevalence of dengue fever18. It was decided a priori that if publication bias were present it would not be adjusted for, since we believed that the prevalence estimates of interest would likely be published even if substantially different from previously reported estimates. In the case of multinational studies, we considered data from each country as one study before the meta-analysis. This was also done in cases of studies including both febrile and non-febrile patients included in the same study. To assess the robustness of our findings, we conducted a sensitivity analysis considering only studies that used a confirmation analysis by PRNT.
Results
Study selection and characteristics
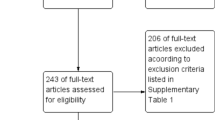
In total, we identified 2,554 records, of which 77 studies were included (Fig. 1). The final list of included studies is in the Appendix. Agreement between review authors for study selection based on title and abstract (κ = 0.86) and data extraction (κ = 0.78) were moderate to high. No study had high risk of bias. Twenty-two (29%) had moderate and 54 (71%) had low risk of bias. Twenty-six and 50 studies were conducted in apparently healthy and febrile participants respectively. Sixty-four and 12 studies were prospective and retrospective respectively. All studies were cross-sectional and conducted from 2000 to 2017. Proportion of males varied from 0% to 97.2% (61 studies). Data were only from sub-Saharan Africa. In total, 38 studies were from Eastern, 27 from Western, 10 from Central, and 1 from Southern Africa. Data were from 23 countries: Angola (n = 1), Burkina-Faso (n = 2), Cameroon (n = 3), Comoros (n = 1), Côte d’Ivoire (n = 4), Democratic Republic of the Congo (n = 1), Djibouti (n = 3), Ethiopia (n = 3), Gabon (n = 4), Ghana (n = 3), Guinea (n = 1), Kenya (n = 8), Mali (n = 1), Mayotte (n = 1), Namibia (n = 1), Nigeria (n = 12), Sao Tome and Principe (n = 1), Senegal (n = 1), Sierra Leone (n = 3), South Sudan (n = 1), Sudan (n = 10), Tanzania (n = 9), Zambia (n = 1), and Zimbabwe (n = 1). Mean/median age of participants varied from 1.2 to 47.9 years (38 studies). Individual characteristics of included studies are in the Supplementary Table 3 (Appendix).
Prevalence of dengue virus infection in africa
In total, 80,977 participants were included in the 76 studies. The prevalence of DENV infection varied widely depending on clinical presentation and biomarkers of infection considered (Table 1). The IgG seroprevalence in febrile participants was 24.8% (95%CI 13.8–37.8) and 15.6% (95%CI 9.9–22.2) in apparently healthy individuals without significant difference (p = 0.165) (Fig. 2; Table 1). Sensitivity analysis considering only study with DENV infection confirmation by PNRT test yielded findings in the range of that of crude analysis (Table 1).
The prevalence of IgM seroprevalence in febrile participants 10.8% (95%CI 3.8–20.6) was higher compared to apparently healthy participants 3.5% (95%CI 0.8–7.8) without significant difference, p = 0.084 (Fig. 3; Table 1).
The DENV RNA prevalence in febrile participants 8.4% (95%CI 3.7–14.4) was significantly higher compared to apparently healthy participants 0.0% (95%CI 0.0–0.5), p < 0.0001 (Fig. 4; Table 1).
We found substantial heterogeneity for all meta-analyses (Table 1). Publication bias was detected for IgG seroprevalence in febrile participants (p = 0.0007, Table 1) as depicted in the funnel plot (Supplementary Fig. 1, Appendix). There was no publication bias for other analyses (Table 1 and Supplementary Figs 1–5, Appendix).
Prevalence of dengue virus infection in sub-regions of africa
There was a wide variation of DENV infection prevalence between sub-regions in Africa. There was difference in the DENV infection IgG seroprevalence between regions for participants with fever, varying from 3.6% in Eastern Africa to 52.6% in Western Africa, p < 0.0001 (no data from Southern Africa; Supplementary Fig. 6). There was a difference in the DENV infection IgG seroprevalence between regions in apparently healthy participants, varying from 8.0% in Southern Africa to 38.6% in Central Africa, p < 0.0001 (Supplementary Fig. 7). The IgM seroprevalence in febrile participants varied from 6.6% in Western Africa to 35.2% in Central Africa, p < 0.001 (Supplementary Fig. 8). Among apparently healthy participants, this prevalence varied from 0.1% in Central Africa to 5.0% in Eastern Africa, p = 0.044 (Supplementary Fig. 9). For RNA prevalence in febrile participants, the prevalence varied from 5.3% in Eastern Africa to 12.2% in Central Africa, without significant difference, p = 0.270 (Supplementary Fig. 10). There were not enough studies to perform subgroup analysis for RNA prevalence among apparently healthy participants.
Discussion
This first systematic review with meta-analysis of the prevalence of DENV infection among people residing in Africa depicted a relatively high prevalence with substantial heterogeneity depending on clinical presentation, viral markers considered, and geographical distribution.
The seroprevalence obtained in this study is in the range of that found in the review by Humphrey and colleagues conducted in urban areas of Middle East and North Africa, with majority of the studies from Red Sea region9. Humphrey and colleagues reported a seroprevalence in general population of 25% ranging from 0 to 62% in studies conducted from 1941 to 20159. However, this previous review did not differentiate type of seroprevalence between IgG and IgM. In addition, RNA of DENV was not considered.
The high prevalence we found in this review may reflect the weakness of the ongoing public health interventions to control vector transmission of DENV. This can be explained by weak health systems in a low-income context leading to inability to sustain efficient and continuously powerful interventions. In addition, in certain African countries any strategies were implemented to date for vector control5,6,9. The increasing rate of urbanization in the continent can also explain the high DENV infection prevalence. Indeed, high urbanization rate with poor infrastructure in tropical and subtropical regions is associated with high burden of DENV infection2,3. The mechanism would be the increase of mosquitoes breeding sites in these developing countries. The ecological environment and climatological patterns of tropical and subtropical regions where most of African countries are located also favor vector long-life and development19,20. Indeed, in warm and humid climate that favors eggs conservation and development, Aedes albopictus and Aedes aegypti are active all year long compared to cooler, temperate regions, where they hibernate over winter21,22,23,24. Another explanation and one of the most important for the high prevalence is the interaction between all factors evocated above in addition to the facilitation of international travelling due to development of transportation that favor the expansion of dengue vectors in Africa25,26,27,28. One of DENV vectors, Aedes albopictus, originally came from tropical and subtropical region of Southeast Asia. The presence of this vector in Africa has been facilitated by the development of international transport. For example, the tire trade between Asia, Europe, and Africa was the most favorable factor for the transfer of mosquitoes eggs from Asia or Europe to Africa29,30,31. Definitively, in the last decades there is an increasing urbanization, increasing human exchange between countries and ecological imbalance favoring the infection of vectors, and then of other never infected humans.
We found a higher prevalence with IgG compared to IgM and RNA markers. RNA is detectable in patients only from infection up to six days after the onset of the fever. IgM are detectable five days after the onset of fever up to three months while IgG appear on the tenth day after the onset of fever and can persist for several years3. The prevalence was not surprisingly higher in febrile compared to apparently healthy individuals, although not significant. This may be explained by the fact that the viral markers are more prevalent during febrile phase (first seven days). We found a higher prevalence of IgG in the febrile patients compared to apparently healthy. As IgG can stay several years in the serum, an episode of fever can occur in an individual who already had a previous infection (with IgG and IgM production), especially in the endemic context for DENV like Africa. During a new acute infection (a second and others after), the secondary immunological response is faster with a higher IgG production32.
There was also a heterogeneity in prevalence according to geographical areas. Since not all regions and countries were represented, this finding needs a cautious interpretation. In addition, the UNSD of the Africa continent is only an administrative subdivision and could not efficiently map the epidemiology. We found that western Africa had the highest IgG seroprevalence in febrile participants and the highest IgM seroprevalence in apparently healthy individuals, that western and central Africa had the highest IgG seroprevalence in apparently healthy individuals, and that central Africa had the highest IgM seroprevalence and RNA prevalence in febrile participants. Higher prevalence in both western and central Africa may be the reflection of weak quality of infrastructures in urban areas in these regions, weaker healthcare systems, and optimal ecological environment and climatological patterns for lifecycle of DENV. However, studies are needed to deeply investigate the heterogeneity of epidemiology of DENV infection between and within countries of the continent. It would be better to have the geospatial distribution of DENV vectors in the continent to better understand the distribution of DENV infection among humans. Heterogeneity in the prevalence of dengue infection in Africa may also be due to the difference of the immunity of human across the continent and the heterogeneity of vectors distribution33,34,35. In addition, in some African countries, both epidemic vectors Aedes aegypti and Aedes albopictus are found and only Aedes aegypti in other countries34,36.
The findings of this review have important inference for clinical practice, researchers, and health policies for the continent. To date, there is no effective antiviral treatment for dengue fever7. With the high prevalence we found, especially among patients presenting with fever, implementation of routine diagnostic for people living in endemic areas or for people presenting fever with history of a travel in dengue endemic area may be explored37,38. This can be motivated by the fact that, proper medical management of early detected can lower fatality rates below 1% and there is no specific treatment against DENV infection7. It would be also interesting to explore the implementation of routine diagnostic of DENV infection in endemic countries with the aim to make differential diagnosis with other infectious diseases (like malaria) with close clinical presentation. However, for endemic areas with most of them locating in developing countries like in Africa, it will be difficult to implement routine diagnostic of DENV infection at a large scale as most of countries have weak health systems with low funding. Our findings call for cost-effective strategies to curb the burden DENV infection in Africa since severe dengue fever can rapidly lead to serious illness and death, especially among children7. To date, the efficacy of dengue vaccine has been accessed in eleven countries with immune response of 59% of vaccinated people39, suggesting that high quality studies are needed to have highly effective vaccine and antiretroviral treatment to rapidly curb the burden of dengue fever in Africa. Another approach for curbing the burden of dengue fever is vector control. For curbing the burden of vector-borne disease, WHO suggests an integrated vector control. This vector control should be environmentally safe when using insecticides for the elimination of mosquitoes Laval from their artificial and natural habitats. This is to ensure the protection of individuals, communities, and households in an ecological context to be effective and efficient10,40.
This review has some limitations. They may be a heterogeneity between diagnostic tools used in different included studies. Other sources of heterogeneity not explored in this study, because they are not reported in primary studies, include the difference in the prevalence of DENV in vectors, the difference in the immunity of humans, the difference in serotypes and genotypes of DENV, and ecosystem of different study places across the continent. All countries were not represented in this systematic review. This can hinder the generalization of our findings. Although there were some limitations, this is to the very best of our knowledge, the first review with meta-analysis summarizing data on the epidemiology of DENV infection in febrile and apparently healthy populations living in Africa. We have performed a comprehensive search of the literature and included independent reviewers for study selection and data extraction to avoid biases. Almost three quarters of the included studies were assessed as having low risk of bias. This suggest that we can be confident in the quality of findings this review. In addition, our main findings were in the range to that of sensitivity analysis considering only studies with PNRT for DENV confirmation; highlighting the robustness of our findings.
We can definitely conclude that the prevalence of DENV infection is high in the African continent although this infectious disease is considered as neglected tropical disease. It is therefore necessary that dengue fever deserves more attention from healthcare workers, researchers, and health policy makers in the continent. In the absence to date of effective antiviral treatment for dengue fever, strategies to curb its burden should therefore focus on prevention including vector control.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
WHO. Dengue, https://www.afro.who.int/health-topics/dengue (2017).
Amarasinghe, A., Kuritsky, J. N., William Letson, G. & Margolis, H. S. Dengue virus infection in Africa. Emerging Infectious Diseases 17, 1349–1354, https://doi.org/10.3201/eid1708.101515 (2011).
Simmons, C. P., Farrar, J. J., Nguyen, V. V. & Wills, B. Dengue. The New England journal of medicine 366, 1423–1432, https://doi.org/10.1056/NEJMra1110265 (2012).
Messina, J. P. et al. Global spread of dengue virus types: mapping the 70 year history. Trends in microbiology 22, 138–146, https://doi.org/10.1016/j.tim.2013.12.011 (2014).
Guo, C. et al. Global Epidemiology of Dengue Outbreaks in 1990–2015: A Systematic Review and Meta-Analysis. Frontiers in cellular and infection microbiology 7, 317, https://doi.org/10.3389/fcimb.2017.00317 (2017).
Villar, L. A., Rojas, D. P., Besada-Lombana, S. & Sarti, E. Epidemiological trends of dengue disease in Colombia (2000–2011): a systematic review. PLoS Negl Trop Dis 9, e0003499, https://doi.org/10.1371/journal.pntd.0003499 (2015).
WHO. Dengue and severe dengue, https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (2019).
Pabalan, N. et al. Associations of tumor necrosis factor-alpha-308 polymorphism with dengue infection: A systematic review and meta-analysis. Acta Trop 173, 17–22, https://doi.org/10.1016/j.actatropica.2017.05.007 (2017).
Humphrey, J. M. et al. Dengue in the Middle East and North Africa: A Systematic Review. PLoS Neglected Tropical Diseases 10, https://doi.org/10.1371/journal.pntd.0005194 (2016).
Simo, F. B. N. et al. Chikungunya virus infection prevalence in Africa: a contemporaneous systematic review and meta-analysis. Public health 166, 79–88, https://doi.org/10.1016/j.puhe.2018.09.027 (2019).
Division., U. N. S. Methodology: Standard country or area codes for statistical use (M49), https://unstats.un.org/unsd/methodology/m49/ (2019).
Thomas, S. J. et al. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: How alterations in assay conditions impact performance. The American journal of tropical medicine and hygiene 81, 825–833, https://doi.org/10.4269/ajtmh.2009.08-0625 (2009).
Hoy, D. et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. Journal of clinical epidemiology 65, 934–939, https://doi.org/10.1016/j.jclinepi.2011.11.014 (2012).
Barendregt, J. J., Doi, S. A., Lee, Y. Y., Norman, R. E. & Vos, T. Meta-analysis of prevalence. J Epidemiol Community Health 67, 974–978, https://doi.org/10.1136/jech-2013-203104 (2013).
Cochran, W. G. The Combination of Estimates from Different Experiments. Biometrics 10, 101–129, https://doi.org/10.2307/3001666 (1954).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 21, 1539–1558, https://doi.org/10.1002/sim.1186 (2002).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 315, 629–634, https://doi.org/10.1136/bmj.315.7109.629 (1997).
Seagroatt, V. & Stratton, I. Bias in meta-analysis detected by a simple, graphical test. Test had 10% false positive rate. BMJ (Clinical research ed.) 316, 470; author reply 470–471 (1998).
Mohd-Zaki, A. H., Brett, J., Ismail, E. & L’Azou, M. Epidemiology of dengue disease in Malaysia (2000–2012): a systematic literature review. PLoS Negl Trop Dis 8, e3159, https://doi.org/10.1371/journal.pntd.0003159 (2014).
Bravo, L., Roque, V. G., Brett, J., Dizon, R. & L’Azou, M. Epidemiology of dengue disease in the Philippines (2000–2011): a systematic literature review. PLoS Negl Trop Dis 8, e3027, https://doi.org/10.1371/journal.pntd.0003027 (2014).
Gratz, N. G. Critical review of the vector status of Aedes albopictus. Med Vet Entomol 18, 215–227, https://doi.org/10.1111/j.0269-283X.2004.00513.x (2004).
Hanson, S. M. & Craig, G. B. Jr. Aedes albopictus (Diptera: Culicidae) eggs: field survivorship during northern Indiana winters. J Med Entomol 32, 599–604, https://doi.org/10.1093/jmedent/32.5.599 (1995).
Hawley, W. A., Pumpuni, C. B., Brady, R. H. & Craig, G. B. Jr. Overwintering survival of Aedes albopictus (Diptera: Culicidae) eggs in Indiana. J Med Entomol 26, 122–129, https://doi.org/10.1093/jmedent/26.2.122 (1989).
Ryan, S. J., Carlson, C. J., Mordecai, E. A. & Johnson, L. R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis 13, e0007213, https://doi.org/10.1371/journal.pntd.0007213 (2019).
Lutomiah, J. et al. Dengue Outbreak in Mombasa City, Kenya, 2013–2014: Entomologic Investigations. Plos Neglected Tropical Diseases 10, https://doi.org/10.1371/journal.pntd.0004981 (2016).
Fournet, F. et al. The Influence of Urbanization Modes on the Spatial Circulation of Flaviviruses within Ouagadougou (Burkina Faso). International Journal of Environmental Research and Public Health 13, https://doi.org/10.3390/ijerph13121226 (2016).
Kamgang, B. et al. Genetic structure of the tiger mosquito, aedes albopictus, in Cameroon (central Africa). PLoS ONE 6, https://doi.org/10.1371/journal.pone.0020257 (2011).
Paupy, C. et al. Comparative Role of Aedes albopictus and Aedes aegypti in the Emergence of Dengue and Chikungunya in Central Africa. Vector-Borne and Zoonotic Diseases 10, 259–266, https://doi.org/10.1089/vbz.2009.0005 (2010).
Krueger, A. & Hagen, R. M. Short communication: First record of Aedes albopictus in Gabon, Central Africa. Tropical Medicine and International Health 12, 1105–1107, https://doi.org/10.1111/j.1365-3156.2007.01893.x (2007).
Althouse, B. M. et al. Impact of climate and mosquito vector abundance on sylvatic arbovirus circulation dynamics in senegal. American Journal of Tropical Medicine and Hygiene 92, 88–97, https://doi.org/10.4269/ajtmh.13-0617 (2015).
LaBeaud, A. D. et al. Arbovirus prevalence in mosquitoes, Kenya. Emerg Infect Dis 17, 233–241, https://doi.org/10.3201/eid1702.091666 (2011).
John, A. L. & Rathore, A. P. S. Adaptive immune responses to primary and secondary dengue virus infections. Nature reviews. Immunology 19, 218–230, https://doi.org/10.1038/s41577-019-0123-x (2019). St.
Xavier-Carvalho, C., Cardoso, C. C., de Souza Kehdy, F., Pacheco, A. G. & Moraes, M. O. Host genetics and dengue fever. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases 56, 99–110, https://doi.org/10.1016/j.meegid.2017.11.009 (2017).
Wearing, H. J. & Rohani, P. Ecological and immunological determinants of dengue epidemics. Proc Natl Acad Sci USA 103, 11802–11807, https://doi.org/10.1073/pnas.0602960103 (2006).
Hanley, K. A. et al. Fever versus fever: The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infection, Genetics and Evolution 19, 292–311, https://doi.org/10.1016/j.meegid.2013.03.008 (2013).
Weetman, D. et al. Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int J Environ Res Public Health 15, https://doi.org/10.3390/ijerph15020220 (2018).
Ayukekbong, J. A., Oyero, O. G., Nnukwu, S. E., Mesumbe, H. N. & Fobisong, C. N. Value of routine dengue diagnosis in endemic countries. World journal of virology 6, 9–16, https://doi.org/10.5501/wjv.v6.i1.9 (2017).
Jaenisch, T. et al. Dengue expansion in Africa-not recognized or not happening? Emerg Infect Dis 20, https://doi.org/10.3201/eid2010.140487 (2014).
da Costa, V. G., Marques-Silva, A. C., Floriano, V. G. & Moreli, M. L. Safety, immunogenicity and efficacy of a recombinant tetravalent dengue vaccine: a meta-analysis of randomized trials. Vaccine 32, 4885–4892, https://doi.org/10.1016/j.vaccine.2014.07.008 (2014).
WHO. Dengue control: Control strategies, https://www.who.int/denguecontrol/control_strategies/en/ (2019).
Author information
Authors and Affiliations
Contributions
F.B.N.S., J.J.B., S.K. and M.D. conceived the study and together with M.S.N., E.T. and P.F.M. designed the protocol. J.J.B. conceived and performed the search strategy and literature search. F.B.N.S., J.J.B. and S.K. selected studies and extracted relevant information. J.J.B. performed analysis and synthetized data. F.B.N.S. and J.J.B. wrote the first draft. J.J.B., S.K., M.S.N., E.T., P.F.M., and M.D. revised and reviewed successive versions of the manuscript. F.B.N.S., J.J.B., S.K., M.S.N., E.T., P.F.M. and M.D. approved the final version of the manuscript. M.D. is the guarantor of the review.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simo, F.B.N., Bigna, J.J., Kenmoe, S. et al. Dengue virus infection in people residing in Africa: a systematic review and meta-analysis of prevalence studies. Sci Rep 9, 13626 (2019). https://doi.org/10.1038/s41598-019-50135-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-50135-x
This article is cited by
-
A dengue virus infection in Ethiopia: a systematic review and meta-analysis
BMC Infectious Diseases (2024)
-
Epidemiological investigation of dengue fever outbreak and its socioeconomic determinants in Banadir region, Somalia
BMC Infectious Diseases (2024)
-
Determinants and prevalence of symptomatic dengue fever among adults in the Central Region of Burkina Faso: a hospital-based cross-sectional study
BMC Infectious Diseases (2024)
-
Spatiotemporal and meteorological relationships in dengue transmission in the Dominican Republic, 2015–2019
Tropical Medicine and Health (2023)
-
Detection of dengue virus infection in children presenting with fever in Hawassa, southern Ethiopia
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.