Abstract
Gyroviruses (GyVs) are small, single-stranded, circular DNA viruses in the genus Gyrovirus, which consists of the chicken anemia virus (CAV) prototype and nine other viral species. These different GyV species have been reported in chickens, humans, mice, and companion animals. To date, CAV has been identified in the feces of domestic cats, while the circulation of other GyV species in cats is currently unknown. In the present study, 197 fecal samples were collected from pet cats in northeast China, and samples were screened for different GyV species by PCR. Twelve GyV strains were identified from the feces of pet cats. These included 4 positive for CAV, 3 for HGyV/AGV2, 3 for GyV3 and 2 positive for GyV6. The complete genome sequences of the 12 cat-sourced GyV strains showed 93.9–99.7% nucleotide identities to the homologous reference GyV strains. Phylogenetic analyses based on the complete genomes, VP1, VP2 and VP3 genes showed the identical classification of GyV species with previous reports. Moreover, one and four unique amino acid substitutions were identified in the VP1 protein of the cat-sourced HGyV/AGV2 and GyV6 strains, respectively, and one substitution was also observed in the VP2 protein of one GyV6 strain identified in this study. In conclusion, our investigation demonstrates that the diverse GyV species were circulating in domestic cats, and provides the first molecular evidence for the circulation of HGyV/AGV2, GyV3 and GyV6 in domestic cats. These cat-origin GyVs possessed considerable genetic diversity. This study also raises the possibility that domestic cats, as reservoirs for gyroviruses, may inadvertently disseminate viruses to other species, e.g., humans and chickens.
Similar content being viewed by others
Introduction
Gyrovirus (GyV) is a small, non-enveloped, icosahedral virus with a single-stranded, negative-sense, circular DNA genome, approximately 2.3-kb in length. The GyV genome contains three partial overlapping open reading frames (ORFs) encoding structural protein VP1, non-structural protein VP2 and VP3 (also named as Apoptin), respectively1. Of these, VP1 is the most important viral structural protein determining viral replication, infection ability and virulence2,3. Both VP1 and VP2 can induce neutralizing antibodies4. GyVs belong to the genus Gyrovirus originally classified in the family Circoviridae. Because GyVs are not either structurally or genetically related to the members of the family Circoviridae, the genus Gyrovirus was reassigned to the family Anelloviridae according to the recent viral taxonomy report of International Committee on Taxonomy of Virus (ICTV)5. To date, the Gyrovitus genus only includes one officially recognized species, chicken anemia virus (CAV). CAV was first isolated from bursa of Fabricius of 1-day old chickens in 1979 in Japan and had been commonly found in chickens worldwide6,7, leading to significant losses in the poultry industry.
In 2011, two GyVs genetically related to CAV, human gyrovirus (HGyV) and avian gyrovirus 2 (AGV2), were found in the surface of healthy French adults’ skin8 and in the diseased chickens from Brazil9, respectively. Because HGyV shared more than 93% nucleotide identities with AGV2 in VP1-VP3 gene, they are classified as the same species, named as HGyV/AGV2. Since 2011, nine novel, unofficially recognized GyV species have been identified in different hosts. HGyV/AGV2 has been identified in sera and tissues of chickens10,11,12, commercial poultry vaccines13, the blood of healthy blood donors14, HIV-infected patients and organ transplant recipients15, and the skin of healthy humans8. The third gyrovirus named GyV3 was identified for the first time in the feces of diarrhoeic children from Chile in 201216 and had been also found in chickens with transmissible viral proventriculitis and human feces in China17,18. GyV4 was found in chicken meat and human stools in Hong Kong18. In 2013, GyV5 and GyV6 were first detected in the feces of Tunisian children with diarrhea19. Two additional species, GyV7 and GyV8 were reported to infect chicken20 and fulmar (sea bird)21, respectively. The ninth gyrovirus (GyV9) was found in the feces of a diarrhoeic adult in 2015 in the USA22. Recently, a newly described gyrovirus, GyV10, was identified in the sera of a crested screamer (Chauna torquata) with severe neurologic disease23. All gyrovirus species can be phylogenetically divided into three clades, A (CAV, HGyV/AGV2, GyV3, GyV6, GyV7, GyV9 and GyV10), B (GyV4 and GyV5) and C (GyV8).
Gyroviruses are not only reported to infect human and avian species, but also found in companion animals. Feher, et al. investigated the fecal virome of pet ferrets by metagenomic sequencing, and found the genome of CAV, HGyV/AGV2, GyV3 and GyV4 in the ferret feces24. Moreover, CAV had also been detected from the feces of domestic cats25 and dogs26,27 in China. These findings suggest that companion animals may be the reservoirs for gyrovirus. However, the circulation of other gyrovirus species in companion animals is currently unknown and the further investigation is required.
In this study, we investigate the prevalence of different GyV species in the feces of domestic cats in five cities in northeast China, and identify 12 cat feces-derived gyrovirus strains, including 4 CAVs, 3 HGyV/AGV2 strains, 3 GyV3 strains and 2 GyV6 strains. Moreover, the complete genome sequences of these 12 gyrovirus strains are amplified and sequenced by inverse PCR, and are also analyzed using various phylogenetic methods to describe the genomic characteristics. Based on our investigation, we demonstrate that the diverse gyrovirus species were circulating in domestic cats, and these cat-origin gyroviruses possess considerable genetic diversity.
Results
Epidemiology of gyrovirus in domestic cats
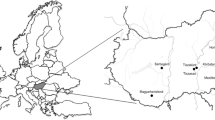
Between January 2016 and November 2017, a total of 197 fecal samples were collected from Jinzhou (n = 17), Shenyang (n = 36), Changchun (n = 85), Jilin (n = 33) and Harbin (n = 26) in northeast China. According to the PCR-based detection, twelve fecal samples, including one collected from Shenyang and Harbin each, two from Jilin and eight from Changchun, were positive for gyrovirus, with an apparent prevalence of 6.1% (12/197). Of these gyrovirus-positive samples, four fecal samples were positive for CAV, three were positive for HGyV/AGV2, three were positive for GyV3 and two were positive for GyV6, respectively (Table 1). One CAV-positive sample, two GyV3-positive samples and one GyV6-positive sample were detected from domestic cats with diarrhea, other eight gyrovirus-positive samples were from healthy cats. Healthy cats had a high gyrovirus positive rate (8.7%, 8/92) than diarrhoeic cats (3.8%, 4/105). We also detected the chicken DNA in 12 gyrovirus-positive feces, and found the chicken DNA in two CAV-positive samples (17JL0310 and 17SY0902) and one HGyV/AGV2-positive sample (16CC1103) (Table 1). These three fecal samples were collected from healthy cats. Copy numbers in gyrovirus-positive cats ranged 62,483 copies/ml to 1,317,593 copies/ml (Table 1). For CAV and HGyV/AGV2 positive samples, the viral loads were higher in fecal samples which tested to be positive for chicken DNA. While the viral copy numbers in cats with diarrhea (391,652–1,317,593 copies/ml, mean 825,998 copies/ml) are higher than that in healthy cats (95,113–558,771 copies/ml, mean 326,942 copies/ml) for GyV3 and GyV6 positive samples (Fig. 1).
Genomic analysis and sequences identities of the 12 gyrovirus strains
The complete cat-sourced gyrovirus genome sequences were verified using inverse PCR. Ten complete circular genome sequences (17CC0509, 17SY0902, 17JL0310, 17JL0314, 16CC1103, 17CC0315, 17CC0810, 17CC0704, 17CC0711 and 17HRB0905) and two near full-length genome sequences (17CC0301 and 17CC1116) were acquired and deposited in GenBank under accession numbers MK089240-MK089251. The length of the genome of 17CC0509, 17SY0902, 17JL0310 and 17JL0314 was 2298-bp, similar to the genome size of the CAV reference strains isolated from chickens, dogs and mice. Three partial overlapping ORFs, VP1 (450-aa), VP2 (217-aa) and VP3 (122-aa), were identified in these four CAV strains (Fig. 2). Comparative genomic analysis showed that the higher nucleotide identities were found between the four sequences and 22 CAV sequences retried from GenBank, with a mean value of 97.4% (Table 2). 17JL0310, 17JL0314 and 17SY0902 shared the highest nucleotide identities with CAV strains SH11 (99.8%, accession no. DQ141670), HN9 (99.1%, accession no. DQ141672) and 98D02152 (99.4%, accession no. AF311892) isolated from chicken, whereas 17CC0509 displayed the highest pairwise nucleotide identity (99.3%) with CAV isolated from human feces in Beijing, China (accession no. JQ690762). All four sequences displayed the lowest pairwise nucleotide identities (94.7–95.8%) with the CAV strain, 3711 (accession no. EF683159).
The genome of 16CC1103, 17CC0315 and 17CC0810 was 2375-bp long, encoding VP1 (460-aa), VP2 (231-aa) and VP3 (124-aa), respectively (Fig. 2). Compared to the gyrovirus reference sequences, the three strains were more closely related to the HGyV/AGV2 reference strains, with a mean nucleotide identity of 96.6% (Table 2). The strain 16CC1103 shared the highest nucleotide identity (99.7%) with Brazilian chicken-sourced AGV2 strain, Ave3 (accession no. HM590588), whilst it shared the lowest identity (95.0%) with AGV2 strain G17 (accession no. KJ452213) isolated from ferret fecal samples. Contrary to 16CC1103, 17CC0315 and 17CC0810 shared the highest nucleotide identities (99.2% and 98.9%) to the ferret-sourced HGyV/AGV2 strains G13 (accession no. KJ452214) and G17, respectively, while both of the two strains shared the lowest identities with Ave3 isolated from either a chicken in Brazil.
For 17CC0704, 17CC0711 and 17HRB0905, the length of the complete genome was 2356-bp, with three overlapping ORFs for the VP1 gene (1392-bp), VP2 gene (720-bp) and VP3 gene (378-bp) (Fig. 2). The three strains shared more than 99.1% nucleotide identities with GyV3 reference strains. And, more remarkable, 17CC0704 and 17CC0711 were more closely related to chicken-source GyV3 strain, SDAU-1 (accession no. MG366592), isolated in China, with the high nucleotide identities of 99.6–99.7%, whereas 17HRB0905 shared a higher identity of 99.5% to human-sourced GyV3 strain, FecGy (accession no. JQ308210).
Furthermore, the nearly complete genome sequences of 17CC0301 and 17CC1116, with length of 2220-bp, were acquired in the present study. Both 17CC0301 and 17CC1116 showed three overlapping ORFs, encoding VP1 (453-aa), VP2 (225-aa) and VP3 (111-aa) (Fig. 2), and showed the highest nucleotide identities (98.7% and 99.1%) with the GyV6 strain, Tu789 (accession no. KF294862), isolated from human feces in Tunisia in 2013.
Phylogenetic analysis
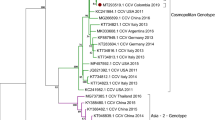
A phylogenetic tree based on these 12 gyrovirus genome nucleotide sequences and 40 representative genome nucleotide sequences from ten gyrovirus species was constructed using different methods. The Bayesian tree showed that the 12 gyrovirus sequences were classified into different groups, supported by the topology and the high posterior probability (Fig. 3). 17CC0509, 17SY0902, 17JL0310 and 17JL0314 were placed within the group CAV, which included 23 CAV reference strains isolated from different hosts (including chicken, human, cat, dog and mouse); 16CC1103, 17CC0315 and 17CC0810 were more closely related to 7 HGyV/AGV2 reference strains identified in human, chicken and ferret, clustering within the group HGyV/AGV2; 17CC0704, 17CC0711 and 17HRB0905 were classified within the group GyV3 of which 17CC0704, 17CC0711 and chicken-sourced GyV3 strain SDAU-1 formed a small branch, while 17HRB0905 and human-sourced GyV3 strain FecGy were clustered within another branch; 17CC0301, 17CC1116 and GyV6 reference strain Tu789 cluster together, belonging the group GyV6. This classification was also supported by the ML phylogenetic analysis (Supplementary Fig. S1).
Bayesian phylogenetic tree constructed based on the whole genome nucleotide sequences of the 12 cat-origin gyroviruses and 40 reference gyroviruses. Branch lengths are measured in nucleotide substitutions. Numbers above branches indicate the posterior probability from the Bayesian approach. The tree is rooted at the midpoint of the longest branch. Cat-origin CAV, HGyV/AGV2, GyV3 and GyV6 strains identified in the present study are shown in blue, purple, red and green color, respectively.
We then constructed the phylogenetic trees based on the deduced amino acid sequences of VP1, VP2 and VP3 genes using the Bayesian analysis with the JTT model (Fig. 4). The phylogenetic analyses based on VP1, VP2 and VP3 amino acid sequences showed the identical classification with that based on the complete genome sequences. The cat sourced CAV strain 17JL0310 shared 100% nucleotide and amino acid identities with the reference strain SH11 (accession no. DQ141670) in the VP2 and VP3 gene. 17JL0314 shared 99.7–99.8% nucleotide and 100% amino acid identities with reference strain isolated from feline feces in China (accession no. KC414026) in the VP2 and VP3 genes (Supplementary Table S4). 17JL0310 and 17SY0902 shared higher nucleotide and amino acid identities with the chicken-sourced CAV strain SH11 in the VP1, VP2 and VP3 genes. While 17CC0509 and 17JL0314 shared higher pairwise identities with human-sourced and cat-sourced CAV strains. In the Bayesian trees based on the VP1, VP2 and VP3 genes, all HGyV/AGV2 strains cluster within two distinct subgroups with >0.98 posterior probability. 16CC1103 shared the highest amino acid identity with the strain Ave3 (accession no.HM590588) isolated from chicken, while 17CC0315 and 17CC0810 were more closely related to the strain G17 (accession no.KJ452213) isolated from the feces of pet ferrets. All GyV3 strains identified in this study were 100% similar to GyV3 reference strains isolated from chicken meats and human feces based on the amino acid sequences of the VP1 gene, and shared 100% amino acid identities with GyV3 strain SDAV1 identified from chicken meats in the VP3 gene. The two cat-sourced GyV6 strains were 100% similar to GyV6 reference strain in both nucleotide and amino acid sequences of VP3 gene. Moreover, all of the gyroviruses could be further grouped into three distinct clades A, B and C in the Bayesian trees based on the amino acid sequences of VP1, VP2 and VP3 gene, similar to the previous reports. All cat-sourced gyrovirus strains belonged to the gyrovirus clade A.
Phylogenetic analysis based on the VP1 (a), VP2 (b) and VP3 (c) amino acid sequences of cat-origin and reference gyroviruses using MrBayes. Branch lengths are measured in nucleotide substitutions. Numbers above branches indicate the posterior probability from the Bayesian approach. The tree is rooted at the midpoint of the longest branch. Cat-origin CAV, HGyV/AGV2, GyV3 and GyV6 strains identified in the present study are shown in blue, purple, red and green color, respectively. The gyrovirus species and phylogenetic clades were showed in the tree.
We analyzed the recombination events for the 12 cat-origin gyrovirus strains using five methods in this study, but no recombination breakpoint was found in these strains.
Molecular characterization
To further analyze molecular characteristics of the 12 complete genome sequences of gyroviruses isolated from cats in this study, we systematically compared the amino acid polymorphisms of the 12 cat-sourced gyroviruses and representative gyrovirus strains isolated from different hosts (Fig. 5). The amino acid polymorphisms were found in the 19, 28, 6 and 5 amino acid sites in VP1-VP3 genes of cat feces-derived CAV, HGyV/AGV2, GyV3 and GyV6 strains, respectively. No amino acid substitutions were identified to be commonly possessed only by the cat feces-origin CAV and GyV3 isolates. A previous study had demonstrated that amino acid residues 139 and 144 of the CAV VP1 protein played an important role in viral growth and spread, as Gln139 and/or Gln144 were associated with a decreased rate of spread of CAV isolates28. In the 4 cat-sourced CAV isolates, the VP1 residues 139 and 144 of 17CC0509 and 17JL0310 were both glutamines, suggesting that the growth and spread rates of these two strains might be relatively low, while 17JL0314 and 17SY0902 carried amino acid residues carried Lys139 and Glu144 in the VP1 protein. It was also demonstrated that the position 394 in VP1 protein of CAV was a major genetic determinant of virulence, with glutamine (Glu) and histidine (His) representing high and low pathogenicity, respectively2,3. All of the cat-sourced CAV strains identified in the present study contained a glutamine at position 394, suggesting that they might be highly pathogenic. In all three cat-sourced HGyV/AGV2 isolates, the arginine (Arg) at position 209 of the VP1 gene was replaced by serine (Ser) in 17CC0315 and 17CC0810. Furthermore, there were four unique amino acid mutations (Ile76Phe, Thr149Gla, Pro292Ser and Thr380Gln) in VP1 and one mutation (Glu7Gly) in VP2 of cat-origin GyV6 isolates, which had not been previously reported. Three out of these five substitutions, including Thr149Gla, Pro292Ser and Thr380Gln, were simultaneously observed in the VP1 gene of 17CC0301 and 17CC1116.
Viral isolation
Three cell lines (CRFK, Caco-2 and MDCC-MSB1) were co-cultured with gyrovirus-positive fecal supernatants for 4 days and passaged to the fifth generation. No CPE were observed in the cultured cells, and no gyrovirus DNA was detected in culture lysates and supernatant by qPCR.
Discussion
The members of the genus Gyrovirus, which consists of the CAV prototype and nine other unofficially recognized species (HGyV/AGV2, GyV3, GyV4, GyV5, GyV6, GyV7, GyV8, GyV9 and GyV10), have been identified in chickens10,11,17,18, humans8,15,16,19,22, sea birds21, mice25, ferrets24,29, dogs26 and cats25. To date, there is only one report that CAV circulating in domestic cats in China25. Whether other gyrovirus species are circulating in cats is currently unknown. In this study, twelve gyrovirus strains were identified in the fecal samples of domestic cats that were collected from four cities (Shenyang, Jilin, Changchun and Harbin) in northeast China, with an apparent prevalence of 6.1% (12/197). The multiple alignment based on the full-length sequences of the gyrovirus genomes showed that the 12 cat-sourced gyroviruses includes 4 CAVs, 3 HGyV/AGV2 strains, 3 GyV3 strains and 2 GyV6 strains. To our knowledge, this study is the first report of HGyV/AGV2, GyV3 and GyV6 detection in domestic cats. So far only three gyrovirus species, including CAV, HGyV/AGV2 and GyV3 were reported in China. CAV was first isolated from sera of chickens in 199630 and was first identified in pediatric fecal samples in 2012. HGyV/AGV2 was detected for the first time in chickens and healthy humans in China in 201512. Moreover, GyV3 was first isolated from commercial broiler chickens in 201817. Our investigation also provides for the first time the molecular evidence that GyV6 is circulating in China.
Previous studies had demonstrated that CAV can cause atrophy of bone marrow hematopoietic tissue and lymphoid tissues in young chickens, and lead to severe anemia and immunosuppression31,32. However, the potential of nine other gyrovirus species is unknown. Maggi, et al. found that HGyV may infect HIV-infected patients and organ transplant recipients15. Phan, et al. reported that the high positive rate of CAV and GyV3 in feces from diarrhoeic and healthy Chilean children16. GyV5 and GyV6 were also isolated from the feces of diarrhoeic children in Tunisia19. This evidence was inadequate to demonstrate that gyrovirus could cause immunosuppression and/or gastrointestinal disease in mammalians (e.g., humans). Our investigation showed that CAV, GyV3 and GyV6 were detected in domestic cats with or without diarrhea, while HGyV/AGV2 was only isolated from healthy cats. The positive rate of gyroviruses DNA in healthy cats (8.7%) was higher than that in diarrhoeic cats (3.8%), suggesting the gyrovirus may be non-pathogenic for domestic cats. However, for GyV3 and GyV6 positive samples, higher viral copy numbers (391,652–1,317,593 copies/ml, mean 825,998 copies/ml) were observed in cats with diarrhea, suggesting GyV3 and GyV6 may be related to the diarrhea of cats. More studies are needed to further investigate the pathogenic potential of different gyrovirus species in cats.
Comparison of the VP1-VP3 amino acid sequences between the 12 cat-origin gyrovirus strains and the homologous reference gyrovirus sequences indicated that the amino acid polymorphisms were existed in cat-origin gyroviruses, but no unique amino acid substitutions were found in cat-sourced CAV and GyV3 isolates. It had been demonstrated that the amino acid at position 394 of the CAV VP1 protein was a major genetic determinant of virulence, and the amino acids at position 139 and 144 of the CAV VP1 protein play an important role in viral growth and spread3,28. All of the CAV strains identified in this study possessed glutamine at position 394, suggesting that they might be highly pathogenic. 17CC0509 and 17JL0310 possessed both glutamines at the VP1 residues 139 and 144, which suggested these two CAV strains might have low growth and spread rates. Moreover, a unique amino acid mutation was found at position 209 of the VP1 gene in 17CC0315 and 17CC0810, and four unique amino acid mutations in VP1 and one mutation in VP2 were identified in three cat-sourced GyV6 strains. Whether these unique amino acid substitutions is due to the change in hosts or these mutations made gyrovirus easier to spread to different hosts is unclear and needs to be further studied. The amino acid polymorphisms analysis suggested that these cat-origin gyroviruses possessed considerable genetic diversity.
Genomic analysis showed that the cat-sourced gyroviruses have the same genome organization with the homologous reference gyrovirus strains, and shared more than 94.0% nucleotide pairwise identities to the homologous reference gyrovirus sequences from chickens, humans and pet ferrets. Phylogenetic analyses based on the complete gene sequences, VP1, VP2 and VP3 showed that all of the cat-sourced gyroviruses were divided into different gyrovirus species, clustering within gyrovirus clade A, which consists of CAV, HGyV/AGV2, GyV3, GyV6, GyV7, GyV9 and GyV10. In the gyrovirus clade A, CAV, HGyV/AGV2, GyV3, GyV7 and GyV10 had been reported to infect chickens; CAV, HGyV/AGV2, GyV3, GyV6 and GyV10 had been detected in human skin or feces. Interestingly, we found that some cat-sourced gyrovirus strains were more closely related to the reference strains isolated from chickens, while others shared higher nucleotide and amino acid identities with the homologous reference gyrovirus sequences identified in humans. These findings suggested that these diverse cat-origin gyroviruses might originated from gyrovirus-infected chickens or humans carrying gyroviruses via the fecal oral route, and domestic cats might be not the natural hosts of gyroviruses. In the present study, we detected the chicken DNA in 12 gyrovirus-positive feces, and found the chicken DNA in two CAV-positive samples (17JL0310 and 17SY0902) and one HGyV/AGV2-positive sample (16CC1103). Moreover, the three chicken-DNA-positive samples (395,804–1,051,657 copies/ml, mean 730,193 copies/ml) had higher viral copy numbers than chicken-DNA-negative samples (62,483–628,377 copies/ml, mean 327,221 copies/ml). Even more remarkably, 17JL0310, 17SY0902 and 16CC1103 shared higher nucleotide and amino acid identities with chicken-sourced gyrovirus strains, while other cat-sourced CAV and HGyV/AGV2 strains identified in chicken-DNA-negative samples were phylogenetically related to human/ferret-sourced gyroviruses. Chicken meat and chicken powder are usually used to make commercial food for cats. Domestic cats may be reservoirs for gyrovirus through ingest these commercial chicken foods from gyrovirus-infected chickens. This could be an explanation why the CAV and HGyV/AGV2, widely circulating in chickens, were identified in the feces of cats. Similarly, domestic cats could contact gyrovirus via the contact with gyrovirus-infected humans. This study reveals that domestic cats may be potential reservoir for gyroviruses, and raises the potential threat of gyrovirus to the health of domestic cats and other mammals.
Furthermore, to isolate the cat-origin gyroviruses, we selected three cell types originating from cats (CRFK cell line), humans (Caco-2 cell line) and chickens (MDCC-MSB1 cell line). Unfortunately, no gyrovirus replication was detected in any of these cell lines. To our knowledge, only CAV could be cultured in MDCC-MSB1 cell line33, while other attempts to isolate the other gyrovirus species have failed. Because of the failure of virus isolation, there is no evidence that the gyrovirus could replicate in domestic cats.
In conclusion, we identified 12 cat feces-derived gyrovirus strains, including 4 CAVs, 3 HGyV/AGV2 strains, 3 GyV3 strains and 2 GyV6 strains, and described the complete genome of the 12 gyroviruses. Our investigation demonstrates that the diverse gyrovirus species were circulating in domestic cats, and provides the first molecular evidence for the circulation of HGyV/AGV2, GyV3 and GyV6 in domestic cats. These cat-origin gyroviruses possessed considerable genetic diversity. This study also raises the possibility that domestic cats, as reservoirs for gyroviruses, may inadvertently disseminate viruses to other species, e.g., humans and chickens.
Material and Methods
Clinical specimens
A total of 197 fecal samples were collected from 105 diarrhoeic cats and 92 healthy cats in five cities, including Jinzhou, Shenyang, Changchun, Jilin and Harbin, in northeast China during 2016–2017. All samples were stored at −70 °C until further use.
DNA extraction and gyrovirus detection
Individual fecal sample was homogenized in phosphate-buffered saline (PBS) at a concentration of approximately 0.5 g/ml using a vortex oscillator (Thermo, USA), and then were centrifuged at 10,000 × g for 10 min to collect the supernatant. Total DNA was extracted from 250 μl of supernatant for each sample using AxyPrepTM Body Fluid Viral DNA/RNA Miniprep Kit (CORNING, China) according to the manufacturer’s instruction. Ten pairs of specific primers of which 6 pairs were described in previous papers18,20,22,25 and 4 pairs were designed using Primer 5.0 to detect different gryovirus species. The primer sequences and amplification conditions are shown in Supplementary Table S1. The sizes of amplicons were 535-bp for CAV, 293-bp for HGyV/AGV2, 412-bp for GyV3, 285-bp for GyV4, 475-bp for GyV5, 473-bp for GyV6, 224-bp for GyV7, 371-bp for GyV8, 1841-bp for GyV9 and 414-bp for GyV10. The amplified products were separated after electrophoresis on 1.5% agarose gels at 160 V for 20 min, and were visualized using a gel documentation system (Wealtec, USA). Furthermore, a conventional reverse transcription PCR (RT-PCR) with forward primer 5′-CTGAAGTACCCCATTGAACACG-3′ and the reverse primer 5′-ACAGGACTCCATACCCAAGAAAG-3′ targeting a 618-bp fragment of chicken β-actin gene was used to evaluate the chicken DNA in gyrovirus-positive fecal samples.
Quantitative PCR for the quantification of the viral DNA load
To quantify viral load in fecal samples, quantitative PCR (qPCR) was performed with TB GreenTM Fast qPCR mix (Takara, Japan) and ABI 7500 Fast Real-time PCR system (Invitrogen, USA), with the forward and reverse primers for VP1 gene of CAV, HGyV/AGV2, GyV3 and GyV6 (Supplementary Table S2). A 25 μl PCR mixture was used for per reaction and contained 12.5 l of 2 × TB premix Ex TaqII, 20 pmol of forward primer, 20 pmol of reverse primer and 1 μg template DNA or 2 μl diluted standard DNA. DNA was amplified using the ABI 7500 Fast Real-time PCR system using the following procedure: 95 °C for 5 min, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The standard curve was plotted from the results of parallel PCRs performed on ten-fold serial dilutions (107–102 copies/ml) of standard DNA. Reactions for each sample were performed in triplicate. DNA absolute quantities of gyrovirus in fecal samples were calculated by normalization to the standard curve, and were presented as the means from three repeated reactions per sample.
Amplification, cloning and sequencing of the gyrovirus genomes
To further analyze the genomic characteristics, the full-length genomes of different gyrovirus species were amplified by inverse PCR (primers sequences were shown in Supplementary Table S3). Three pairs of primers were used to verify the CAV genome. The amplicons were 843-bp, 989-bp and 802-bp in length, respectively, to cover the entire CAV genome26. To map the HGyV/AGV2 genome, three primer pairs were used to amplify 733-bp, 981-bp and 802-bp fragments encompassing the entire genome10. For the amplification of the GyV3 genome, two primer pairs targeting the 431-bp and 2203-bp regions were used for inverse PCR17. Moreover, two pairs of specific primers were designed based on the published GyV6 sequences in GenBank (KF294862) to amplify the complete GyV6 genome. The amplified fragments were 1846-bp and 594-bp, respectively. The PCR amplification was carried out in a total volume of 50 μl of reaction mixture containing 25 μl of Premix Ex Taq (Takara, Japan), 2 μl of 10 pM forward and reverse primers, 5 μl of sample DNA and 16 μl of RNase free water using a thermal cycler (BIOER, China). The amplification programs were shown in Supplementary Table S3. All PCR products were analyzed by 1.0% agarose gel electrophoresis, and visualized using a gel documentation system (Wealtec, USA).
The amplification fragments with the expected sizes for each primer pair were purified using AxyPrep DNA gel Extraction Kit (CORNING, China), and cloned into a PMD-18T vector (Takara, China) according to the manufacturer’s instruction. Then, the ligated products were transformed into Trans-T1 competent cells (Transgen Biotech, China). Plasmid DNA was extracted using AxyPrep Plasmid Miniprep kit (CORNING, China), and was sent to Sangon Biotech (Shanghai, China) for Sanger sequencing. Cloning and sequencing were performed at least three times for each PCR fragment of each sample.
Genomic, phylogenetic and recombination analyses
The nucleotide sequences of the gyrovirus-positive samples were assembled using Seqman program in Lasergene 7.0 software package (DNASTAR Inc., Wisconsin). The ORFs of the complete gyrovirus genome were predicted using ORF finder (https://www.ncbi.nlm.nih.gov/orffinder/) and were further verified using Gene Marks (http://opal.biology.gatech.edu/GeneMark/genemarks.cgi). Multiple sequences alignment between the gyrovirus genomes identified in this study and the gyrovirus reference sequences from different hosts were performed using Clustal W method. Percentage pairwise identities based on the nucleotide and the deduced amino acid sequences were calculated using Bioedit and SDT34. Phylogenetic analysis of the 12 cat-sourced gyroviruses and 40 reference sequences obtained from GenBank was performed using different methods. Maximun likelihood (ML) analysis was performedi in MEGA7.0 using the Kimura 2-parameter substitution model, and the branch robustness was evaluated by a bootstrap of 1,000 replicates. Additionally, the Bayesian trees based on the amino acid sequences of VP1, VP2 and VP3, and the genome nucleotide sequences were conducted using the Jones Taylor Thornton (JTT) amino acid substitution model and General Time Reversible (GTR) nucleotide substitution model, respectively. Four independent chains were run for 20 million steps with trees and parameters sampled every 2000 steps, and the first 10% were removed as burn-in. Two GyV4 strains (accession no. KJ452215 and JX310702) and one GyV5 strain (accession no. KF294861) were used as outgroup sequences. The trees were visualized using Figtree v1.4.3 and annotated with the support of the posterior probability from the Bayesian approach.
Recombination analysis was performed using GENECONV, BootAcan, RDP, MaxChi and SiScan methods in Recombination Detection Program (RDP) 4.0 software to identify putative parental sequences with significance set at P values < 0.01 and the sliding window size set as 30 bp. Only recombination event supported by no fewer than three independent methods were regarded as positive. Then the putative recombination events were further identified using SimPlot analysis in SimPlot 3.5 software.
Virus isolation
The fecal supernatants of gyrovirus-positive samples were filtered through a 0.22-μm membrane (Millipore, Ireland) for virus isolation. Then, 500 μl of the filtrate were inoculated onto CRFK (originating from cats), Caco-2 (originating from humans) and MDCC-MSB1 (originating from chicken) cells (approximate 1 × 107 cells per culture flask). Three repetitions were set for each sample on the same cell line. After incubating for 1 hour, the inocula were removed and replaced with MEM which supplemented with 2% foetal calf serum, and continued incubating at 37 °C with 5% CO2. Cultures were directly inspected daily by microscopy for cytopathic effects (CPE) until 4 days postinoculation. The cultures were frozen and used for further passage. Passaging did not stop until the fifth generation, after which the sample was considered negative if no CPE was observed. Culture lysates and supernatant were also collected for gyrovirus qPCR.
Ethics statement
Written informed consent was obtained from all pets’ owners before collecting fecal samples in this study. Sample collection was performed by veterinary assistant of our lab according to the approved guidelines and related regulations. All of the experimental procedures were reviewed and approved by the Ethics Committee and Institutional Review Board of Use Committee of Jilin Agricultural University.
Data Availability
The whole genomes of the identified viruses in the present study were summited to the GenBank database under accession numbers of MK089240-MK089251.
References
Hino, S. & Prasetyo, A. A. Relationship of Torque teno virus to chicken anemia virus. Curr Top Microbiol Immunol 331, 117–130, https://doi.org/10.1007/978-3-540-70972-5_8 (2009).
Todd, D., Scott, A. N. J., Ball, N. W., Borghmans, B. J. & Adair, B. M. Molecular basis of the attenuation exhibited by molecularly cloned highly passaged chicken anemia virus isolates. Journal of Virology 76, 8472–8474, https://doi.org/10.1128/Jvi.76.16.8472-8474.2002 (2002).
Yamaguchi, S. et al. Identification of a genetic determinant of pathogenicity in chicken anaemia virus. J Gen Virol 82, 1233–1238, https://doi.org/10.1099/0022-1317-82-5-1233 (2001).
Koch, G., van Roozelaar, D. J., Verschueren, C. A., van der Eb, A. J. & Noteborn, M. H. Immunogenic and protective properties of chicken anaemia virus proteins expressed by baculovirus. Vaccine 13, 763–770, https://doi.org/10.1016/0264-410X(94)00034-K (1995).
Rosario, K. et al. Revisiting the taxonomy of the family Circoviridae: establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch Virol 162, 1447–1463, https://doi.org/10.1007/s00705-017-3247-y (2017).
Schat, K. A. Chicken anemia virus. Curr Top Microbiol Immunol 331, 151–183, https://doi.org/10.1007/978-3-540-70972-5_10 (2009).
Yuasa, N., Yoshida, I. & Taniguchi, T. Isolation of a reticuloendotheliosis virus from chickens inoculated with Marek’s disease vaccine. Natl Inst. Anim Health Q (Tokyo) 16, 141–151 (1976).
Sauvage, V. et al. Identification of the first human gyrovirus, a virus related to chicken anemia virus. J Virol 85, 7948–7950, https://doi.org/10.1128/JVI.00639-11 (2011).
Rijsewijk, F. A. et al. Discovery of a genome of a distant relative of chicken anemia virus reveals a new member of the genus Gyrovirus. Arch Virol 156, 1097–1100, https://doi.org/10.1007/s00705-011-0971-6 (2011).
Yao, S. et al. Novel characteristics of the avian gyrovirus 2 genome. Sci Rep 7, 41068, https://doi.org/10.1038/srep41068 (2017).
Yao, S. et al. Avian gyrovirus 2 in poultry, China, 2015–2016. Emerg Microbes Infect 5, e112, https://doi.org/10.1038/emi.2016.113 (2016).
Ye, J. et al. Avian Gyrovirus 2 DNA in Fowl from Live Poultry Markets and in Healthy Humans, China. Emerg Infect Dis 21, 1486–1488, https://doi.org/10.3201/eid2108.150203 (2015).
Varela, A. P. et al. Chicken anemia virus and avian gyrovirus 2 as contaminants in poultry vaccines. Biologicals 42, 346–350, https://doi.org/10.1016/j.biologicals.2014.08.002 (2014).
Biagini, P., Bedarida, S., Touinssi, M., Galicher, V. & de Micco, P. Human gyrovirus in healthy blood donors, France. Emerg Infect Dis 19, 1014–1015, https://doi.org/10.3201/eid1906.130228 (2013).
Maggi, F. et al. Human gyrovirus DNA in human blood, Italy. Emerg Infect Dis 18, 956–959, https://doi.org/10.3201/eid1806.120179 (2012).
Phan, T. G. et al. A third gyrovirus species in human faeces. J Gen Virol 93, 1356–1361, https://doi.org/10.1099/vir.0.041731-0 (2012).
Li, G. et al. Emergence of gyrovirus 3 in commercial broiler chickens with transmissible viral proventriculitis. Transbound Emerg Dis 65, 1170–1174, https://doi.org/10.1111/tbed.12927 (2018).
Chu, D. K. et al. Characterization of a novel gyrovirus in human stool and chicken meat. J Clin Virol 55, 209–213, https://doi.org/10.1016/j.jcv.2012.07.001 (2012).
Gia Phan, T. et al. Divergent gyroviruses in the feces of Tunisian children. Virology 446, 346–348, https://doi.org/10.1016/j.virol.2013.08.020 (2013).
Zhang, W., Li, L., Deng, X., Kapusinszky, B. & Delwart, E. What is for dinner? Viral metagenomics of US store bought beef, pork, and chicken. Virology 468–470, 303–310, https://doi.org/10.1016/j.virol.2014.08.025 (2014).
Li, L. et al. A gyrovirus infecting a sea bird. Arch Virol 160, 2105–2109, https://doi.org/10.1007/s00705-015-2468-1 (2015).
Phan, T. G. et al. A new gyrovirus in human feces. Virus Genes 51, 132–135, https://doi.org/10.1007/s11262-015-1210-0 (2015).
Goldberg, T. L., Clyde, V. L., Gendron-Fitzpatrick, A., Sibley, S. D. & Wallace, R. Severe neurologic disease and chick mortality in crested screamers (Chauna torquata) infected with a novel Gyrovirus. Virology 520, 111–115, https://doi.org/10.1016/j.virol.2018.05.014 (2018).
Feher, E. et al. Molecular detection and characterization of human gyroviruses identified in the ferret fecal virome. Arch Virol 159, 3401–3406, https://doi.org/10.1007/s00705-014-2203-3 (2014).
Zhang, X. et al. Identification of a chicken anemia virus variant-related gyrovirus in stray cats in china, 2012. Biomed Res Int 2014, 313252, https://doi.org/10.1155/2014/313252 (2014).
Li, Y. et al. Genomic Characterization of Recent Chicken Anemia Virus Isolates in China. Front Microbiol 8, 401, https://doi.org/10.3389/fmicb.2017.00401 (2017).
Fang, L. et al. Genetic Analysis of Two Chicken Infectious Anemia Virus Variants-Related Gyrovirus in Stray Mice and Dogs: The First Report in China, 2015. Biomed Res Int 2017, 6707868, https://doi.org/10.1155/2017/6707868 (2017).
Renshaw, R. W. et al. A hypervariable region in VP1 of chicken infectious anemia virus mediates rate of spread and cell tropism in tissue culture. Journal of Virology 70, 8872–8878, https://doi.org/10.1016/S0166-0934(96)02147-7 (1996).
Feher, E., Pazar, P., Lengyel, G., Phan, T. G. & Banyai, K. Sequence and phylogenetic analysis identifies a putative novel gyrovirus 3 genotype in ferret feces. Virus Genes 50, 137–141, https://doi.org/10.1007/s11262-014-1128-y (2015).
Zhou, W., Yang, B., Shen, B., Han, S. & Zhou, J. A serologic survey of antibody against chicken infectious anemia virus by indirect immunofluorescent assay in domestic poultry in China. Avian Dis 40, 358–360, https://doi.org/10.1080/03079458808436450 (1996).
Adair, B. M. Immunopathogenesis of chicken anemia virus infection. Dev Comp Immunol 24, 247–255, https://doi.org/10.1016/S0145-305X(99)00076-2 (2000).
Taniguchi, T., Yuasa, N., Maeda, M. & Horiuchi, T. Chronological observations on hemato-pathological changes in chicks inoculated with chicken anemia agent. Natl Inst Anim Health Q (Tokyo) 23, 1–12, https://doi.org/10.1258/002367783781062334 (1983).
Yuasa, N. Propagation and infectivity titration of the Gifu-1 strain of chicken anemia agent in a cell line (MDCC-MSB1) derived from Marek’s disease lymphoma. Natl Inst Anim Health Q (Tokyo) 23, 13–20, https://doi.org/10.1101/gad.1012602 (1983).
Muhire, B. M., Varsani, A. & Martin, D. P. SDT: A Virus Classification Tool Based on Pairwise Sequence Alignment and Identity Calculation. Plos One 9, https://doi.org/10.1371/journal.pone.0108277 (2014).
Acknowledgements
This study was supported by the Research Project of the National Key Research and Development Plan of China (Grant No. 2016YFD0501002) and National Key R&D Program of China (Grant No. 2017YFD0501703).
Author information
Authors and Affiliations
Contributions
J.T.N., S.S.Y. and G.X.H. conceived the experiments, S.S.Y., J.T.N. and Y.B.G. performed the experiments; J.T.N., G.Y.D. and Y.L.Z. analyzed the data, H.D., Y.L.Z., H.L.H. and K.W. contributed samples/reagents/analysis tools, S.S.Y., K.W. and J.T.N. wrote the manuscript, All authors reviewed the manuscript, J.T.N., S.S.Y. and K.W. revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Niu, JT., Yi, SS., Dong, GY. et al. Genomic Characterization of Diverse Gyroviruses Identified in the Feces of Domestic Cats. Sci Rep 9, 13303 (2019). https://doi.org/10.1038/s41598-019-49955-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49955-8
This article is cited by
-
Serological investigation of Gyrovirus homsa1 infections in chickens in China
BMC Veterinary Research (2022)
-
A novel gyrovirus in a common pheasant (Phasianus colchicus) with poult enteritis and mortality syndrome
Archives of Virology (2022)
-
Kinetic analysis of pathogenicity and tissue tropism of gyrovirus 3 in experimentally infected chickens
Veterinary Research (2021)
-
Taxonomic updates for the genus Gyrovirus (family Anelloviridae): recognition of several new members and establishment of species demarcation criteria
Archives of Virology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.