Abstract
Glucose transporter-1 (GLUT1) has been proposed as a prognosticator in various cancers associated with therapeutic resistance and immune evasion; however little data is available on the role of GLUT1 in cervical cancer. Most cervical cancers are caused by human papilloma virus (HPV), but studies on the treatment response and prognosis depending on the HPV subtype, are conflicting. This hypothesis-generating study aims to investigate the prognostic impact of GLUT1 in cervical cancer, in conjunction with HPV subtype. Clinicopathologic factors, along with mRNA expression data were obtained using The Cancer Genome Atlas database. Tumor HPV status and immune cell scores were extracted from previous publications. In total, 298 patients were analyzed. High GLUT1 expression was associated with old age, squamous cell carcinoma, high tumor stage, pelvic lymph node metastases, and low hysterectomy rate. Multivariate survival analysis revealed that high GLUT1 expression (Hazard ratio (HR) 2.57, p = 0.002) and HPV16 subtype (HR 0.56, p = 0.033) were independent prognostic factors for overall survival. In the subgroup analysis, poor prognostic impact of high GLUT1 expression was maintained in HPV16-positive group (p < 0.001), but not in HPV16-negative group (p = 0.495). Decreased immune cell scores of CD8+ T cells, B cells, and Th1 cells by high GLUT1 expression were observed only in HPV16-positive group. In conclusion, these results suggested that GLUT1 expression and HPV16 subtype might have an independent prognostic value in cervical cancer. GLUT1-mediated immunomodulation might be an important cause of treatment failure, especially in HPV16-positive group.
Similar content being viewed by others
Introduction
Cervical cancer is still occurring worldwide at a considerable frequency despite significant advances in the screening of pre-invasive lesion, as well as modern prevention strategies with the high-risk human papilloma virus (HPV) vaccination1. Although HPV infection has been considered as an important cause of cervical cancer, studies on the treatment response and prognosis according to the different HPV subtypes are conflicting2,3,4. Several studies suggested that HPV genotypes might be associated with the prognosis of cervical cancer patients treated with radiotherapy5,6. In addition, cancer linked to the high-risk HPV is considered to be more immunogenic because of the production of viral proteins7. Although clinical stage, lymph node metastasis, lymphovascular invasion, and tumor size are commonly used to predict the prognosis, patients with similar prognostic factors often experience very different therapeutic responses in actual clinics. Further stratification based on novel biologic markers may help clinicians developing individualized strategies for heterogeneous group of patients.
HPV infection could promote certain cancer hallmarks including immune evasion and epithelial-mesenchymal transition, increasing the activity of glycolytic enzymes8,9. Altered tumor metabolic status has been recognized as an important contributing factor to chemo- or radio-resistance. Among the glycolytic enzymes, glucose transporter-1 (GLUT1), which facilitates glucose transport across the plasma membrane, has been reported to be a poor prognosticator in various cancers10,11,12. Previous meta-analysis suggested that the overexpression of GLUT1 was associated with enhanced invasiveness, proliferative potential, and decreased patients’ survival13. However, little data is available for the prognostic significance of GLUT1 in cervical cancer.
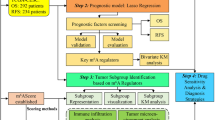
Therefore, to elucidate the effect of the combination of HPV genotypes and tumor metabolism, mediated by GLUT1, on the treatment outcomes and tumor microenvironment in cervical cancer, we analyzed these biomarkers in patients with cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), using The Cancer Genome Atlas (TCGA) database.
Results
Patients’ characteristics
Differential GLUT1 mRNA expression levels in tumor and normal tissues are shown according to TCGA classification in Supplemental Table 1. CESC ranked second among the pan-cancers in terms of GLUT1 gene differential fold change (tumor over normal tissues).
The characteristics of the patients included in this study are presented in Supplemental Table 2. Specifically, the mean age was 48 years (range 20–88 years). Squamous cell carcinoma was found in 247 (82.9%) patients, and adenocarcinoma was found in the remaining 51 patients (17.1%). About half of the patients had clinical stage I disease. The patients with diverse stage distribution received heterogeneous treatments including hysterectomy, radiotherapy, and/or chemotherapy. In terms of HPV infection, HPV16 was found in 164 (55.0%) patients, HPV18 in 39 (13.1%) patients, and other HPV subtypes in 70 (23.5%) patients. Only 18 patients were negative for all types of HPV (6.0%). Available follow-up data showed that recurrence occurred in 48 (16.1%) patients and death in 72 (24.2%) patients.
Upon subdivision of the patients into two groups based on the GLUT1 expression level, we found significant differences in characteristics (Table 1). High GLUT1 expression was associated with age ≥50, squamous cell carcinoma, high tumor stage, pelvic lymph node metastases, low tumor grade, and low hysterectomy rate. On the contrary, when the patients were divided into two groups based on HPV16 positivity, we did not find association with clinicopathologic factors, except for a low tumor grade and age <50 (Supplemental Table 3).
Survival analysis
The results of the univariate analyses for OS and PFS are listed in Table 2. Four factors appeared to be associated with OS in univariate analysis: clinical stage, pelvic lymph node (LN) metastases, hysterectomy, and GLUT1 expression. However, all factors except GLUT1 expression were excluded from the Cox proportional hazard model, due to the issue of multicollinearity in relation to GLUT1 expression (all p-value <0.05 by Pearson correlation test). Other than that, age, histology, and tumor grade were entered into the multivariate model to adjust the potential confounding effects, although they did not significantly affect OS in the univariate analysis. Multivariate survival analysis revealed that high GLUT1 expression (HR 2.57, p = 0.002) and HPV16 subtype (HR 0.56, p = 0.033) were independent prognostic factors for OS (Table 3). Kaplan-Meier curves of overall survival based on GLUT1 expression or HPV16 positivity in all study patients were shown in Fig. 1. Five-year OS rates were 51.7% in GLUT1-high group and 68.7% in GLUT1-low group (p < 0.001).
On the other hand, HPV16 positivity and pelvic LN metastases were significant prognostic factors for PFS in univariate analysis. However, none of them maintained statistical significance in multivariate analysis.
Exploratory subgroup analysis assessing the impact of GLUT1 on
Supplemental Fig. 1 plotted HR and 95% CI after comparing OS based on the GLUT1 expression, between each patient subgroups. The significance of high GLUT1 expression varies depending upon several factors. Specifically, by focusing on HPV16 positivity, the poor prognostic impact of high GLUT1 expression was maintained only in HPV16-positive group (HR 3.326, 95% CI 1.700–6.509, p < 0.001), and not in HPV16-negative group (HR 1.293, 95% CI 0.617–2.708, p = 0.495). Survival curves based on GLUT1 expression and HPV16 status were shown in Fig. 2.
To further elucidate the cause of the differential impact of GLUT1 overexpression on OS between HPV16-positive and -negative patients, possible differences in the tumor immune microenvironment were investigated. As shown in Table 4, decreased immune cell scores of T cells, CD8+ T cells, B cells, Th1 cells, and exhausted CD8+ cells induced by high GLUT1 expression were observed only in the HPV16-positive group, whereas no significant differences was found in the HPV16-negative group.
Discussion
In the present study, we demonstrated that high GLUT1 expression and HPV16 subtype are independent prognostic factors in cervical cancer. Interestingly, poor prognostic impact as well as differences in the tumor immune microenvironment linked to high GLUT1 expression were observed only in HPV16-positive group.
GLUTs are plasma membrane transporters that facilitate glucose transport across the cell membrane. Upregulation of GLUTs is present in most cancer cells and can be one of the initial carcinogenic changes14. Among GLUT family, it is known that high GLUT1 expression level is associated with advanced tumor stage, squamous histology, high tumor grade, depth of invasion, and poor differentiation in various cancers10,11,15,16. Our results also validated these findings in TCGA cervical cancer cohort.
Until now, few studies have evaluated the prognostic value of GLUT1 in cervical cancer reporting conflicting results. Kim et al. investigated hypoxia and metabolic markers in cervical cancer demonstrating that the high expression of HIF-1α and c-Met was associated with low OS, whereas the high expression of GLUT1 and CA9 did not show significant impact on survival17. On the contrary, Kanjanapan et al. recently showed that high GLUT1 expression was associated with low PFS (HR 2.8, p = 0.049) and OS (HR 5.0, p = 0.011) in 60 patients with locally advanced cervical cancer18, and these findings are consistent with our study. These previous studies were based on immunohistochemistry results, which might be subjective, therefore may produce different results. In our study, we quantitatively assessed GLUT1 mRNA expression level since there was no GLUT1 protein expression data in the TCGA database.
We hypothesized that one of the reasons of the above conflicting results for the prognostic role of GLUT1 in cervical cancer may result from the difference in the proportion of HPV16-positive patients. In our study, HR for high GLUT1 expression was 3.326 in the HPV16-positive group (p < 0.001) and 1.293 in the HPV16-negative group (p = 0.495), showing that the role of GLUT1 may differ depending on HPV16 status. Our study is the first report suggesting a differential prognostic impact of GLUT1 according to the carcinogenic virus type. Carcinogenic viruses can regulate tumor metabolic profiles via direct or indirect interaction with GLUT119. According to above findings, HPV16-related cancer may be more addicted to the glycolysis pathway through its oncoprotein E6/E7, thus GLUT1 expression level could have an important prognostic value in HPV-positive cervical cancer20,21. HPV16 E7 protein can alter the activity of pyruvate kinase, which enables deriving metabolic energy mostly from glycolytic processes rather than from oxidative phosphorylation20. Moreover, the expression changes of E6/E7 significantly promote the expression of hypoxia-inducible factor 1-alpha (HIF-1α) which activates the transcription of transporters and enzymes regulating the glycolysis and the pentose phosphate shunt21. On the other hand, HPV16-negative tumors may have an alternative metabolic dependency. For example, HPV18 cancers have higher unspliced transcripts of the E6 oncoprotein than HPV16 cancers, suggesting different functional implications of E6 and E7, according to the HPV genotypes22. Determining unrevealed prognostic determinants of HPV16-negative cervical cancer would be critical in developing further strategies for enhancing treatment response.
Previous studies estimated that HPV16 accounts for about half of all cervical cancers and HPV18 accounts for an additional 15%, although there are at least 15 known oncogenic HPV types4,23,24. These proportions are very similar to our patient characteristics shown in Supplemental Table 2, and this similarities support our presumptive methods’ relevance. Several studies suggested that HPV types might be a useful prognostic factor in cervical cancer. Ghong et al. reported that patients with HPV16-positive cancer had better diseaes-free survival (HR 0.41; p = 0.0019)24. Schwartz et al. demonstrated that patients with HPV18-related cancers showed higher cervical cancer specific mortality (HR 2.5) compared with those with HPV16-related cancers3. Similarly, we found that HPV16 subtype was an independent prognostic factor for OS after adjusting for clinical factors in the multivariate analysis. The reason why HPV16 subtype is associated with a better OS is largely unknown. However, it has been suggested that HPV16-positive cells have increased sensitivity to cancer therapies, a higher level of tumor apoptosis, a slower growth rate, and an enhanced immune response towards the virus in several cancer cells, providing a possible explanation for the increased radiation sensitivity of cervical cancer with HPV16 positivity25,26.
To further investigate the phenotype of GLUT1 overexpression in HPV16-positive cervical cancer, we examined the effects of GLUT1 overexpression on tumor immune microenvironment, based on the hypothesis that GLUT1-mediated immune evasion in the HPV16-positive cervical cancer could affect the treatment outcome27,28. Our results revealed that individual immune cell scores of T cells, CD8+ T cells, and B cells were significantly reduced in high GLUT1 expression group of HPV16-positive cervical cancer. It is known that metabolic perturbations within tumor induced by high GLUT1 level modulate tumor immune microenvironment by suppressing T cells; this effect is due to a metabolic competition and to the generation of metabolic byproducts29,30. GLUT1-mediated immune escape from cytotoxic T cells of tumor cells may be the cause of reduced survival.
Our results raise the possibility of considering GLUT1 as a potential novel target of HPV16-induced cervical cancer. Similar to the immune checkpoint blockade, strategies to reverse GLUT1-mediated immune escape could also be considered as potential treatment. Clinically, GLUT1 has not been directly targeted since specific and potent inhibitors are still not found14. Zhao et al. have shown that the inhibition of GLUT1 by the small-molecule inhibitor WZB117, could increase the radiosensitivity of breast cancer cells31. Other novel inhibitors of GLUT1 are under development14.
The present study has several limitations. First, there is a possibility that the integration of HPV information and the immune cell scores from different studies may be an obstacle in the interpretation of the main grouping, despite they all used the same TCGA data. Second, the generalization of our findings could be restricted by the lack of a suitable external validation cohort. Lastly, the relatively small number of patients as well as the heterogeneity of the treatment modalities might also have affected the prognostic impact of the analyzed variables.
Conclusions
Despite of these limitations, our results suggested that GLUT1 expression and HPV16 subtype might have an independent prognostic value in cervical cancer. This study will provide new hypothesis and strategies for the sensitization of cancer cells to radiotherapy or chemotherapy through the regulation of glucose metabolism. Pre-treatments testing the HPV status may make personalized treatment possible. GLUT1-mediated immunomodulation might be an important cause of treatment failure, especially in the HPV16-positive group; thus, the incorporation of GLUT1 inhibitors may be helpful to increase treatment efficacy. Further proof-of-concept study is warranted to identify novel immunometabolic targets to overcome therapeutic resistance.
Methods
Patient population and data acquisition
All methods were in accordance with the relevant guidelines and regulations, and were approved by the Ethical Review Committee of the Seoul Metropolitan Government Seoul National University Boramae Medical Center. As the data were de-identified in the TCGA database, the ethics committee approved that the requirement of informed consent was waived. This study complied with TCGA publication guidelines and policies (http://cancergenome.nih.gov/publications/publicationguidelines). Clinicopathologic factors and mRNA expression data were obtained from TCGA database, using TCGA-biolinks package32. Clinical information including tumor stage, tumor histology, disease extent, and treatment course were collected and analyzed. In total, 298 cervical cancer patients with appropriate survival data were included. The sample types of the included patients were all primary solid tumors except for two metastatic tissues.
HPV status and genotype
Tumor HPV status and genotype were determined according to the supplemental information from a previous TCGA publication, which calculated them using MassArray and RNA-sequencing22. For patients who did not fulfill the inclusion criteria for the above study, their HPV status and genotype were determined using another previous publication from TCGA, which used the number of normalized reads mapping to each HPV reference (DNA cut-off of 50 normalized counts of HPV aligned reads)33.
Immune cell scores
Immune cell scores were extracted from a previous TCGA study, which calculated these scores in 9986 RNASeq samples from 24 different tumor types34. Briefly, each immune cell score was calculated from specific 60 marker genes, whose expression levels could classify 14 immune cell populations. These results were highly reproducible and concordant with flow cytometry and immunohistochemistry results34.
Statistical analysis
To compare the clinicopathologic features between two groups, student’s t-test and chi-square test were applied to continuous variables and categorical variables, respectively. Using clinical follow-up data version 4.0, the overall survival (OS) and the progression-free survival (PFS) were defined from the date of initial diagnosis to the date of death or the last follow-up and to the date of disease progression, respectively. Survival estimates were calculated by the Kaplan-Meier method and compared using the log-rank test. The dichotomous cut-off point discriminating the OS between GLUT1-high and -low patients, was determined by the maximal chi-square test, which divided all the patients into two groups based on the median z-score of 0.165. Statistically significant factors in univariate analysis were checked for collinearity, using Pearson correlation coefficient. Factors with a p-value < 0.05 on univariate analysis or factors considered clinically important (e.g., HPV16 genotype) were subjected to the multivariate analysis. In multivariate analysis, Cox proportional hazards regression models were applied to adjust the potential confounding effects of associated variables and also were used to calculate hazard ratios (HR) and 95% confidence intervals (CI). R 3.3.0 statistical package (https://www.r-project.org/) was used for all statistical analyses.
Data Availability
The raw datasets of the current study are available in the Supplemental Dataset.
References
Vu, M., Yu, J., Awolude, O. A. & Chuang, L. Cervical cancer worldwide. Curr. Probl. Cancer 42, 457–465, https://doi.org/10.1016/j.currproblcancer.2018.06.003 (2018).
Huang, L.-W., Chao, S.-L. & Hwang, J.-L. Human papillomavirus-31-related types predict better survival in cervical carcinoma. Cancer 100, 327–334 (2004).
Schwartz, S. M. et al. Human papillomavirus and prognosis of invasive cervical cancer: a population-based study. J. Clin. Oncol. 19, 1906–1915 (2001).
Tong, S. Y., Lee, Y. S., Park, J. S. & Namkoong, S. E. Human papillomavirus genotype as a prognostic factor in carcinoma of the uterine cervix. Int. J. Gynecol. Cancer 17, 1307–1313 (2007).
Wang, C.-C. et al. Clinical effect of human papillomavirus genotypes in patients with cervical cancer undergoing primary radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 78, 1111–1120 (2010).
Hall, J. S. et al. Poor prognosis associated with human papillomavirus α7 genotypes in cervical carcinoma cannot be explained by intrinsic radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 85, e223–229 (2013).
Lowy, D. R. & Munger, K. Prognostic implications of HPV in oropharyngeal cancer. N. Engl. J. Med. 363, 82–84 (2010).
Martínez-Ramírez, I. et al. Regulation of Cellular Metabolism by High-Risk Human Papillomaviruses. Int. J. Mol. Sci. 19, E1839 (2018).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Kawamura, T. et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer 92, 634–641 (2001).
Mori, Y. et al. Glucose transporter type 1 expression are associated with poor prognosis in patients with salivary gland tumors. Oral Oncol. 43, 563–569 (2007).
Cho, H., Lee, Y. S., Kim, J., Chung, J.-Y. & Kim, J.-H. Overexpression of glucose transporter-1 (GLUT-1) predicts poor prognosis in epithelial ovarian cancer. Cancer Invest. 31, 607–615 (2013).
Yu, M. et al. The prognostic value of GLUT1 in cancers: a systematic review and meta-analysis. Oncotarget 8, 43356–43367 (2017).
Qian, Y., Wang, X. & Chen, X. Inhibitors of glucose transport and glycolysis as novel anticancer therapeutics. World J. Transl. Med. 3, 37–57 (2014).
Chang, M. S. et al. High-risk human papillomavirus load and biomarkers in cervical intraepithelial neoplasia and cancer. APMIS 122, 427–436 (2014).
Kim, E. et al. Significance of 18F-FDG PET Parameters According to Histologic Subtype in the Treatment Outcome of Stage III Non-small-cell Lung Cancer Undergoing Definitive Concurrent Chemoradiotherapy. Clin. Lung Cancer 20, e9–23, https://doi.org/10.1016/j.cllc.2018.08.018 (2019).
Kim, B. W. et al. Prognostic assessment of hypoxia and metabolic markers in cervical cancer using automated digital image analysis of immunohistochemistry. J. Transl. Med. 11, 185 (2013).
Kanjanapan, Y. et al. Glut-1 expression in small cervical biopsies is prognostic in cervical cancers treated with chemoradiation. Clin. Transl. Radiat. Oncol. 2, 53–58 (2017).
Noch, E. & Khalili, K. Oncogenic Viruses and Tumor Glucose Metabolism: Like Kids in a Candy Store. Mol. Cancer Ther. 11, 14–23 (2012).
McLaughlin-Drubin, M. E. & Münger, K. The Human Papillomavirus E7 Oncoprotein. Virology 384, 335–344 (2009).
Fan, R. et al. Overexpression of HPV16 E6/E7 mediated HIF-1α upregulation of GLUT1 expression in lung cancer cells. Tumour Biol. 37, 4655–4663 (2016).
Cancer Genome Atlas Research Network et al. Integrated genomic and molecular characterization of cervical cancer. Nature 543, 378–384 (2017).
Roden, R. & Wu, T.-C. How will HPV vaccines affect cervical cancer? Nat. Rev. Cancer 6, 753–763 (2006).
Chong, G. O. et al. Prognostic value of pre-treatment human papilloma virus DNA status in cervical cancer. Gynecol. Oncol. 148, 97–102 (2018).
Arends, M. J., Wyllie, A. H. & Bird, C. C. Human papillomavirus type 18 is associated with less apoptosis in fibroblast tumours than human papillomavirus type 16. Br. J. Cancer 72, 646–649 (1995).
Albers, A. et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 65, 11146–11155 (2005).
Ho, P.-C. & Liu, P.-S. Metabolic communication in tumors: a new layer of immunoregulation for immune evasion. J. Immunother. Cancer 4, 4 (2016).
Tang, L. et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. CR 37, 87 (2018).
Anderson, K. G., Stromnes, I. M. & Greenberg, P. D. Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies. Cancer Cell 31, 311–325 (2017).
Ganti, K. et al. Interaction of the Human Papillomavirus E6 Oncoprotein with Sorting Nexin 27 Modulates Endocytic Cargo Transport Pathways. PLoS Pathog. 12, e1005854 (2016).
Zhao, F., Ming, J., Zhou, Y. & Fan, L. Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation. Cancer Chemother. Pharmacol. 77, 963–972 (2016).
Colaprico, A. et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 44, e71 (2016).
Banister, C. E., Liu, C., Pirisi, L., Creek, K. E. & Buckhaults, P. J. Identification and characterization of HPV-independent cervical cancers. Oncotarget 8, 13375–13386 (2017).
Danaher, P. et al. Gene expression markers of Tumor Infiltrating Leukocytes. J. Immunother. Cancer 5, 18 (2017).
Acknowledgements
The results showed in the present study are in part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/. The authors would like to acknowledge the patients who provided tissue specimens to TCGA. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2017R1A2B4003535).
Author information
Authors and Affiliations
Contributions
Conception and design: Byoung Hyuck Kim and Ji-Hyun Chang. Collection and assembly of data: Byoung Hyuck Kim. Data analysis and interpretation: Byoung Hyuck Kim and Ji-Hyun Chang. Manuscript writing: Byoung Hyuck Kim and Ji-Hyun Chang. Final approval of manuscript: Byoung Hyuck Kim and Ji-Hyun Chang.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, B.H., Chang, J.H. Differential effect of GLUT1 overexpression on survival and tumor immune microenvironment of human papilloma virus type 16-positive and -negative cervical cancer. Sci Rep 9, 13301 (2019). https://doi.org/10.1038/s41598-019-49928-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49928-x
This article is cited by
-
Metabolic reprogramming in cervical cancer and metabolomics perspectives
Nutrition & Metabolism (2021)
-
Prognostic implication of human papillomavirus types in cervical cancer patients: a systematic review and meta-analysis
Infectious Agents and Cancer (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.