Abstract
The development of chimeric antigen receptor (CAR) and bispecific T-cell engager (BiTE) has led to the successful application of cancer immunotherapy. The potential reactivity mediated by CAR- and BiTE-redirected T cells needs to be assessed to facilitate the application of these treatment options to a broader range of patients. Here, we have generated CAR and BiTE possessing the same single chain fragment variable (scFv) specific for the HLA-A2/NY-ESO-1157-165 complex (A2/NY-ESO-1157). Using HLA-A2+NY-ESO-1+ myeloma cells and peptides presented by HLA-A2 molecules as a model, both sets of redirected T cells recognized and killed HLA-A2+NY-ESO-1+ myeloma cells in an A2/NY-ESO-1157-specific manner in vitro. Moreover, CAR- and BiTE-activated T cells showed similar functional avidity, as assessed by cytokine production and killing activity, both displaying antitumor reactivity against HLA-A2+NY-ESO-1+ myeloma cells in vivo. Interestingly, cross-reactivity for homologous peptides presented by HLA-A*02:01 and NY-ESO-1157 peptide presented by HLA-A2 alleles was not identical between CAR- and BiTE-redirected T cells, probably due to structural differences of modified antibodies. These results have demonstrated that both antitumor CAR- and BiTE-activated T cells have comparable potential to recognize tumors, while paying attention to unknown off-target reactivity that would differ for each antibody-based modality even if the same scFv was employed.
Similar content being viewed by others
Introduction
Clinical trials have demonstrated that T-cell therapy can suppress tumor growth even in patients with refractory malignancies1. Checkpoint blockade therapy has been shown to induce clinical responses in patients with hematological malignancies such as Hodgkin’s lymphoma, as well as solid tumors2,3,4,5,6. However, non-specific activation of patients’ T cells induces not only cytotoxic activity against tumors but also unwanted toxicity against normal tissues, resulting in immune-related adverse events7,8,9. Therefore, immunotherapy utilizing T cells that can accurately segregate tumor cells from normal cells is needed for safe and effective T-cell therapy.
A series of tumor antigens including tumor-specific antigens (TSAs) and tumor-associated antigens (TAAs) have been identified10. Peptides derived from these antigens are produced in a proteasome-dependent manner, and are naturally processed and presented by MHC molecules. Antitumor T cells recognize MHC/peptide complexes selectively presented on tumor cells via their T-cell receptor (TCR), and thus exhibit target-specific reactivity. On the basis of this mechanism of T-cell antigen recognition, adoptive transfer therapy has been developed using T cells redirected with TCRs specific for human leukocyte antigen (HLA)/peptide complexes expressed by tumor cells, and clinical trials have shown promise in patients with advanced tumors11,12,13,14,15,16,17,18. However, TCR-transduced T cells are laborious to generate, and exhibit cross-reactivity induced by the introduced TCR itself and/or mispairing between the introduced TCR and endogenous TCR19,20,21. To overcome these issues associated with TCR-T cell therapy, recent technical advances have facilitated the generation of antibody-based antitumor receptors that can activate T cells with tumor specificity. For antibody modification, single chain fragment variables (scFvs) generated on the basis of the primary structure of variable regions derived from monoclonal antibodies specific for tumor antigens expressed as surface proteins or HLA/peptide complexes are utilized. Each chimeric antigen receptor (CAR) encodes an scFv followed by a transmembrane domain derived from a co-stimulatory molecule, such as CD28, 4-1BB, and an intracellular domain of the CD3ζ chain22,23. One of the bispecific antibodies (BsAb), bispecific T-cell engager (BiTE), possesses an antitumor scFv linked with an anti-human CD3ε scFv24. CAR- and BiTE-redirected T cells recognize and kill tumor cells, and clinical trials using CAR- and BiTE-redirected T cells have shown great success in the treatment of refractory CD19+ malignancies25,26,27,28,29,30. In contrast, there are not many CAR and BiTE specific for HLA/peptide complexes expressed by refractory tumor cells that can target intracellular antigens31,32. To clarify the benefits and risks of antitumor CAR and BiTE, direct comparison of reactivities mediated by CAR- and BiTE-redirected T cells possessing the same scFv recognizing an HLA/peptide complex as a model would reveal in more detail their target-specific reactivity and cross-reactivity by changing specific peptides and HLA alleles. Such knowledge would help facilitate the application of these treatment options to a broader range of patients with refractory malignancies.
Multiple myeloma still remains incurable despite the development of new therapeutic agents33. T-cell therapy utilizing CAR and/or BiTE reactive with myeloma cells expressing surface antigens such as BCMA, CD19, CD138, and integrin β7 has advanced, and a series of clinical trials have been ongoing34,35,36,37. Unlike the above surface antigens, NY-ESO-1 is a cancer-testis antigen expressed intracellularly by tumors but not by normal tissues, except for testis lacking the expression of endogenous HLA38. Myeloma cells express NY-ESO-1 abundantly, and importantly, an NY-ESO-1157-165 peptide (SLLMWITQC) presented by an HLA-A*02:01 molecule (A2/NY-ESO-1157) has been demonstrated39,40. In a clinical trial, adoptive therapy using T cells modified with TCR specific for A2/NY-ESO-1157 in combination with autologous stem cell transplantation successfully induced clinical responses in patients with advanced myeloma15. T cells redirected with A2/NY-ESO-1157-specific CAR have been established and assessed for their anti-myeloma reactivity41,42, although their precise target-specificity and cross-reactivity are still unknown. In addition, A2/NY-ESO-1157-specific BiTE has yet to be generated or compared for T-cell reactivity with target cells in comparison with CAR-T cells.
In the present study, to clarify the characteristics of antitumor CAR and BiTE more precisely for expansion of these treatment modalities, we generated both CAR and BiTE carrying the same A2/NY-ESO-1157-specific scFv. Employing A2/NY-ESO-1157-specific CAR and BiTE and myeloma cells as a model, we investigated the potential target-specific reactivity and cross-reactivity of CAR- and BiTE-redirected T cells for NY-ESO-1157 peptide and its homologous peptides presented by the HLA-A2 molecule as well as HLA-A2+NY-ESO-1+ myeloma cells in vitro and in vivo, and compared them directly.
Results
Target specificity of A2/NY-ESO-1157-specific CAR- and BiTE-redirected peripheral blood T cells
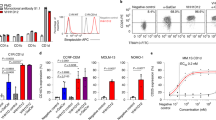
First, we generated peripheral blood T cells redirected with A2/NY-ESO-1157 CAR. As shown in Fig. 1a, a 2nd generation CAR encoding an A2/NY-ESO-1157-specific scFv derived from clone 3M4E5 followed by the CD28 transmembrane domain and cytoplasmic domain of the CD3ζ chain was synthesized. Both A2/NY-ESO-1157 CAR-transduced CD8+ T cells and CD4+ T cells were successfully and reproducibly stained with A2/NY-ESO-1157 tetramer (Fig. 1b,c). These CAR-CD8+ T cells and CD4+ T cells produced multiple cytokines in response to A2/NY-ESO-1157 but not A2/HIV Gag77 (Fig. 1d). These results indicate that A2/NY-ESO-1157 CAR-redirected T cells are able to distinguish NY-ESO-1157 peptide from irrelevant peptide and exhibit A2/NY-ESO-1157-specific reactivity.
A2/NY-ESO-1157-specific CAR redirects peripheral blood T cells. (a) Second generation CAR possessing an A2/NY-ESO-1157-specific scFv (clone 3M4E5) followed by a transmembrane domain of CD28 and a cytoplasmic domain of CD3ζ was generated. (b) The A2/NY-ESO-1157-specific CAR was transduced into peripheral blood T cells. These CAR-T cells were stained with 5 μg/mL A2/NY-ESO-1157 tetramer or A2/HIV Gag77 tetramer. Representative staining of A2/NY-ESO-1157 CAR-CD8+ T cells (left) and CD4+ T cells (right) obtained from donor 1 is shown. (c) A2/NY-ESO-1157 CAR-CD8+ T cells (left) and CD4+ T cells (right) derived from 5 out of 5 donors were reproducibly stained with A2/NY-ESO-1157 tetramer. (d) A2/NY-ESO-1157 CAR-CD8+ T cells (left) and CD4+ T cells (right) established from donor 1 were cocultured with T2 cells loaded with 10 μg/mL NY-ESO-1157 peptide or HIV Gag77 peptide. The experiments were performed in triplicate and error bars show the SD. *P < 0.05; ***P < 0.001; ****P < 0.0001; n.s., not significant. ΔNGFR-positive cells were gated and analyzed.
Next, we generated a BiTE, in which an A2/NY-ESO-1157-specific scFv was fused with human CD3ε-specific scFv derived from clone OKT3 via a linker sequence (Fig. 2a). The A2/NY-ESO-1157-specific BiTE bound to A2/NY-ESO-1157, but not to A2/HIV Gag77 (Fig. 2b, bottom left). A control BiTE bound to neither A2/NY-ESO-1157 nor A2/HIV Gag77 (Fig. 2b, top left). On the other hand, both BiTEs bound to CD3+ Jurkat cells but not to CD3− Jurkat 76 cells, indicating that the A2/NY-ESO-1157 BiTE was able to bind to the CD3 molecule as well as A2/NY-ESO-1157 on the cell surface (Fig. 2b, right). We also tested whether freshly isolated peripheral blood T cells recognized A2/NY-ESO-1157 in the presence of the A2/NY-ESO-1157 BiTE. Peripheral blood CD8+ T cells and CD4+ T cells derived from 5 out of 5 donors we tested appeared to significantly produce a panel of cytokines against A2/NY-ESO-1157 in the presence of the A2/NY-ESO-1157 BiTE, but not the control BiTE (Fig. 2c). These results clearly indicate that the A2/NY-ESO-1157 BiTE successfully redirected peripheral blood T cells with A2/NY-ESO-1157 specificity. Importantly, a BiTE appeared to be unable to activate T cells only by binding to the CD3 molecule, suggesting that engagement of T cells with target cells via BiTE is necessary for stimulation of T cells.
A2/NY-ESO-1157-specific BiTE engages peripheral blood T cells with target cells. (a) A2/NY-ESO-1157-specific BiTE containing an A2/NY-ESO-1157-specific scFv (clone 3M4E5) and a human CD3-specific scFv was generated. A hexahistidine (6xHis) tag was inserted at the C-terminus of the construct. Purified 1 μg control BiTE and A2/NY-ESO-1157 BiTE were similarly detected by immunoblotting (IB) using anti-His mAb. The full-length blotting image is shown in Supplementary Fig. S4 (top). (b) 10 μg/mL A2/NY-ESO-1157 peptide or HIV Gag77 peptide was loaded onto T2 cells. After floating peptides had been removed, the cells were stained with 10 μg/mL A2/NY-ESO-1157 BiTE or control BiTE (left). To see the binding of each BiTE to the CD3 molecule, CD3+ Jurkat cells and CD3− Jurkat 76 cells were stained with each BiTE (right). PE-conjugated anti-His mAb was utilized to detect the binding of BiTEs. Dotted lines show the isotype control. (c) Freshly isolated peripheral blood T cells derived from 5 different donors were individually incubated with T2 cells pulsed with 10 μg/mL NY-ESO-1157 peptide or HIV Gag77 peptide in the presence of 1 μg/mL A2/NY-ESO-1157 BiTE or control BiTE. Cytokine production by peripheral blood T cells was measured by intracellular cytokine staining via flow cytometry. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; n.s., not significant.
A2/NY-ESO-1157 CAR- and BiTE-redirected T cells recognize HLA-A2+NY-ESO-1+ myeloma cells
Next, we investigated whether A2/NY-ESO-1157 CAR- and BiTE-redirected T cells recognize target cells endogenously expressing A2/NY-ESO-1157. NY-ESO-1 expression of six myeloma cell lines expressing or not expressing HLA-A*02:01 was examined. Three out of six myeloma cell lines we tested overexpressed NY-ESO-1 mRNA and NY-ESO-1 protein (Fig. 3). A2/NY-ESO-1157 CAR-CD8+ T cells and CD4+ T cells recognized HLA-A2+NY-ESO-1+ U266 cells, but neither HLA-A2+NY-ESO-1− KMS26 cells nor HLA-A2−NY-ESO-1− KMS34 cells (Supplementary Fig. S1). It appeared that the target-specific cytokine release occurred mainly from CAR-transduced CD8+ T cells, suggesting that binding of the CD8 molecule to the HLA class I molecule is able to enhance the cytoplasmic signals of the CAR-T cells (Fig. 4a, left). Peripheral blood CD8+ T cells and CD4+ T cells significantly produced cytokines against U266 cells in the presence of A2/NY-ESO-1157 BiTE (Fig. 4b, left and Supplementary Fig. S1).
Myeloma cells express NY-ESO-1. Expression of NY-ESO-1 mRNA and NY-ESO-1 protein was measured by qRT-PCR (top) and Western blotting (bottom). Data were normalized using hHPRT1 for qRT-PCR and β-actin for Western blotting. The expression of NY-ESO-1 mRNA in U266 cells is shown as 1.0, and the expression levels in other cells are calculated relative to this value. Error bars show the SD. Among six myeloma cell lines we tested, three were HLA-A*02:01-positive, and three were HLA-A*02:01-negative, as indicated at the bottom. The full-length blotting images are displayed in Supplementary Fig. S4 (bottom).
A2/NY-ESO-1157 CAR- and BiTE-redirected T cells recognize myeloma cells in an A2/NY-ESO-1157-specific manner. (a) A2/NY-ESO-1157 CAR-transduced CD8+ T cells and CD4+ T cells were incubated with the indicated target cells, and their cytokine production was measured by intracellular cytokine assay. The HLA-A2 (A2) and NY-ESO-1 (NY) positivity of each myeloma cell line used is also shown. The experiments were performed in triplicate, and ΔNGFR-positive cells were gated and analyzed. The experiments were repeated twice, and representative data obtained from donor 1 are shown. Error bars depict the SD. (b) Freshly isolated peripheral blood T cells derived from 5 different donors were incubated with the indicated target cells in the presence of 5 μg/mL A2/NY-ESO-1157 BiTE or control BiTE. Cytokine production was assessed by intracellular cytokine staining. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; n.s., not significant.
We also assessed whether CAR- and BiTE-redirected T cells indeed recognize naturally processed and presented A2/NY-ESO-1157 in target cells. For this purpose, K562 cells, which lack expression of endogenous HLA and NY-ESO-1, were transduced with the HLA-A*02:01 gene with or without the NY-ESO-1 gene. The level of HLA-A2 expression was similar among K562/A2, K562/A2/NY-ESO-1, and U266 cells; on the other hand, NY-ESO-1 expression by K562/A2/NY-ESO-1 cells was higher than that by U266 cells (Supplementary Fig. S2). Cytokine production by CAR- and BiTE-redirected CD8+ T cells and CD4+ T cells against K562/A2/NY-ESO-1 cells was more abundant in comparison to that against U266 cells (Fig. 4). Importantly, CAR- and BiTE-redirected CD8+ T cells and CD4+ T cells segregated K562/A2/NY-ESO-1 cells from K562/A2 cells (Fig. 4a,b, right and Supplementary Fig. S1). We also confirmed that CAR- and BiTE-redirected T cells killed NY-ESO-1157 peptide-pulsed T2 cells, K562/A2/NY-ESO-1 cells, and HLA-A2+NY-ESO-1+ U266 cells, but not other control cells (Fig. 5). Cytotoxicity against HLA-A2+NY-ESO-1+ myeloma cells mediated by CAR-T cells was more efficient than that mediated by BiTE-redirected T cells in vitro, although it must be noted that we employed freshly isolated peripheral blood T cells for experiments using BiTEs. These results indicate that BiTE-redirected T cells as well as CAR-redirected T cells possess sufficient avidity to recognize endogenously processed and presented A2/NY-ESO-1157, resulting in cytokine production and specific killing of HLA-A2+NY-ESO-1+ myeloma cells.
A2/NY-ESO-1157 CAR- and BiTE-redirected T cells kill HLA-A2+NY-ESO-1+ target cells in vitro. (a) A2/NY-ESO-1157 CAR-transduced T cells were incubated with 51Cr-labeled T2 cells pulsed with 10 μg/mL NY-ESO-1157 peptide or HIV Gag77 peptide (left). These responder cells were also cocultured with 51Cr-labeled K562 transfectants (middle) or myeloma cell lines (right) at the indicated E/T ratio. (b) Freshly isolated peripheral blood T cells were also incubated with 51Cr-labeled target cells as described above in the presence of A2/NY-ESO-1157 BiTE or control BiTE (1 μg/mL for peptide-pulsed target cells or 5 μg/mL for other target cells) at the indicated E/T ratio. All the experiments were performed in triplicate and error bars depict the SD. The results obtained from donor 1 are displayed.
A2/NY-ESO-1157 BiTE-redirected T cells as well as CAR-T cells show sufficient functional avidity to kill HLA-A2+NY-ESO-1+ myeloma cells in vivo
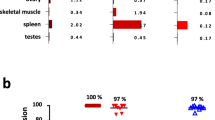
Since we detected sufficient cytokine production and specific lysis mediated by CAR- and BiTE-redirected T cells against endogenously expressed A2/NY-ESO-1157 in target cells, we directly compared the functional avidity of CAR-redirected T cells with that of BiTE-redirected T cells. To compare this under the same conditions, we prepared similarly activated T cells derived from the same donor as responder cells for the CAR and BiTE experiments. The functional avidity of BiTE-redirected CD8+ T cells and CD4+ T cells measured in terms of cytokine production and killing activity was approximately similar to that of CAR-redirected CD8+ T cells and CD4+ T cells (Fig. 6a). We then compared the in vivo antitumor effects of CAR-redirected T cells with that of BiTE-redirected T cells. CAR- and BiTE-redirected T cells with a similar CD4/CD8 ratio were prepared for in vivo side-by-side experiments (Supplementary Fig. S3). Using in vivo bioluminescence imaging assays, we confirmed that U266 cells were successfully engrafted in NOG mice on Day 11. On Day 13 and Day 18, CAR-T cells or control T cells were injected intravenously into tumor-bearing mice. The same number of similarly activated T cells were administered to NOG mice followed by intravenous injection of an A2/NY-ESO-1157 BiTE or a control BiTE for direct comparison. On Day 20, tumor suppression was achieved by treatment with A2/NY-ESO-1157 CAR-T cells but not control T cells. Antitumor effects induced by responder cells were obtained with the A2/NY-ESO-1157 BiTE, but not the control BiTE (Fig. 6b). On Day 15, tumor growth was significantly suppressed by treatment with the T cells in combination with A2/NY-ESO-1157 BiTE, but not with CAR-T cells. In contrast, on Day 20, there was a tendency for CAR-T cells to suppress tumor growth more effectively (Fig. 6b). Because of xenoreactivity, we were unable to observe antitumor effects of both sets of redirected T cells for over 20 days in our experiments. However, these results suggest that CAR- and BiTE-redirected T cells successfully kill HLA-A2+NY-ESO-1+ tumors in vivo. BiTE is able to engage T cells with tumor cells and induce antitumor responses at tumor sites in the early stage.
A2/NY-ESO-1157 CAR- and BiTE-redirected T cells show sufficient functional avidity to induce anti-myeloma reactivity in vivo. (a) Functional avidities of A2/NY-ESO-1157 CAR- and BiTE-redirected CD8+ T cells and CD4+ T cells were assessed using T2 cells pulsed with graded concentrations of NY-ESO-1157 peptide as stimulator cells. Side-by-side experiments using similarly activated T cells derived from donor 1 as responder cells were performed. CAR- and BiTE-redirected T cells were incubated with target cells for 16 hours after adding BFA to measure IFN-γ production, and for 5 hours to examine target-specific lysis, respectively. % IFN-γ secreting ability (left, middle) and % killing activity (right) of CAR- and BiTE-redirected T cells are shown. The reactivity of responder cells against T2 cells pulsed with 10 μg/mL (IFN-γ) or 1 μg/mL (killing) NY-ESO-1157 peptide is considered as 100%, and each reactivity is calculated relative to this value. The experiments were repeated twice, and representative data are shown. Error bars show the SD. (b) Two million U266/SLR cells were intravenously injected into NOG mice. After engraftment of U266/SLR cells had been confirmed by bioluminescence imaging assays on Day 11, 5.0 × 106 CAR-T cells or control T cells were injected twice on Days 13 and 18 (top). Similarly activated bulk T cells were injected twice on Days 13 and 18 followed by intravenous injection of 10 μg A2/NY-ESO-1157 BiTE or control BiTE on Days 13, 14, 18, and 19 (bottom). These experiments were performed side-by-side. Measured photon counts obtained from a mouse in both the prone and supine positions were aggregated, and calculated as arbitrary units for comparison between the groups. The experiments were repeated twice using distinct donor-derived T cells (donor 1), and representative data are displayed. Error bars indicate the SD. ****P < 0.0001; n.s., not significant.
A2/NY-ESO-1157 CAR- and BiTE-redirected T cells possess potent cross-reactivity
Clinical trials have indicated the risks of cross-reactivity of gene-modified antitumor T cells with normal cells19,20. The cross-reactivity of CAR- and BiTE-redirected T cells has also been suggested21; however, their characteristics have yet to be investigated in detail. Therefore, we examined the potential cross-reactivity of A2/NY-ESO-1157 CAR- and BiTE-redirected T cells using peptides homologous to NY-ESO-1157. CAR- and BiTE-redirected T cells derived from the same donor were prepared. A single alanine substitution in the NY-ESO-1157 peptide was performed to determine the important amino acids recognized by CAR- and BiTE-redirected T cells. Since the responsiveness of these T cells to peptides in which the second, fourth, fifth, and sixth amino acids were substituted was reduced, as shown in Fig. 7a, we searched for homologous peptides containing the xLxMWIxxx sequence using the ScanProsite tool. Nine different peptides homologous to the original NY-ESO-1157 were synthesized (Supplementary Table S1). A2/NY-ESO-1157 CAR- and BiTE-redirected T cells responded differently to some of them. In addition, CAR- and BiTE-redirected CD8+ T cells showed a tendency to be more cross-reactive than CD4+ T cells. Although proteins containing cross-reactive peptides recognized by A2/NY-ESO-1157 scFv, such as SLC13A2, TAS2R8, LYPLAL1 as shown in Fig. 7b, are likely to be expressed in normal tissues, their expression level does not appear to be higher than that of NY-ESO-1 expressed by tumor cells, at least on the basis of an in silico search. Moreover, it is unknown whether these peptides with HLA-A*02:01 could be naturally processed and presented by normal tissues.
A2/NY-ESO-1157 CAR- and BiTE-redirected T cells show potential cross-reactivity. (a) A2/NY-ESO-1157 CAR-transduced CD8+ T cells and CD4+ T cells were incubated with T2 cells pulsed with 9 different alanine-substituted peptides at 5 μg/mL (left). Similarly, freshly isolated peripheral blood T cells were cocultured with T2 cells loaded with 5 μg/mL alanine-substituted peptides in the presence of 1 μg/mL A2/NY-ESO-1157 BiTE (right). IFN-γ production (%) was measured using intracellular cytokine assays. Each response for alanine-substituted peptides was compared with that for the original NY-ESO-1157 peptide. *P < 0.05; **P < 0.01. (b) A2/NY-ESO-1157 CAR-T cells (left) or BiTE-redirected T cells (right) were stimulated with T2 cells individually pulsed with 10 μg/mL irrelevant HIV Gag77 peptide, original NY-ESO-1157 peptide, and 9 different peptides homologous to NY-ESO-1157. IFN-γ production (%) was examined similarly to (a). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. (c) A2/NY-ESO-1157 CAR-transduced CD8+ T cells and CD4+ T cells were stimulated with K562/A*02:01 or K562/A*02:06 cells pulsed with 10 μg/mL NY-ESO-1157 peptide or HIV Gag77 peptide (top). Freshly isolated peripheral blood T cells were also incubated with the same targets in the presence of 10 μg/mL NY-ESO-1157 BiTE or control BiTE (bottom). IFN-γ production (%) by T cells was similarly examined. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; n.s., not significant. All the experiments from (a) to (c) were performed in triplicate, and ΔNGFR-positive cells among CAR-T cells were gated and analyzed. The experiments were repeated twice, and representative data obtained from donor 1 are displayed. Error bars depict the SD.
We also tested the cross-reactivity of CAR- and BiTE-redirected T cells with NY-ESO-1157 peptide presented by different HLA-A2 alleles. In this study, an HLA-A*02:06 molecule was employed, as NY-ESO-1157 peptide can bind to HLA-A*02:06 as well as to HLA-A*02:0118. A2/NY-ESO-1157 CAR-T cells recognized NY-ESO-1157 peptide pulsed onto HLA-A*02:01 and HLA-A*02:06 with different magnitude (Fig. 7c, top). Interestingly, peripheral blood T cells were reactive with NY-ESO-1157 peptide presented by HLA-A*02:01 and HLA-A*02:06 in the presence of A2/NY-ESO-1157 BiTE comparably (Fig. 7c, bottom). Although collectively, cross-reactivity for HLA-A2/peptide complexes would aid the application of these treatment modalities to both HLA-A*02:01- and HLA-A*02:06-positive patients, careful attention to any unknown off-target reactivity with normal tissues will be necessary.
Discussion
Since previous studies had employed scFvs recognizing surface antigens that were not HLA/peptide complexes, comparison of target-specific reactivity and cross-reactivity mediated by CAR- and BiTE-redirected T cells was difficult. To our knowledge, this is the first study to have precisely assessed target-specific reactivity and cross-reactivity of CAR-T cells and BiTE-redirected T cells side-by-side, especially using the same A2/NY-ESO-1157-specific scFv and specific peptides presented by HLA-A2 molecules as a model. CAR- and BiTE-redirected T cells recognized naturally processed and presented A2/NY-ESO-1157 and killed HLA-A2+NY-ESO-1+ myeloma cells in an A2/NY-ESO-1157-specific manner. In our experiments, CAR- and BiTE-redirected T cells showed similar functional avidity, as assessed by cytokine production and killing activity, and thereby both displayed cytotoxicity for HLA-A2+NY-ESO-1+ myeloma cells in vivo. Cross-reactivity for homologous peptides presented by HLA-A*02:01 and NY-ESO-1157 peptide presented by HLA-A2 alleles was not identical between CAR- and BiTE-redirected T cells.
Stone and colleagues have prepared CAR and BiTE possessing the same scFv specific for a glycopeptide epitope presented by murine fibrosarcoma cells43. They quantified the number of epitopes on the surface of target cell lines using a monoclonal antibody, and compared the cytokine production capacity and killing activity of CAR- and BiTE-redirected T cells against a couple of target cell lines presenting different amounts of glycopeptide epitopes. They concluded that CAR-transduced T cells have greater sensitivity than BiTE-treated T cells in vitro. Hoseini and colleagues have also investigated the functional capabilities of T cells redirected with CAR or BsAb possessing the same scFv specific for disialoganglioside GD2. Using similarly activated T cells, they found that BsAb-redirected T cells show greater survival and rapidly induce tumor regression in vivo in comparison with CAR-T cells44. When we precisely measured the functional avidity of CAR- and BiTE-redirected T cells using similarly activated T cells as responder cells and graded the concentrations of NY-ESO-1157 peptide, both sets of redirected T cells showed approximately similar avidity, resulting in sufficient reactivity against HLA-A2+NY-ESO-1+ myeloma cells in vivo. Interestingly, antitumor effects induced by BiTE-redirected T cells were seen in the early phase relative to those induced by CAR-T cells. A2/NY-ESO-1157 tetramer staining of CAR-T cells ranged from bright to dim (Fig. 1b), whereas BiTE was able to engage infused T cells with tumor cells equivalently. In addition, engagement of T cells with tumor cells by BiTE induces cytolytic synapses similar to those formed by binding of TCRs to HLA/peptide complexes, relative to those induced by CAR-T cells45,46. These characteristics may account for the difference in antitumor effects between BiTE-redirected T cells and CAR-transduced T cells in the early stage. On the other hand, CAR-T cells had a tendency to show delayed antitumor responses when compared with BiTE-activated T cells, suggesting that in the context of optimal delivery of intravenously injected BiTE into tumor sites to engage T cells with tumor cells in vivo, BiTE-redirected T cells have the potential to suppress myeloma, which is a slowly progressive disease. These factors should be considered when applying BiTE to patients, even though CAR- and BiTE-activated T cells display similar functional avidity.
CAR- and BiTE-redirected T cells differed in their recognition of NY-ESO-1157 peptide bound to an HLA-A*02:01 or HLA-A*02:06 molecule. This type of cross-reactivity allows us to broaden the range of patients who can be treated with T cells redirected with CAR or BiTE specific for HLA/peptide complexes. On the other hand, these cells showed different degrees of cross-reactivity with some homologous peptides presented by HLA-A*02:01. Previous studies have revealed that scFvs showing cross-reactivity with different peptides presented by an HLA molecule show atypical orientations, tilting toward α1-helix or α2-helix and forming few direct contacts with the peptide47. However, this A2/NY-ESO-1157-specific scFv was obviously dependent on homologous peptides as well as NY-ESO-1157 peptide along with an HLA-A2 allele48, suggesting that an A2/NY-ESO-1157 scFv cross-reactive with HLA-A2/peptide complexes adopts conformations differing from scFvs possessing high cross-reactivity47. The difference in the magnitude of cross-reactivity of CAR- and BiTE-redirected T cells might be explained by the structures of CAR and BiTE, which are affected by the transmembrane/intracellular domain composed of a CAR or a CD3-specific scFv containing a BiTE. This means that other types of modified antibodies containing the same scFv might display different cross-reactivity with target antigens. Crystal structure analyses of CAR and BiTE possessing an identical scFv for the target antigens would be warranted to clarify the underlying mechanism.
CAR- and BiTE-redirected CD8+ T cells showed a greater tendency to possess enhanced target-specific reactivity and cross-reactivity than CD4+ T cells. Some studies have demonstrated that T-cell cross-reactivity as well as target-specific reactivity is enhanced by binding of CD8 to the HLA class I molecule49,50. Although we employed an scFv targeting an HLA class I/peptide complex, CD8+ T cells redirected with CAR or BiTE possessing scFvs specific for other surface antigens may also have displayed enhanced reactivity due to interaction between CD8 and HLA class I without target-specific binding of an scFv to HLA/peptide complexes. Further studies of this issue will be needed.
Our study has revealed the target-specific reactivity of CAR and BiTE and the need for caution regarding unknown off-target adverse events caused by cross-reactivity of CAR, BiTE, or other antibody-based modalities. The clinical application of antibody-based T-cell therapy can be expanded, since scFvs can be broadly applied to both cell therapy and other biological reagents, unlike other T-cell therapies. Moreover, using flow cytometry or immunohistochemistry, scFvs that bind to an HLA/peptide complex can be utilized to assess whether target HLA/peptide complexes are naturally processed and presented by tumor cells before the introduction of T-cell therapy. This would make it possible to select patients who would benefit most from those therapies. For clinical application and diagnostics, optimization of A2/NY-ESO-1157 scFv will be necessary. However, the most important outcome of this study is that, using an A2/NY-ESO-1157 scFv and myeloma cells as a model, we have demonstrated both the capacity and the risk of antitumor CAR- and BiTE-redirected T cells by direct comparison for further development of antibody-based T-cell therapy.
Materials and Methods
Cells
K562 cells, which lack expression of endogenous HLA and NY-ESO-1, were purchased from the American Type Culture Collection (ATCC) and cultured with RPMI1640 supplemented with 10% fetal calf serum (FCS). T2 cells endogenously expressing HLA-A*02:01 as one of the HLA alleles were cultured with RPMI1640 supplemented with 10% FCS. The U266 cell line was a generous gift from Dr. Ryosuke Uchibori and Dr. Keiya Ozawa, Jichi Medical University. Other myeloma cell lines were kindly provided by Dr. Takemi Ohtsuki, Kawasaki Medical University. Myeloma cell lines were cultured in RPMI1640 supplemented with 10% FCS. Plat-A cells (kindly provided by Dr. Toshio Kitamura, Institute of Medical Sciences, University of Tokyo) were maintained in DMEM supplemented with 10% FCS, 1 μg/mL puromycin and 10 μg/mL blasticidin, as described previously51. PG13 cells were cultured in DMEM supplemented with 10% FCS. Jurkat 76 cells, which lack the expression of endogenous TCR and CD3 (generous gift from Dr. Mirjam Heemskerk, Leiden University Medical Center) were cultured in RPMI1640 supplemented with 10% FCS52. Human peripheral blood mononuclear cells (PBMCs) were isolated from healthy volunteers using Ficoll-Paque (GE Healthcare) and stored until use. All the experiments using human samples were approved by the Certified Review Board, Ehime University. All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was given by all donors.
Genes
Variable regions derived from immunoglobulin light and heavy chains of an HLA-A*02:01-restricted NY-ESO-1157-165-specific monoclonal antibody (mAb) (clone: 3M4E5) were linked with a linker sequence to generate the scFv48,53. A second generation A2/NY-ESO-1157 CAR gene encoding the scFv followed by a transmembrane region of CD28 and an intracellular domain of the CD3ζ chain was synthesized by GeneArt (Thermo Fisher Scientific). To detect CAR-transduced T cells, a CAR gene was fused with a truncated nerve growth factor receptor (ΔNGFR) gene via a furin cleavage site, an SGSG spacer sequence, and a codon-optimized P2A sequence54,55,56. A BiTE gene was also generated by GeneArt. A human CD3ε-specific scFv derived from a CD3ε-specific mAb (clone: OKT3) was fused with an A2/NY-ESO-1157-specific scFv or an anti-human IgG scFv for control via an SGSG linker sequence. A hexahistidine (6xHis) tag was inserted at the C-terminus of the construct with an SGSG linker sequence for BiTE purification and detection. All genes were individually integrated into a pMX retroviral vector for transduction57.
Transfectants
The experiments were reviewed and approved by the institutional review board at Ehime University. K562 cells were transduced with the HLA-A*02:01 or HLA-A*02:06 gene to establish K562/A*02:01 (K562/A2) or K562/A*02:06 cells. In some experiments, K562/A2 cells were transduced with the NY-ESO-1 gene to establish K562/A2/NY-ESO-1 cells. U266 cells were transduced with the luciferase gene (SLR: stable luciferase red) to establish U266/SLR cells for in vivo bioluminescence imaging assays. Human peripheral blood T cells were stimulated with 100 IU/mL human IL-2 and 50 ng/mL anti-human CD3ε mAb (clone OKT3) for 2 days before transduction. Then, T cells were cultured in RPMI1640 supplemented with 10% AB type human serum (Sigma Aldrich) and retrovirally transduced with an A2/NY-ESO-1157 CAR gene or a control gene (ΔNGFR alone) to establish gene-modified T cells as described previously55,56. To perform the ELISA experiments, NGFR-positive T cells labeled with FITC-conjugated anti-human NGFR mAb (clone ME20.4) were collected by FACSAria II (Becton Dickinson). When needed, further selection of CD8- or CD4-positive T cells was performed using microbeads (Miltenyi Biotec). To confirm the killing activity of CAR-T cells in vitro and in vivo, gene-modified T cells were isolated using PE-conjugated anti-human NGFR mAb (clone ME20.4) in combination with anti-PE microbeads (Miltenyi Biotec). 293 T cells were retrovirally transduced with an A2/NY-ESO-1157 BiTE gene or a control BiTE gene to produce supernatants containing BiTE. The BiTEs were purified using a His GraviTrap column and a Vivaspin column (GE Healthcare) for the following experiments.
Flow cytometry
T cells were stained with PC5-conjugated anti-human CD8 mAb (clone B9.11), FITC-conjugated anti-human CD4 mAb (clone OKT4), and V450-conjugated anti-human NGFR mAb (clone C40-1457). Biotinylated HLA-A*02:01/NY-ESO-1157 monomer and HLA-A*02:01/HIV Gag77 monomer (MBL) were multimerized using PE-conjugated streptavidin (Thermo Fisher Scientific), and utilized for detection of CAR-transduced T cells as described elsewhere55,56. Myeloma cell lines and K562 transfectants were stained with PE-conjugated anti-HLA-A2 mAb (clone BB7.2). They were also stained with anti-human NY-ESO-1 mAb (clone E978) and PE-conjugated AffiniPure F(ab’)2 goat anti-mouse IgG (Fcγ specific) (Jackson ImmunoResearch). To confirm target-specific binding of BiTE, target cells were stained with purified BiTE in combination with PE-conjugated anti-His mAb (clone GG11-8F3.5.1) for detection. Human CD3+ T cells were collected from freshly isolated PBMCs with anti-CD3 microbeads (Miltenyi Biotec) for in vitro experiments using BiTEs unless otherwise specified. To investigate A2/NY-ESO-1157-specific cytokine production by CAR- and BiTE-redirected T cells, 3.0 × 105 responder cells were incubated with 5.0 × 104 target cells. Brefeldin A (BFA) was added after 2 hours, and the cells were incubated for an additional 4 hours for CAR-T cells and 16 hours for BiTE-redirected T cells to produce cytokines, unless otherwise specified. Then, PE-conjugated anti-human TNF-α mAb (clone Mab11), APC-conjugated anti-human IL-2 mAb (clone MQ1-17H12), and PC7-conjugated anti-human IFN-γ mAb (clone B27) together with other surface markers as described above were utilized for detection. All samples were analyzed using a Gallios flow cytometer (Beckman Coulter) and FlowJo Version 7.6.5 software (Becton Dickinson). ΔNGFR-positive cells were gated to assess CAR-T cells.
Enzyme-linked immunosorbent assays (ELISA)
IFN-γ production by 1.0 × 105 CAR- and BiTE-redirected T cells against 2.0 × 104 target cells was measured by ELISA. Briefly, anti-human IFN-γ capture mAb (clone 1D1K) was coated onto a Nunc MaxiSorp flat-bottom 96-well plate (Thermo Fisher Scientific) overnight at 4 °C. Collected supernatants were added to the plate and incubated for 2 hours at room temperature. The plate was then incubated with biotin-conjugated anti-human IFN-γ detection mAb (clone 7-B6-1) for 1 hour at room temperature. Then, streptavidin-conjugated alkaline phosphatase was added to each well. One-step pNPP solution (Thermo Fisher Scientific) was utilized for detection. The absorbance at 405 nm was measured by FlexStation3 (Molecular Devices).
Quantitative analyses of NY-ESO-1 mRNA
Total RNA and cDNA were prepared as described previously58. NY-ESO-1 mRNA was measured by quantitative real-time PCR (qRT-PCR) using QuantiTect SYBR Green PCR kit (Qiagen). Human hypoxanthine phosphoribosyltransferase 1 (hHPRT1) mRNA was utilized as an internal control. NY-ESO-1 mRNA expression was normalized to the levels of hHPRT1 mRNA, and the relative expression values were calculated using the 2−ΔΔCt method. The following primer sets were used for the experiments: NY-ESO-1 forward, TTCTGAAGGAGTTCACTGTGTCC, and reverse, AGGGAGGCTGAGCCAAAAAC; hHPRT1 forward, GGCAGTATAATCCAAAGATGGTCAA, and reverse, GTCAAGGGCATATCCTACAACAAAC.
Immunoblotting assays
Immunoblotting assays were performed as described previously59,60. Cells were extracted with lysis buffer (20 mM Tris-HCL, pH 7.5, 1 mM EDTA, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM phenylmethyl sulfonyl fluoride, and 1 μg/mL aprotinin). After centrifugation at 12,000 rpm for 20 minutes at 4 °C, the supernatant was collected as the lysate. Purified BiTEs were used directly for immunoblotting. Following SDS-PAGE, protein was transferred to a polyvinylidene difluoride membrane and incubated with a blocking buffer (Nakarai Tesque). The membranes were incubated with the indicated primary antibodies (anti-NY-ESO-1 mAb: clone E978, anti-β-actin mAb: clone AC-15, anti-His mAb: clone AD1.1.10) overnight at 4 °C, washed and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Fc specific) secondary antibody (Sigma Aldrich) for 1 h at room temperature. After washing the membrane, the signal was detected using enhanced chemiluminescence (GE Healthcare).
Cytotoxicity assays
Standard cytotoxicity assays were performed as described previously56. Briefly, 5.0 × 103 target cells were labeled with chromium-51 (51Cr) for 1.5 hours at 37 °C. CAR- and BiTE-redirected T cells were incubated with target cells at various E/T ratios for 5 hours (CAR) and 16 hours (BiTE), respectively, unless otherwise specified. The supernatants were collected and their cpm counts were measured. The percentage of specific lysis was calculated as follows: (experimental release cpm − spontaneous release cpm)/(maximal release cpm − spontaneous release cpm) × 100 (%).
In vivo murine experiments
All the murine experiments in this study were approved by the Ehime University Animal Care Committee. All methods were performed in accordance with the relevant guidelines and regulations. Five-week-old NOD/Shi-scid,IL-2RγΚΟ Jic (NOG) mice were purchased from In-Vivo Science Inc. and subjected to 1.5 Gy of irradiation. Then, two million U266/SLR cells were intravenously injected into NOG mice. After engraftment, five million redirected T cells, control T cells, or T cells with BiTEs were intravenously injected into each mouse. Tumor sizes were measured by AEQUORIA-2D/8600 bioluminescence imaging assays (Hamamatsu Photonics).
In silico analyses
The ScanProsite tool (http://prosite.expasy.org/scanprosite/) was utilized to search for human-derived peptide sequences homologous to NY-ESO-1157 peptide (SLLMWITQC) based on the results of alanine scanning assays. GeneCards (https://www.genecards.org/) was used to search for the expression of proteins containing peptides homologous to NY-ESO-1157. The binding affinity of peptides for HLA-A*02:01 was predicted by netMHC4.0 (http://www.cbs.dtu.dk/services/NetMHC/).
Statistical analyses
Statistical analyses were performed with GraphPad Prism 6.0 h. Welch’s t test (two-sided) or paired t test (two-sided) was employed to examine whether two groups were significantly different for a given variable. Comparisons between more than two groups were performed by two-way ANOVA with Sidak’s multiple comparisons test. P values of <0.05 were considered statistically significant.
Data Availability
All data supporting the conclusions of this study are included in the manuscript and its supplementary files, or are available from authors on request.
References
Houot, R., Schultz, L. M., Marabelle, A. & Kohrt, H. T-cell-based Immunotherapy: Adoptive Cell Transfer and Checkpoint Inhibition. Cancer Immunol Res 3, 1115–1122 (2015).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363, 711–723 (2010).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366, 2443–2454 (2012).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372, 311–319 (2015).
Davids, M. S. et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med 375, 143–153 (2016).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Johnson, D. B. et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 375, 1749–1755 (2016).
June, C. H., Warshauer, J. T. & Bluestone, J. A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 23, 540–547 (2017).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 378, 158–168 (2018).
Ilyas, S. & Yang, J. C. Landscape of Tumor Antigens in T Cell Immunotherapy. J Immunol 195, 5117–5122 (2015).
Morgan, R. A. et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006).
Johnson, L. A. et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–546 (2009).
Parkhurst, M. R. et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 19, 620–626 (2011).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29, 917–924 (2011).
Rapoport, A. P. et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 21, 914–921 (2015).
Kageyama, S. et al. Adoptive Transfer of MAGE-A4 T-cell Receptor Gene-Transduced Lymphocytes in Patients with Recurrent Esophageal Cancer. Clin Cancer Res 21, 2268–2277 (2015).
Tawara, I. et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood 130, 1985–1994 (2017).
D’Angelo, S. P. et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 (c259)T Cells in Synovial Sarcoma. Cancer Discov 8, 944–957 (2018).
Linette, G. P. et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122, 863–871 (2013).
Morgan, R. A. et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36, 133–151 (2013).
Hinrichs, C. S. & Restifo, N. P. Reassessing target antigens for adoptive T-cell therapy. Nat Biotechnol 31, 999–1008 (2013).
Sadelain, M., Brentjens, R. & Riviere, I. The basic principles of chimeric antigen receptor design. Cancer Discov 3, 388–398 (2013).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Kontermann, R. E. & Brinkmann, U. Bispecific antibodies. Drug Discov Today 20, 838–847 (2015).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365, 725–733 (2011).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371, 1507–1517 (2014).
Kochenderfer, J. N. et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 33, 540–549 (2015).
Schuster, S. J. et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med 377, 2545–2554 (2017).
Park, J. H. et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med 378, 449–459 (2018).
Kantarjian, H. et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med 376, 836–847 (2017).
Dao, T. et al. Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat Biotechnol 33, 1079–1086 (2015).
Akahori, Y. et al. Antitumor activity of CAR-T cells targeting the intracellular oncoprotein WT1 can be enhanced by vaccination. Blood 132, 1134–1145 (2018).
Rollig, C., Knop, S. & Bornhauser, M. Multiple myeloma. Lancet 385, 2197–2208 (2015).
Guo, B. et al. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J Cellular Immunother 2, 28–35 (2016).
Berdeja, J. G. et al. First-in-human multicenter study of bb2121 anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: Updated results. J Clin Oncol 35, 3010–3010 (2017).
Hosen, N. et al. The activated conformation of integrin beta7 is a novel multiple myeloma-specific target for CAR T cell therapy. Nat Med 23, 1436–1443 (2017).
Garfall, A. L. et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight 3, 120505, https://doi.org/10.1172/jci.insight.120505 (2018).
Jungbluth, A. A. et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer 92, 856–860 (2001).
van Rhee, F. et al. NY-ESO-1 is highly expressed in poor-prognosis multiple myeloma and induces spontaneous humoral and cellular immune responses. Blood 105, 3939–3944 (2005).
Purbhoo, M. A. et al. Quantifying and imaging NY-ESO-1/LAGE-1-derived epitopes on tumor cells using high affinity T cell receptors. J Immunol 176, 7308–7316 (2006).
Schuberth, P. C. et al. Effector memory and central memory NY-ESO-1-specific re-directed T cells for treatment of multiple myeloma. Gene Ther 20, 386–395 (2013).
Maus, M. V. et al. An MHC-restricted antibody-based chimeric antigen receptor requires TCR-like affinity to maintain antigen specificity. Mol Ther Oncolytics 3, 1–9 (2016).
Stone, J. D., Aggen, D. H., Schietinger, A., Schreiber, H. & Kranz, D. M. A sensitivity scale for targeting T cells with chimeric antigen receptors (CARs) and bispecific T-cell Engagers (BiTEs). Oncoimmunology 1, 863–873 (2012).
Hoseini, S. S., Dobrenkov, K., Pankov, D., Xu, X. L. & Cheung, N. K. Bispecific antibody does not induce T-cell death mediated by chimeric antigen receptor against disialoganglioside GD2. Oncoimmunology 6, e1320625, https://doi.org/10.1080/2162402X.2017.1320625 (2017).
Offner, S., Hofmeister, R., Romaniuk, A., Kufer, P. & Baeuerle, P. A. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol 43, 763–771 (2006).
Xiong, W. et al. Immunological Synapse Predicts Effectiveness of Chimeric Antigen Receptor Cells. Mol Ther 26, 963–975 (2018).
Mareeva, T., Martinez-Hackert, E. & Sykulev, Y. How a T cell receptor-like antibody recognizes major histocompatibility complex-bound peptide. J Biol Chem 283, 29053–29059 (2008).
Stewart-Jones, G. et al. Rational development of high-affinity T-cell receptor-like antibodies. Proc Natl Acad Sci USA 106, 5784–5788 (2009).
Wooldridge, L. et al. CD8 controls T cell cross-reactivity. J Immunol 185, 4625–4632 (2010).
Spear, T. T., Foley, K. C., Garrett-Mayer, E. & Nishimura, M. I. TCR modifications that enhance chain pairing in gene-modified T cells can augment cross-reactivity and alleviate CD8 dependence. J Leukoc Biol 103, 973–983 (2018).
Morita, S., Kojima, T. & Kitamura, T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther 7, 1063–1066 (2000).
Heemskerk, M. H. et al. Redirection of antileukemic reactivity of peripheral T lymphocytes using gene transfer of minor histocompatibility antigen HA-2-specific T-cell receptor complexes expressing a conserved alpha joining region. Blood 102, 3530–3540 (2003).
Held, G. et al. Dissecting cytotoxic T cell responses towards the NY-ESO-1 protein by peptide/MHC-specific antibody fragments. Eur J Immunol 34, 2919–2929 (2004).
Kim, J. H. et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 6, e18556, https://doi.org/10.1371/journal.pone.0018556 (2011).
Nakatsugawa, M. et al. Specific roles of each TCR hemichain in generating functional chain-centric TCR. J Immunol 194, 3487–3500 (2015).
Ochi, T. et al. Optimization of T-cell Reactivity by Exploiting TCR Chain Centricity for the Purpose of Safe and Effective Antitumor TCR Gene Therapy. Cancer Immunol Res 3, 1070–1081 (2015).
Kitamura, T. New experimental approaches in retrovirus-mediated expression screening. Int J Hematol 67, 351–359 (1998).
Asai, H. et al. Co-introduced functional CCR2 potentiates in vivo anti-lung cancer functionality mediated by T cells double gene-modified to express WT1-specific T-cell receptor. PLoS One 8, e56820, https://doi.org/10.1371/journal.pone.0056820 (2013).
Ochi, T. et al. Aurora-A kinase: a novel target of cellular immunotherapy for leukemia. Blood 113, 66–74 (2009).
Yamashita, Y. et al. HLA-DP(84Gly) constitutively presents endogenous peptides generated by the class I antigen processing pathway. Nat Commun 8, 15244, https://doi.org/10.1038/ncomms15244 (2017).
Acknowledgements
This work was supported by Grant-in-Aid for Research Activity Start-up of JSPS, Grant Number JP16H07025 (to T.O.), Research Scholarship of The Uehara Memorial Foundation (to T.O.), and Grant-in-Aid for Scientific Research (B) of JSPS, Grant Number JP18H02839 (to M.Y.). We thank Dr. Kenji Kameda, Division of Analytical Bio-Medicine, Advanced Research Support Center (ADRES), Ehime University, and Ms. Atsuko Hiura, Department of Hematology, Clinical Immunology, and Infectious Diseases, Ehime University Graduate School of Medicine, for their technical assistance.
Author information
Authors and Affiliations
Contributions
M.M. and T.O. designed the project, performed the experimental works, analyzed the results, and wrote the paper. K.T., H.A., and T.S. analyzed and discussed the results. H.F. and T.I. discussed the results and provided supportive comments. K.T. and M.Y. supervised this study, provided financial supports, and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maruta, M., Ochi, T., Tanimoto, K. et al. Direct comparison of target-reactivity and cross-reactivity induced by CAR- and BiTE-redirected T cells for the development of antibody-based T-cell therapy. Sci Rep 9, 13293 (2019). https://doi.org/10.1038/s41598-019-49834-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49834-2
This article is cited by
-
Nanoparticle T-cell engagers as a modular platform for cancer immunotherapy
Leukemia (2021)
-
A single-chain antibody generation system yielding CAR-T cells with superior antitumor function
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.