Abstract
The assessment of Ki-67 in early-stage breast cancer has become an important diagnostic tool in planning adjuvant therapy, particularly for the administration of additional chemotherapy to hormone-responsive patients. An accurate determination of the Ki-67 index is of the utmost importance; however, the reproducibility is currently unsatisfactory. In this study, we addressed the predictive/prognostic value of Ki-67 index assessed by using the most reproducible methods, which were identified in the pilot phase. Paraffin blocks obtained from patients with moderately differentiated, estrogen receptor (ER)-positive early-stage breast cancer in Switzerland, who were originally randomized to the treatment arms with and without chemotherapy in the IBCSG VIII-IX trials, were retrieved. Of these 344 randomized patients, we identified 158 patients (82 treated with and 76 treated without chemotherapy) for whom sufficient tumour tissue was available. The presence of Ki-67 was assessed visually by counting 2000 cells at the periphery (A) and estimating the number of positive cells in five different peripheral regions (C), which was determined to be the most reproducible method identified the pilot phase. The prognostic and predictive value was assessed by calculating the breast cancer-free interval (BCFI) and overall survival (OS) rate. Ki-67 was considered a numerical and categorical variable when different cut-off values were used (10%, 14%, 20% and 30%). An mRNA-based subtyping by using the MammaTyper kit with the application of a 20% Ki-67 immunohistochemistry (IHC) cut-off equivalent was also performed. 158 of 344 randomized patients could be included in the Ki-67 analysis. The mean Ki-67 values obtained by using the two methods differed (A: 21.32% and C: 16.07%). Ki-67 assessed by using method A with a cut-off of 10% was a predictive marker for OS, as the hazard ratio (>10% vs. <=10%) in patients with chemotherapy was 0.48 with a 95% confidence interval of [0.19–1.19]. Further, the HR of patients treated without chemotherapy was 3.72 with a 95% confidence interval of [1.16–11.96] (pinteraction=0.007). Higher Ki-67 index was not associated with outcome and using the 10% Ki-67 cut-off there was an opposite association for patients with and without chemotherapy. Ki-67 assessments with IHC significantly correlated with MammaTyper results (p=0.002). The exact counting method (A) performed via a light-microscope revealed the predictive value of Ki-67 assessment with a 10% cut-off value. Further analyses employing image analyses and/or mRNA-based-assessments in larger populations are warranted.
Similar content being viewed by others
Introduction
The assessment of proliferation by estimating the Ki-67 labelling index has increasingly become an integral biomarker of early-stage breast cancer1,2,3,4,5,6,7,8,9,10,11,12,13. The decision on further adjuvant hormonal therapy with additional chemotherapy in luminal A- and B-like breast cancers is based on the progesterone receptor status and Ki-67 labelling index1,2,3,7,10,12,13,14,15. Recent indications for preoperative chemotherapy, including patients with luminal-type breast cancers, increasingly include the Ki-67 labelling index as a biomarker for this therapy choice9,16,17. The issues of reproducibility and the choice of the best method for measuring the Ki-67 labelling index have been the subject of several pathology studies and were included in several oncological/senological guidelines4,5,8,11,18,19,20,21,22,23,24,25,26,27,28. Difficulties in reproducing the Ki-67 labelling index are particularly crucial in the intermediate range proliferative luminal B-like breast cancers, as published data are reaching a consensus on this delicate issue and advising caution with the use of this biomarker4,5,8,11,18,19,20,22,23,24,25,26,27,28. Data have accumulated and results supporting or refuting the superiority of a digital or visual analysis are approximately equal, basically suggesting that both methodologies can be applied for routine diagnostic purposes3,22,23,27,28,29.
We previously conducted a reproducibility study (SAKK 28/12 pilot phase) testing different Ki-67 methods using visual and digital analyses to identify the most reproducible method regarding intra- and inter-rater reliability. In the SAKK 28/12 validation phase, the chosen methods from the pilot phase were subjected to further analysis using a prospective clinical cohort comprised of paraffin blocks obtained from patients who were initially enrolled in the IBCSG VIII and IX trials11,13,22,23,24,25,26,27,28,30,31.
The aim of this study was to correlate the immunohistochemical Ki-67 labelling index obtained using the two most reproducible methods from the SAKK 28/12 study with clinical data such as overall survival (OS) and the breast cancer-free interval (BCFI)27. Additionally, we determined the Ki-67 index with an mRNA-based assessment using MammaTyper and correlated the mRNA levels with OS and BCFI. The reason to include mRNA-based subtyping and Ki-67 mRNA values was the high interobserver reliability and interclass correlation reported previously in mRNA-based subtyping32.
Methods
Objectives of the study
The main goal of the validation phase of SAKK 28/12 is to determine the prognostic/predictive value of Ki-67, which was assessed by using the most reproducible methods, as identified in the pilot phase (methods A and C), for predicting OS and BCFI27. In the pilot phase, two assessing methods resulted in an almost equally high inter-observer reliability, which were both chosen for further validation in this study. These methods were: A (exact counting as the original recommendation) and C (estimating resp. eyeballing in central and peripheral regions)27.
Additionally, we aim to assess the association between mRNA-based subtyping and assessments based on methods A/C. Furthermore, we are also interested in determining the associations between the Ki-67 mRNA level and OS/BCFI.
Materials and Methods
We retrieved residual paraffin blocks collected before study treatment from patients who were enrolled in the IBCSG VIII and IX studies and registered in Switzerland.
The designs of IBCSG Trials VIII and IX have been described in detail elsewhere33,34. IBCSG Trials VIII and IX were randomized clinical trials that compared the effectiveness of adjuvant endocrine therapy alone and sequential chemotherapy followed by endocrine therapy for node-negative invasive breast cancer among pre- and peri-menopausal (Trial VIII) and post-menopausal (Trial IX) women33,34. The breast cancer-free interval was defined as the length of time from the date of randomization to any invasive breast cancer relapse (including ipsilateral or contralateral breast recurrence) or was censored at date of the last follow-up or death without relapse. OS was defined as the length of time from the date of randomization to death from any cause or censored at the last known date the patient was alive33,34.
Briefly, from 1990–1999, in Trial VIII, 1063 pre- and peri-menopausal women with node-negative early breast cancer were randomly assigned to endocrine therapy with 24 months of goserelin alone, six cycles of chemotherapy with classical cyclophosphamide, methotrexate and 5-fluorouracil (CMF), or a sequence of 6 cycles of CMF followed by 18 months of goserelin. Similarly, from 1988–1999, in Trial IX, 1669 eligible post-menopausal women were randomly assigned to endocrine therapy with 5 years of 20 mg of tamoxifen daily or 3 cycles of CMF followed by tamoxifen to complete 5 years therapy. In each trial, randomization was stratified according to the locally determined ER status. Patient follow-up, vital status and the date of any relapse or recurrence are recorded in the IBCSG database. The median follow-up from randomization in Trial VIII is 12 years and in Trial IX 13 years33,34. Ethical approval was obtained in participating countries according to national regulations.
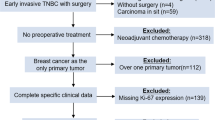
Originally, 660 patients in Switzerland, all with G2 tumors, were randomized in the IBCSG VIII and IX studies, and 344 of these patients met the inclusion criteria (as ER positive, G2). Clinical outcome data was available only for the patients who met the inclusion criteria in the study (Fig. 1). Paraffin blocks from 158 of 344 Swiss patients were retrieved from the archives of Swiss pathology institutions and contained sufficient amounts of invasive breast cancer tissues; 82 of these patients were randomized to the treatment arm with chemotherapy. Eight pathology institutions (University Hospital Lausanne, University Hospital Basel, University Hospital Bern, University Hospital Geneva, University Hospital Zurich, Cantonal Hospital St. Gallen, Cantonal Hospital Graubünden and Cantonal Hospital Locarno Switzerland) that originally participated the IBCSG VIII and IX studies provided paraffin blocks. Patients selected for this study had a moderately differentiated hormone receptor-positive breast cancer (with a negative Her2 status available in the original studies). Morphology was controlled by preparing a fresh haematoxylin-eosin (HE)-stained section to confirm the presence of invasive cancer available for further studies. Data on overall survival (OS) and the breast cancer-free interval (BCFI) were provided to the SAKK by the IBCSG.
This project is a part of a retrospective breast cancer study on archived human tissues and was approved by the Ethical Committee of the Canton Zurich (ZH-KEK-2012-553).
Immunohistochemistry for Ki-67
The Ki-67 status was analysed using immunohistochemical staining, as described previously27. Briefly, sections for Ki-67 were stained centrally in the Institute of Pathology and Molecular Pathology, University Hospital Zurich Switzerland according to the following laboratory protocol (the protocol is from the Institute of Pathology and Molecular Pathology, University Hospital Zurich, Switzerland, Laboratory for in situ technology): Two micrometer thick sections were freshly cut from paraffin blocks containing a sufficient amount of invasive carcinoma tissue. Ki-67 staining was performed using the fully automated Benchmark staining system (Ventana Medical Systems) and the primary antibody (rabbit monoclonal anti-Ki-67 human, clone 30-09 Ventana Medical Systems, Inc.).
Interpretation of Ki-67 Immunohistochemistry
As discussed in the pilot phase of the study, the most reproducible methods were applied to this cohort and were conducted by the principle investigator (ZV) of this study, who was blinded to the clinical outcome (OS/BCFI) and performed the evaluations via a light microscope27. The assessment methods designated as the best methods were method A (exact counting) and method C (eyeballing). Both methods were scored by the principle investigator (ZV).
Method A was defined as the original method of counting 2000 invasive cells in randomly selected, high-power magnification (400×) fields at the periphery of the tumor and determining the percentage of Ki-67 staining12.
Method C was defined as an estimating (so-called eye-balling) assessment analysis performed via a light microscope at 20× magnification for five random fields within the tumor (both the periphery and center), which included approximately 500 cells.
Digital analysis was not applied in this study, as none of the digital analysis methods investigated in the pilot phase outperformed the best light microscopic methods, methods A and C, in terms of reproducibility.
Ki-67 values are reported as percentages of the invasive tumour cells. Throughout this paper, the percent symbol will be removed for Ki-67 index to simplify the presentation. Therefore, the Ki-67 index measured by using methods A and C are presented as a number ranging from 0 to 100.
Assessment of the Ki-67 mRNA
All 158 paraffin blocks underwent an assessment of the Ki-67 mRNA using the MammaTyper assay, as described previously32. Briefly, ten micrometer thick, unstained slides were freshly cut from the paraffin blocks at the Institute of Pathology and Molecular Pathology of the University Hospital Zurich and were sent to BioNTech Diagnostics GmbH, Mainz, Germany for the MammaTyper analyses. The mRNA was extracted from the unstained slides with the RNXtract RNA Extraction Kit (BioNTech Diagnostics) and was subsequently measured via the MammaTyper analysis using the same technical procedures described in a previous study32. The mRNA analysis was blinded to the values of the Ki-67 immunostaining and the clinical outcome. The results were obtained from 137 paraffin blocks for the study. The remaining 21 blocks were excluded either due to a low RNA content because of a poor tissue quality or to missing clinicopathological information.
Interpretation of the Ki-67 mRNA assessment
As described above, MammaTyper is a molecular in vitro diagnostic test for the quantitative detection of the mRNA expression of the ERBB2 (HER2), ESR1 (ER), PGR (PR) and MKI67 (marker of proliferation Ki-67) genes. The test is used for the molecular subtyping of breast cancer tissue into the intrinsic subtypes Luminal A-like, Luminal B-like (HER 2-positive or -negative), HER2-positive (non-luminal) and Triple negative (ductal), as defined in the St. Gallen Consensus Conference recommendations utilized since 20111,7,14. The immunohistochemistry cut-off for the differentiation between Luminal A-like and Luminal B-like cancers was prospectively set to 20%, and the MammaTyper kit was designed using this cut-off based on the mRNA values32.
Statistical analysis
To assess the prognostic value of Ki-67 regarding to time-to-event endpoints (OS and BCFI), a Cox regression model with Ki-67 as the only covariable was fitted. To assess the predictive value of Ki-67, a Cox regression model including the Ki-67 index, treatment and the interaction of these two parameters was fitted. P values were calculated for these models by using Wald’s test. For additional research objectives, the Wilcoxon test was used to identify the association between Ki-67 index assessed by using methods A/C and the Ki-67 mRNA, while the log-rank test was used to assess the associations between the Ki-67 mRNA and OS/BCFI. The sample size estimation based on the prognostic value of Ki-67 (assessed by using method A) for BCFI was performed before we received the clinical data from IBCSG and retrieved the residual paraffin blocks. Assuming a rate of BC recurrence of 20%, a Cox regression analysis of Ki-67 with a standard deviation of 7.5 (estimated from the pilot phase of this project) based on a sample of 231 observations achieves 80% power at a 0.05 significance level to detect a hazard ratio of 1.25 and the number of observations accordingly.
PASS 11 was used to calculate the sample size. SAS 9.4 and R 3.3.2 were used for the analyses. Multiple test corrections were not applied to all p values; thus, the results are considered exploratory. The motivation to select the specific Ki-67cut-offs as 10,14,20,30 was based on previously published consensus recommendations1,2,3,7,10,12,13,14,15.
Novelty and Impact statement
Our data draws attention to the fact that Ki-67 cut-off values are methodology and observer dependent, and median Ki-67 values can vary depending on the assessment methods. In our study, the exact counting under a light microscope revealed the predictive relevance of Ki-67 assessment using a 10% cut-off value for predicting OS or BCFI.
Ethical approval and consent to participate
Ethical approval and informed consent from all patients to use the paraffin blocks at the time of IBCSG VIII and IX randomization were obtained according to the national regulations. The retrospective study was approved by the Lead Ethical Committee of the Canton Zurich (ZH-KEK-2012-553). All procedures performed in this study were conducted in accordance with the ethical standards of the institutional and national research committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
All authors, as well the SAKK and the IBCSG scientific committees, read and approved the manuscript prior to submission. The study, including the design and data interpretation, was discussed during the SAKK annual and semiannual meetings.
Results
Summary
158 of 344 Swiss patients randomized in the IBCSG BIG VIII and IX trials with G2 hormone receptor-positive and Her2-negative breast cancer and with available tumor tissue in paraffin blocks were included in this study (Fig. 1). Our results show, as described in details below, that different Ki-67 assessment methodologies have different mean and median values and the methodologies influence the correlation between OS/BCFI and Ki-67 labelling index. We could demonstrate, that a cut-off of 10% using visual Ki-67 IHC assessment was a predictive marker of OS in patients who were not treated with chemotherapy. Moreover, we found that Ki-67 IHC assessments significantly correlated with Ki-67 mRNA measurements.
Descriptive analysis of Ki-67 immunohistochemistry and clinical outcomes
Mean and median Ki-67 values obtained using immunohistochemical Methods A and C
Compared with Method C (mean=16.07 and median=10.00), Method A (mean=21.32 and median=17.70) generally produces a higher Ki-67 value. Range for Ki-67 values in both Method A and C was 1.00 to 90.00. These differences are shown in Fig. 2 as boxplots (A) and in Fig. 2 as a Bland-Altman plot (B).
Frequencies of Ki-67-positive immunohistochemistry using different cut-off values
Using 10, 14, 20 and 30 as cut-off values for Ki-67 staining, the frequencies obtained by using different cut-offs differed between Methods A and C, as shown in Table 1.
Summary statistics of OS and BCFI
The two clinical endpoints were OS and BCFI, which were collected from the Swiss patients in the IBCSG VIII and IX studies, and these values are presented in Table 2. These endpoints show 20 events in the subgroup with chemotherapy and 16 and 17 events, respectively, in the group without chemotherapy.
Estimated OS and BCFI probabilities
Patients in this Swiss collective enrolled in both treatment arms have similar outcomes as the entire IBCSG study population in terms of OS and BCFI. Estimated OS and BCFI probabilities are shown in Fig. 3(A,B).
Prognostic value of Ki-67 immunohistochemistry assessed by using Method A for determining OS/BCFI
Based on the estimated HR, a higher Ki-67 value did not result in significantly higher hazard ratio for OS and BCFI (all p values are greater than 0.05).
In Table 3A, we present the HR estimated using the univariate Cox regression model, which utilizes Ki-67 index assessed by using Method A as a numeric variable (the first row of the table) and a categorical variable based on different cut-off values (the second to fifth rows).
Prognostic value of Ki-67 immunohistochemistry assessed by using Method C for determining OS/BCFI
In Table 3B, we present the HR estimated using the univariate Cox regression model with Ki-67 index assessed by using Method C as the numeric variable (the first row of the table) and a categorical variable based on different cut-off values (the second to fifth rows).
Notably, the HRs, 95% CIs and p values based on cut-off values of 10 and 14 are exactly the same due to the lack of a Ki-67 index when assessed by using Method C at cut-off values ranging from 10 to 15 (see Fig. 2(A)). Therefore, samples with Ki-67 levels equal or less than 14 are exactly the same as samples with Ki-67 levels equal or less than 10.
Predictive value of Ki-67 immunohistochemistry assessed by using Method A for determining OS
By using the cut-off of 10% and Method A, we found significant differences in the OS and predictive value at levels below and above this threshold (p=0.0074). The use of the cut-off of 14% almost reached statistical significance and showed only a trend towards an improved OS (p=0.0554). No other cut-off values produced significant differences. In Table 4(A), we separately presented the HRs and 95% CIs estimated using the univariate Cox regression model of OS with Ki-67 levels assessed by using Method A for the two treatment groups. The p value (*p value) presented in Table 4(A) was calculated for the interaction term based on the multivariate Cox regression model of OS with Ki-67, treatment group and their interaction. Notably, Tables 4(B) and 5 present the data in the same manner. In Fig. 4(A), the OS is stratified by different cut-off values for Ki-67 which were assessed by using Method A and considering treatment allocation. Based on the estimated HR presented in Table 4(A) a higher Ki-67 level (>10%) results in a lower hazard ratio (HR 0.48) for OS in patients treated with chemotherapy. For patients who were not treated with chemotherapy, the opposite effect was observed (HR 3.72). These data show that patients with Ki-67<=10% do not profit from chemotherapy and patients with Ki-67>10% might potentially have a benefit. Using 10% as the cut-off, this effect was statistically significant.
(A) Kaplan-Meier curves show the analyses of the overall survival (OS) of patients stratified by different cut-off values for Ki-67 levels, as measured using Method A and considering treatment allocation. (B) Kaplan-Meier curves show the analysis of the breast cancer-free interval (BCFI) in patients stratified by different cut-off values for Ki-67 levels measured using Method A and considering treatment allocation.
Predictive value of Ki-67 immunohistochemistry assessed by using Method A for determining the BCFI
Ki-67 levels assessed by using Method A did not show a significant ability to predict BCFI in patients stratified by treatment allocation, as shown in Table 4(B) and Fig. 4(B).
Predictive value of Ki-67 immunohistochemistry assessed by using Method C for determining OS
Ki-67 levels assessed by using Method C did not display a significant ability to predict OS in patients stratified by treatment allocation, as shown in Table 5(A) and Fig. 5(A). However, there was a similar tendency for Method C compared to Method A with respect to OS.
(A) Kaplan-Meier curves show differences in the overall survival (OS) of patients stratified by different cut-off values for Ki-67 levels measured using Method C and considering treatment allocation. (B) Kaplan-Meier curves show differences in the breast cancer-free interval (BCFI) in patients stratified by different cut-off values for Ki-67 levels measured using Method C and considering treatment allocation.
Predictive value of Ki-67 immunohistochemistry assessed by using Method C for determining BCFI
Ki-67 levels did not display a significant ability to predict BCFI in patients stratified by treatment allocation, as shown in Table 5(B) and Fig. 5(B).
Correlation between Ki-67 immunohistochemistry and the mRNA-dependent luminal subtype assessment
A significant correlation was observed between the immunohistochemical assessments (methods A and C) and the classification of the intrinsic subtypes as Luminal A-like or Luminal B-like with the MammaTyper kit: Range for Ki-67 values in both Method A and C was 1.00 to 90.00. As shown in Fig. 6, patients with MammaTyper Luminal B-like tumours generally presented higher Ki-67 values in IHC than patients with the Luminal A-like subtype.
Correlation between Ki-67 mRNA-dependent Luminal A- and Luminal B-like subtypes and OS/BCFI
In this cohort, we did not identify any significant correlations between OS/BCFI and the Ki-67 mRNA assessment, as shown in Fig. 7. Notably, the MammaTyper Ki-67 mRNA cut-off corresponds to a 20% IHC cut-off, which was not significant in this cohort, as shown above and determined by using IHC.
Discussion
In the SAKK 28/12 validation phase, we analysed the prognostic and predictive value of Ki-67 immunohistochemistry assessed by using the most reproducible methods selected from the pilot phase of SAKK 28/1227. The old, archived paraffin blocks containing breast cancer tissues from patients in the IBCSG VIII and IX clinical trials and treated at the time of the trials in Switzerland were used in this study, and the corresponding clinical outcomes were also used for this project27,30,31. The median follow-up from randomization in Trial VIII was 12 years and in Trial IX was 13 years33,34. Additionally, we also assessed Ki-67 mRNA levels and analysed the association with clinical outcomes, as we reported in an earlier paper, and Ki-67 mRNA levels are found to be highly reproducible32.
As shown in the present study, a Ki-67 index obtained using immunohistochemical method A (exact counting of tumour cells at the tumour periphery) with a cut-off of 10% predicts the OS of patients treated with chemotherapy. Although other results regarding the predictive and prognostic values were not statistically significant, Ki-67 levels measured by using Method A represent a potential prognostic factor for BCFI based on the estimated HRs and confidence intervals, which indicated a higher risk of recurrence in patients with higher Ki-67 levels.
The identification of the optimal method or methodologies for the assessment of proliferative activity in breast cancer has been the subject of several previous studies in the last decade since the introduction of Ki-67 as a routinely assessed parameter in hormone receptor-positive breast cancer specimens1,2,3,4,5,6,7,8,9,10,11,12. These studies analysed different types of visual assessments and digital analyses to test whether one method outperforms the other or if these methods yield the same results in terms of reproducibility1,2,3,4,5,6,8,9,11,12,13,14. Based on the currently available published data, a trend that both visual and digital analyses result in a similar inter-rater coefficient has been observed, enabling the diagnostic use of both approaches9,15,16,17,18,19. However, the optimal methodology for assessing Ki-67 levels in breast cancer that fulfils the criteria of perfect inter-rater and inter-laboratory reproducibility has not yet been identified4,6,8,10,13,19,23,27. In contrast to midrange proliferative cancers where reproducibility remains an issue, the inter-rater reliability is considerably better for low and high proliferative cancers4,6,8,10,11,13,19,23,27. Intra-tumour heterogeneity and the area chosen for the Ki-67 assessment appear to be the most crucial factors, in addition to pre-analytical inter-laboratory differences at the current time4,5,6,8,10,13,19,23,27. This heterogeneity remains a relevant factor for Ki-67 and gene-signature tests.
The first descriptions of utilizing cut-off values with Ki-67 levels to make clinical decisions and to estimate prognosis were derived from Ki-67 measurements obtained from tumour samples in the IBCSG VIII and IX prospective clinical trials1,2,7,12,14,17,35.
One of the first sources of data on Ki-67 power measured in the IBCSG VIII and IX trials showed that the Ki-67 labelling index does not predict a benefit from adding chemotherapy to endocrine therapy but, rather, indicates a worse disease-free survival rate regardless of the treatment modalities and, thus, provides important prognostic information35.
The median Ki-67 values, as assessed by central pathology, for tumours in IBCSG VIII and IX were 19%35.
Since the original description in 2008, which stated that a cut-off of 14% for the Ki-67 level differentiates between Luminal A and Luminal B tumours, the cut-off value has periodically undergone adjustments, such as shifting from 20% to 30% or being described as simply low and high, depending on the midrange Ki-67 levels measured at a specific pathology institution, and these modifications are still underway even currently1,2,4,6,8,11,13,19,23,27. This change is probably one reason why our study used a lower optimal and significant Ki-67 cut-off value (10%) to assess overall survival, although the median Ki-67 level was 17.7% for method A. Further explanations for our discrepancy from the original definition of 14% are most likely the smaller sample size and the observation of fewer events in both arms of the Swiss subset of the IBCSG VIII and IX cohorts, although applying a 14% cut-off value with Method A almost reached statistical significance in this subset.
The difficulties in defining the optimal cut-off value and the most reproducible Ki-67 assessment methods has led to adjustments in the clinical guidelines as well, as the current recommendations of the St. Gallen 2017 Consensus Conference do not include cut-offs but instead state that low and high Ki-67 categories, in accordance with the midrange Ki-67 values of the specific pathology laboratory, should be applied1,2,8,10,13,19,23,27. Nevertheless, Ki-67 levels greater than 20–25, regardless of the assessment methodology, are probably the best approximate cut-off values to estimate risk of death compared to lower values and to decide whether additional adjuvant chemotherapy should be administered2,12.
Alternative methods to immunohistochemical Ki-67 assessments, such as mRNA-based analyses, were recommended in recent studies, as inter-laboratory reproducibility with ICC values of 0.980–0.998 revealed the excellent agreement of quantitative measurements for Ki-67 levels measured using MammaTyper13,32. Another recent mRNA-based study assessing Ki-67 levels with STRAT4 showed a good correlation with Ki-67 immunohistochemistry at a 30% cut-off36. However, the clinical utility, particularly in the intermediate range, has not yet been confirmed.
In the present study, Ki-67 IHC assessed by using methods A and C correlated well with the mRNA-based assessments of Luminal A-like and Luminal B-like (HER2 negative) tumours, although Methods A and C produced different Ki-67 values, namely, mean values of 21.3 and 16.1, respectively. This observation has been reported in recent studies, showing that different assessment methodologies using both visual and digital measurements result in different mean and median Ki-67 levels4,5,6,23,28,37,38. The lack of any significant correlations between the mRNA-based Ki-67-dependent Luminal A-like and Luminal B-like subtype assessment and OS/BCFI in our study is probably due to the smaller sample size in the Swiss cohort and the focus on grade 2 tumours, which is in contrast to the entirety of the IBCSG VIII and IX clinical trials. Within this restricted cohort, only the 10% Ki-67 IHC cut-off reached significance, while the MammaTyper MKI67 cut-off correlated with a 20% Ki-67 cut-off. Furthermore, intra-tumour heterogeneity and the analysis of different tumour areas in different tumour blocks from the same tumour of each patient differed from the analyses applied in the original IBCGS VIII and IX subsets and should be considered when interpreting divergent results.
The question of the optimal tissue, such as biopsy, surgical specimen or tissue-micro-arrays (TMA), to assess the Ki-67 index in breast cancer is controversial and has been addressed in the literature9,10,25,26,37,38,39. As shown in several previous studies, Ki-67 levels obtained from the same tumour, whether obtained via TMA, core biopsy or surgical specimen, differ due to intra-tumour heterogeneity, which must be considered in clinical practice if different tissue specimens are available37,38,39. In our study, we restricted the analysis to surgical specimens, which was similar to the original IBCSG VIII and IX cohorts. Nevertheless, in daily routine pathological diagnostics, core biopsies are increasingly considered the primary source for Ki-67 assessments regarding both adjuvant therapy decisions and preoperative chemotherapy selection3,8,10,16,17. As described above, optimal Ki-67 cut-off values in core biopsies for predicting the response to preoperative chemotherapy range from 15–30%3,16,17,40.
Conclusions
In summary, different Ki-67 assessment methodologies affect the correlations with overall survival and the breast cancer-free interval in patients with moderately differentiated breast cancer. Based on our results, method A (counting cells using visual assessment) using a cut-off of 10% was a predictive marker of OS in patients who were not treated with chemotherapy. Higher Ki-67 index was not associated with outcome and using the 10% Ki-67 cut-off there was an opposite association for patients with and without chemotherapy.
The results in this study are hypothesis generating and additional validation of these finding appears warranted. The issue of Ki-67 assessment in breast cancer in terms of the methodology and optimal cut-off values, particularly in midrange samples, remains a challenge, and further studies analysing correlations and prospective clinical trials are needed.
Data Availability
All data and study materials are available upon request without any restrictions.
References
Coates, A. S. et al. Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 26(8), 1533–46 (2015).
Curigliano, G. et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 28(8), 1700–12 (2017).
Denkert, C. et al. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast. 24(Suppl 2), S67–72 (2015).
Focke, C. M. et al. Interlaboratory variability of Ki67 staining in breast cancer. Eur J Cancer. 84, 219–27 (2017).
Focke, C. M., Decker, T. & van Diest, P. J. Intratumoral heterogeneity of Ki67 expression in early breast cancers exceeds variability between individual tumours. Histopathology. 69(5), 849–61 (2016).
Focke, C. M., van Diest, P. J. & Decker, T. St Gallen 2015 subtyping of luminal breast cancers: impact of different Ki67-based proliferation assessment methods. Breast Cancer Res Treat. 159(2), 257–63 (2016).
Goldhirsch, A. et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 24(9), 2206–23 (2013).
Leung, S. C. Y. et al. Analytical validation of a standardized scoring protocol for Ki67: phase 3 of an international multicenter collaboration. NPJ Breast Cancer. 2, 16014 (2016).
Luporsi, E. et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 132(3), 895–915 (2012).
Petrelli, F., Viale, G., Cabiddu, M. & Barni, S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 153(3), 477–91 (2015).
Varga, Z. et al. How reliable is Ki-67 immunohistochemistry in grade 2 breast carcinomas? A QA study of the Swiss Working Group of Breast- and Gynecopathologists. PLoS One. 7(5), e37379 (2012).
Viale, G. et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 26(34), 5569–75 (2008).
Yang, C. et al. Ki67 targeted strategies for cancer therapy. Clin Transl Oncol. 20(5), 570–5 (2018).
Goldhirsch, A. et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 22(8), 1736–47 (2011).
Tashima, R. et al. Evaluation of an Optimal Cut-Off Point for the Ki-67 Index as a Prognostic Factor in Primary Breast Cancer: A Retrospective Study. PLoS One. 10(7), e0119565 (2015).
Denkert, C. et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol. 24(11), 2786–93 (2013).
Klauschen, F. et al. Standardized Ki67 Diagnostics Using Automated Scoring–Clinical Validation in the GeparTrio Breast Cancer Study. Clin Cancer Res. 21(16), 3651–7 (2015).
Christgen, M., von Ahsen, S., Christgen, H., Langer, F. & Kreipe, H. The region-of-interest size impacts on Ki67 quantification by computer-assisted image analysis in breast cancer. Hum Pathol. 46(9), 1341–9 (2015).
Christgen, M., Winkens, W. & Kreipe, H. H. Determination of proliferation in breast cancer by immunohistochemical detection of Ki-67. Pathologe. 35(1), 54–60 (2014).
Dowsett, M. et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 103(22), 1656–64 (2011).
Eliasziw, M., Young, S. L., Woodbury, M. G. & Fryday-Field, K. Statistical methodology for the concurrent assessment of interrater and intrarater reliability: using goniometric measurements as an example. Phys Ther. 74(8), 777–88 (1994).
Hida, A. I. et al. Visual assessment of Ki67 using a 5-grade scale (Eye-5) is easy and practical to classify breast cancer subtypes with high reproducibility. J Clin Pathol. 68(5), 356–61 (2015).
Mikami, Y. et al. Interobserver concordance of Ki67 labeling index in breast cancer: Japan Breast Cancer Research Group Ki67 ring study. Cancer Sci. 104(11), 1539–43 (2013).
Niikura, N. et al. Assessment of the Ki67 labeling index: a Japanese validation ring study. Breast Cancer. 23(1), 92–100 (2016).
Polley, M. Y. et al. An international study to increase concordance in Ki67 scoring. Mod Pathol. 28(6), 778–86 (2015).
Polley, M. Y. et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 105(24), 1897–906 (2013).
Varga, Z. et al. Standardization for Ki-67 assessment in moderately differentiated breast cancer. A retrospective analysis of the SAKK 28/12 study. PLoS One. 10(4), e0123435 (2015).
Zhong, F. et al. A Comparison of Visual Assessment and Automated Digital Image Analysis of Ki67 Labeling Index in Breast Cancer. PLoS One. 11(2), e0150505 (2016).
Arihiro, K. et al. Comparison of visual assessment and image analysis in the evaluation of Ki-67 expression and their prognostic significance in immunohistochemically defined luminal breast carcinoma. Jpn J Clin Oncol. 46(12), 1081–7 (2016).
Castiglione-Gertsch, M., Gelber, R. D., O’Neill, A., Coates, A. S. & Goldhirsch, A. Systemic adjuvant treatment for premenopausal node-negative breast cancer. The International Breast Cancer Study Group. Eur J Cancer. 36(4), 549–50 (2000).
International Breast Cancer Study G. Endocrine responsiveness and tailoring adjuvant therapy for postmenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst. 94(14), 1054–65 (2002).
Varga, Z. et al. An international reproducibility study validating quantitative determination of ERBB2, ESR1, PGR, and MKI67 mRNA in breast cancer using MammaTyper(R). Breast Cancer Res. 19(1), 55 (2017).
Aebi, S. et al. Differential efficacy of three cycles of CMF followed by tamoxifen in patients with ER-positive and ER-negative tumors: long-term follow up on IBCSG Trial IX. Ann Oncol. 22(9), 1981–7 (2011).
Karlsson, P. et al. Long-term results of International Breast Cancer Study Group Trial VIII: adjuvant chemotherapy plus goserelin compared with either therapy alone for premenopausal patients with node-negative breast cancer. Ann Oncol. 22(10), 2216–26 (2011).
Viale, G. et al. Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst. 100(3), 207–12 (2008).
Wu, N. C. et al. Comparison of central laboratory assessments of ER, PR, HER2, and Ki67 by IHC/FISH and the corresponding mRNAs (ESR1, PGR, ERBB2, and MKi67) by RT-qPCR on an automated, broadly deployed diagnostic platform. Breast Cancer Res Treat. 172(2), 327–38 (2018).
Knutsvik, G. et al. Evaluation of Ki67 expression across distinct categories of breast cancer specimens: a population-based study of matched surgical specimens, core needle biopsies and tissue microarrays. PLoS One. 9(11), e112121 (2014).
Muftah, A. A. et al. Ki67 expression in invasive breast cancer: the use of tissue microarrays compared with whole tissue sections. Breast Cancer Res Treat. 164(2), 341–8 (2017).
Obermann, E. C., Eppenberger-Castori, S. & Tapia, C. Assessment of proliferation: core biopsy or resection specimen? Discrepancies in breast cancer with low and high proliferation. Pathologe. 33(3), 245–50 (2012).
Bustreo, S. et al. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: a large case series study with a long-term follow-up. Breast Cancer Res Treat. 157(2), 363–71 (2016).
Acknowledgements
We thank Claudia Gürtler, Daniela Weiser and Kornelia Schlombs in Biontech Mainz Germany for performing the MammaTyper assays, critically reading the manuscript and interpreting the results. Furthermore, we thank Meredith Regan and Alain Coats at the IBCSG for providing information on the Swiss subset of patients in the cohort of the IBCSG VIII and IX clinical trials, as well as for a critical interpretation of the results. Z.V. on behalf of SAKK received research grants from Foundation Empiris and the Swiss Cancer League (SCL) for this study. The SAKK was further supported for this study by research agreements with the following institutions: State Secretary for Education, Research and Innovation (SERI), Swiss Cancer Research Foundation (SCS), Swiss Cancer League (SCL).
Author information
Authors and Affiliations
Contributions
Z.V.: Design of study, conducting Ki-67 assessment, writing the manuscript. Q.L.: Design of study, conducting statistical analyses, writing the manuscript. W.J., U.P., T.R., J.C.T.: Providing paraffin blocks and critically reading the manuscript. H.H.: design of study, coordinating of study. D.K.: design of study, conducting statistical analyses, writing the manuscript. B.T.: Design of study, critically reading the manuscript. T.R.: Design of study, critically reading the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Varga, Z., Li, Q., Jochum, W. et al. Ki-67 assessment in early breast cancer: SAKK28/12 validation study on the IBCSG VIII and IBCSG IX cohort. Sci Rep 9, 13534 (2019). https://doi.org/10.1038/s41598-019-49638-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49638-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.