Abstract
Preeclampsia is a common cause of preterm birth and neonatal morbidity, but its relationship with neonatal respiratory distress syndrome (RDS) remains controversial. We conducted a retrospective cohort study with data from very-low-birth-weight (VLBW) infants born in 1997–2014 from the database of the Premature Baby Foundation of Taiwan to evaluate the relationship between maternal preeclampsia and neonatal RDS. In total, 13,490 VLBW infants were enrolled, including 2200 (16.3%) infants born to preeclamptic mothers. The mean (standard deviation) gestational ages were 30.7 (2.5) weeks in the preeclamptic group and 28.6 (2.9) weeks in the control (non-preeclamptic) group. Severe RDS was defined according to the surfactant therapy requirement. The incidence of severe RDS was lower in infants exposed to maternal preeclampsia than in controls [28.9% vs. 44%; odds ratio (OR), 0.52; 95% confidence interval (CI), 0.47–0.57]. However, after adjustment for confounders, the OR for severe RDS development in the preeclampsia group was 1.16 (95% CI, 1.02–1.31). Other factors, such as gestational age, birth weight, female sex, and antenatal receipt of two or more steroid doses were significantly protective against RDS in multivariate regression analysis. This study revealed that maternal preeclampsia slightly increases the risk of severe RDS in VLBW infants.
Similar content being viewed by others
Introduction
Preeclampsia is a systemic syndrome that occurs in 5–10% of pregnant women and is a leading cause of maternal and neonatal morbidity and mortality1. This syndrome poses a great challenge for obstetricians and neonatologists because the optimal timing for delivery is a dilemma. In most cases, preeclampsia occurs at the late preterm or term stage, but about 12% of affected women develop early-onset preeclampsia (at <34 weeks of gestation)2. The etiology of early-onset preeclampsia differs from that of late-onset preeclampsia; abnormal placentation and spiral artery remodeling lead to intrauterine growth restriction and a greater probability of preterm birth3,4,5. The rates of perinatal death and severe neonatal morbidity are much higher in association with early-onset relative to late-onset preeclampsia2.
Respiratory distress syndrome (RDS) is among the most common complications of preterm delivery. Preterm RDS is secondary to surfactant insufficiency, and its incidence is usually related inversely to gestational age (GA). The literature contains conflicting information on the effect of preeclampsia per se on RDS6,7,8,9,10,11,12,13,14,15,16,17,18. Shah et al.12 found a lower incidence of RDS in preterm (<34 gestational weeks) infants born to preeclamptic mother than in a control group. In contrast, Tagliaferro et al.18 found in a cohort study that the risk of severe RDS was increased in extremely premature (23–28 gestational weeks) infants exposed to preeclampsia. Thus, this study aimed to analyze the relationship between maternal preeclampsia and the development of RDS in very-low-birth-weight (VLBW) infants in a large cohort.
Results
Baseline characteristics of study participants
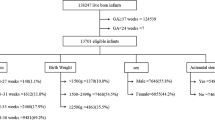
In total, 13,490 VLBW infants, including 2,200 (16.3%) cases born to mothers with preeclampsia, were enrolled in this study. The mean (standard deviation) GAs were 30.7 (2.5) weeks in the preeclamptic group and 28.6 (2.9) weeks in the control group (odds ratio (OR), 1.30; 95% confidence interval (CI), 1.28–1.32; P < 0.001; Table 1). The overall incidence of RDS was about 86%, and that of severe RDS (requiring surfactant use) was 41.2%. The baseline characteristic that differed most between the preeclampsia and control groups was the proportion of small-for-gestational-age (SGA) infants (72.4% vs. 24.9%; OR, 7.91; 95% CI, 7.14–8.77; Table 1). The GA, birth weight, female predominance, and Cesarean section rate were also significantly greater in the preeclamptic group than in the control group (Table 1). The incidence of RDS in infants was lower in the presence than in the absence of preeclampsia (80.9% vs. 87.2%; OR, 0.58; 95% CI, 0.52–0.66; Table 1). The incidence of surfactant use was also significantly lower in the preeclampsia group than in the control group (28.9% vs. 44%; OR, 0.52; 95% CI, 0.47–0.57; Table 1).
Findings of multivariate analysis
In a multivariate logistic regression analysis including GA, birth weight, infant sex, SGA, and antenatal steroid use as potential confounders, preeclampsia was associated with an insignificantly increased risk of RDS (adjusted odds ratio (aOR), 1.12; 95% CI, 0.98–1.29; Table 2), but a significantly increased risk of severe RDS development (aOR, 1.16; 95%CI, 1.02–1.31; Table 3). GA, but not birth weight, was associated negatively with RDS. GA and birth weight were associated inversely with severe RDS, with aOR of 0.68 (95% CI, 0.65–0.7) and 0.94 (95% CI, 0.91–0.97), respectively. Male infants were more likely than females to develop RDS and severe RDS [aOR (95%CI), 1.18 (1.06–1.32) and 1.24 (1.14–1.35), respectively]. SGA and antenatal use of two or more doses of steroids protected against the development of severe RDS [aOR (95% CI), 0.8 (0.69–0.93) and 0.57 (0.53–0.62), respectively].
Findings of subgroup analysis with stratification by SGA
Given the uneven distribution of baseline SGA between the preeclampsia and control groups, and the possible interaction between preeclampsia and SGA in the effect on RDS, we performed a subgroup analysis with stratification by SGA. No significant association between preeclampsia and severe RDS was found in the SGA or non-SGA subgroup (OR (95% CI), 1.12 (0.94–1.34) and 1.15 (0.95–1.40), respectively; Table 4). The trends of other predictor variables were consistent with those revealed by the whole-sample multivariate regression analysis.
Discussion
In this cohort study of VLBW infants, the incidences of RDS and severe RDS were lower in infants whose mothers had preeclampsia; we infer that more protective factors were present in the preeclampsia group than in the control group. This assumption was verified by those of the multivariate analysis, which showed that maternal preeclampsia increased the risk of severe RDS, but not RDS, after adjustment for confounding factors. The high incidence or diagnostic rate of RDS of any grade in very preterm infants in our sample may have obscured a difference in RDS risk between the preeclampsia and control groups. The need for surfactant use, which was generally regarded as rescue therapy in Taiwan during our study period (1997–2014), may more objectively represent the presence and the severity of RDS. GA, birth weight, female sex, and antenatal receipt of two or more doses of steroids were protective factors against RDS in this study.
Our findings are contrary to previous findings that preeclampsia is a protective factor against RDS7,12. Those previous studies promoted the belief that maternal preeclampsia accelerates fetal lung maturation. Some trial results also supported the enhancement of biochemical lung maturation profile by chronic in utero stress, possibly via increased fetal cortisol production8,9. The most recent study to support such a protective effect of preeclampsia on RDS was a large cohort study conducted in the Netherlands10. However, that study was conducted to examine the outcomes of late-onset preeclampsia (34–37 gestational weeks). Our preeclampsia group contained mainly early-onset preeclampsia cases, with a different pathogenesis and worse neonatal outcomes relative to late-onset preeclampsia2.
On the other hand, accumulating data indicate the absence of a protective effect of maternal preeclampsia on the fetal respiratory system6,11,13. Even more, preeclampsia was associated with an increased risk of neonatal RDS in several studies, consistent with our findings14,15,16,17,18,19. In a large national cohort study conducted in the United States, which included 156,681 infants born to mothers with preeclampsia, the greater probability of RDS was found in the preeclamptic group compared to the non-preeclamptic group (6.6% vs. 1.9%)17. Another recent single-center cohort study including infants born at 23–28 gestational weeks also revealed that preeclampsia increased the risk of severe RDS18. Preeclampsia is characterized by an imbalanced maternal angiogenic state, resulting in generalized endothelial dysfunction, increased levels of maternal antiangiogenic factor soluble fms-like tyrosine kinase-1 (sFlt-1), and decreased free circulating levels of the angiogenic factors vascular endothelial growth factor (VEGF) and placental growth factor20,21,22,23. VEGF is important for normal lung vasculature24 and surfactant protein production25,26,27. sFlt-1, an antagonist of VEGF, can impede VEGF signaling and lead to impaired surfactant production. RDS is secondary to surfactant insufficiency, and low VEGF concentrations have been associated with RDS severity in preterm infants28,29,30,31. Similarly, high sFlt-1 and/or low VEGF levels have been observed in neonatal cord blood and tracheal aspirates from infants born to preeclamptic mothers32,33,34,35,36. Higher sFlt-1 concentrations have also been noted in amniotic fluid from preeclamptic mother34. Wang et al.16 demonstrated that preeclampsia was correlated with a higher maternal circulating sFlt-1 level and an increased risk of neonatal RDS. All of these findings imply that preeclampsia creates not only a stressful intrauterine environment, but also an unfavorable state for fetal lung surfactant production. A study of infants born prematurely to mothers with severe preeclampsia or amniotic infection found that non-surviving infants exposed to preeclampsia had a higher proportion of accelerated morphologic lung maturation than non-surviving infants exposed to amniotic infection (40% vs. 5%). However, the remaining survivors in the preeclampsia group needed more respiratory support after the first 24 hours, indicating greater surfactant insufficiency37.
In the present study, baseline characteristics showed a high degree of heterogeneity between the preeclampsia and control groups. Infants born to preeclamptic mothers had significantly greater birth weights and GAs, and the female predominance and SGA proportion were greater in this group than in the control group. SGA status is a common complication of preeclampsia, with a widely ranging incidence of 13.36–52%11,14,38,39,40. In this study, the incidence of SGA in the preeclamptic group was very high, perhaps due to the application of inclusion criteria according to birth weight instead of GA, and thus the enrollment of more SGA infants. Although the association between preeclampsia and severe RDS was insignificant in our subgroup analysis, the trend of increased risk was consistent in both groups. This finding indicates that preeclampsia and SGA do not interact significantly in affecting RDS. In the whole-sample multiple regression analysis, 95% CIs for the association of preeclampsia with severe RDS were very close to 1, which may explain the failure to detect a significant difference in the subgroup analysis in this small sample.
Birth weight and GA are known to correlate negatively with RDS; male sex also contributes to an increased RDS risk41. Amorim et al.42 reported that antenatal corticosteroid therapy could reduce the risk of RDS in the presence of severe preeclampsia at 26–34 weeks of gestation. Our study revealed a weak positive association between preeclampsia and severe RDS, with other covariates also contributing to neonatal RDS development. Thus, the relationship between preeclampsia and RDS could be divergent if all confounding factors are not taken into consideration. This situation may reasonably explain the discrepant results obtained in previous studies, given the small sample and examination of different potentially confounding factors.
The main strength of our study was the examination of a large cohort of VLBW neonates; the mean gestational age was 30 weeks, which indicated that most mothers with preeclampsia had the early-onset form of this condition. We also excluded mother with histories of chronic hypertension, which may alter the pathogenesis and effects relative to those of maternal preeclampsia alone. Another large cohort study of infants delivered at >23 gestational weeks’ (average 37 weeks’) revealed an increased risk of RDS in the presence of maternal hypertensive disorder, and different neonatal outcomes among cases of maternal gestational hypertension, mild chronic hypertension, and mild preeclampsia15.
Our study has some limitations. First, the reliability of our data depends on the accuracy of pediatricians’ records, but the large sample size minimizes the potential effect of this factor. Second, maternal comorbidity, body mass index, and severity of preeclampsia were not recorded in the neonatal-oriented registration database. Third, the type and total dosage of antenatal steroids, and the interval between their use and delivery, were not recorded in the database. Additionally, RDS diagnoses were based on physicians’ subjective interpretations of clinical and chest X-ray data, which may cause bias. For this reason, we took surfactant usage as an objective indicator of severe RDS.
Conclusions
In this population-based cohort study of VLBW infants, we found early-onset maternal preeclampsia slightly increased the risk of severe RDS compare with preterm delivery of other causes, whereas GA, birth weight, SGA, female sex and antenatal use of two or more doses of steroids were prominent protective factors that decreased the risk of RDS.
Methods
Study subjects
This study was based on data on all VLBW infants born from 1997 to 2014 in all 22 neonatal departments in Taiwan, registered in the database of the Premature Baby Foundation of Taiwan. VLBW was defined as birth body weight <1500 g43. The data collected included antenatal and perinatal histories, delivery mode, neonatal histories including diagnoses, complications during hospitalization, and clinical outcomes at discharge. Patient information received by the database coordinator was cross checked with the national birth registry. The exclusion criteria were congenital anomalies, chromosomal anomalies, and maternal chronic hypertension with or without preeclampsia. The cases were divided into the preeclampsia and control (non-preeclampsia) groups. Preeclampsia was defined as diastolic blood pressure ≥90 mm Hg with proteinuria ≥1+ (30 mg/dl) on dipstick testing or non-dependent edema during pregnancy44.
Ethical considerations
Written informed consent was obtained from included subjects’ parents or legal guardians. The study was approved by the Institutional Review Boards of eight participating hospitals (National Taiwan University Hospital, Chang Gung Memorial Hospital, China Medical University Hospital, National Cheng Kung University Hospital, Tri-Service General Hospital, Chung Shan Medical University Hospital, Shin Kong Wu Ho-Su Memorial Hospital, and Kaohsiung Medical University Chung-Ho Memorial Hospital), and Joint Institutional Review Board for the other participating hospitals. All research was performed in accordance with relevant guidelines and regulations.
Outcome variables
RDS was diagnosed by neonatologists in charge of the infants’ care according to clinical symptoms and signs, chest X-ray findings, and arterial blood gas findings. Mainstream surfactant therapy was used as a rescue treatment rather than a prophylactic during the study collection period in Taiwan. Thus, we defined severe RDS according to the requirement for surfactant therapy. Antenatal steroid usage was defined as the receipt of any type of steroid prior to delivery to accelerate fetal lung maturation. SGA status was defined as birth body weight <10th percentile for gestational age45.
Statistical analysis
The chi-squared test and Student’s t-test were used to compare the distributions of categorical and continuous variables, respectively, between groups. A multivariate logistic regression model was used to analyze the association between maternal preeclampsia and RDS risk, with adjustment for potential confounders. The confounders included demographic and clinical variables that differed between the preeclampsia and control groups in univariate analysis. aORs with 95% CIs were calculated to assess the magnitudes of the associations between various factors and RDS risk. Significance was determined by two- tailed P < 0.05. The association between preeclampsia and RDS was further examined in a subgroup analysis stratified according to SGA.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
de Souza Rugolo, L. M. S., Bentlin, M. R. & Trindade, C. E. P. Preeclampsia: effect on the fetus and newborn. NeoReviews 12, e198–e206 (2011).
Lisonkova, S. & Joseph, K. S. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 209, 544 e541–544 e512, https://doi.org/10.1016/j.ajog.2013.08.019 (2013).
von Dadelszen, P., Magee, L. A. & Roberts, J. M. Subclassification of preeclampsia. Hypertens Pregnancy 22, 143–148, https://doi.org/10.1081/PRG-120021060 (2003).
Phipps, E., Prasanna, D., Brima, W. & Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin J Am Soc Nephrol 11, 1102–1113, https://doi.org/10.2215/CJN.12081115 (2016).
Weitzner, O., Yagur, Y., Weissbach, T., Man El, G. & Biron-Shental, T. Preeclampsia: risk factors and neonatal outcomes associated with early- versus late-onset diseases. J Matern Fetal Neonatal Med, 1–5, https://doi.org/10.1080/14767058.2018.1500551 (2018).
Friedman, S. A., Schiff, E., Kao, L. & Sibai, B. M. Neonatal outcome after preterm delivery for preeclampsia. Am J Obstet Gynecol 172, 1785–1788; discussion 1788–1792 (1995).
Yoon, J. J., Kohl, S. & Harper, R. G. The relationship between maternal hypertensive disease of pregnancy and the incidence of idiopathic respiratory distress syndrome. Pediatrics 65, 735–739 (1980).
Gluck, L. & Kulovich, M. V. Lecithin-sphingomyelin ratios in amniotic fluid in normal and abnormal pregnancy. Am J Obstet Gynecol 115, 539–546 (1973).
Kulovich, M. V. & Gluck, L. The lung profile. II. Complicated pregnancy. Am J Obstet Gynecol 135, 64–70 (1979).
Langenveld, J. et al. Neonatal outcome of pregnancies complicated by hypertensive disorders between 34 and 37 weeks of gestation: a 7 year retrospective analysis of a national registry. Am J Obstet Gynecol 205(540), e541–547, https://doi.org/10.1016/j.ajog.2011.07.003 (2011).
Jelin, A. C. et al. Early-onset preeclampsia and neonatal outcomes. The Journal of Maternal-Fetal & Neonatal Medicine 23, 389–392, https://doi.org/10.3109/14767050903168416 (2010).
Shah, D. M., Shenai, J. P. & Vaughn, W. K. Neonatal outcome of premature infants of mothers with preeclampsia. J Perinatol 15, 264–267 (1995).
Hansen, A. R., Barnes, C. M., Folkman, J. & McElrath, T. F. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr 156, 532–536, https://doi.org/10.1016/j.jpeds.2009.10.018 (2010).
Chang, E. Y., Menard, M. K., Vermillion, S. T., Hulsey, T. & Ebeling, M. The association between hyaline membrane disease and preeclampsia. Am J Obstet Gynecol 191, 1414–1417, https://doi.org/10.1016/j.ajog.2004.06.097 (2004).
Cruz, M. O., Gao, W. & Hibbard, J. U. Obstetrical and perinatal outcomes among women with gestational hypertension, mild preeclampsia, and mild chronic hypertension. Am J Obstet Gynecol 205(260), e261–269, https://doi.org/10.1016/j.ajog.2011.06.033 (2011).
Wang, A. et al. Circulating anti-angiogenic factors during hypertensive pregnancy and increased risk of respiratory distress syndrome in preterm neonates. J Matern Fetal Neonatal Med 25, 1447–1452, https://doi.org/10.3109/14767058.2011.640368 (2012).
Stevens, W. et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol 217, 237–248 e216, https://doi.org/10.1016/j.ajog.2017.04.032 (2017).
Tagliaferro, T., Jain, D., Vanbuskirk, S., Bancalari, E. & Claure, N. Maternal preeclampsia and respiratory outcomes in extremely premature infants. Pediatr Res 85, 693–696, https://doi.org/10.1038/s41390-019-0336-5 (2019).
de Souza Rugolo, L. M. S., Bentlin, M. R. & Trindade, C. E. P. Preeclampsia: early and late neonatal outcomes. Neoreviews 13, e532–e541 (2012).
Koga, K. et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 88, 2348–2351, https://doi.org/10.1210/jc.2002-021942 (2003).
Maynard, S. E. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111, 649–658, https://doi.org/10.1172/JCI17189 (2003).
Tsatsaris, V. et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 88, 5555–5563, https://doi.org/10.1210/jc.2003-030528 (2003).
Levine, R. J. et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350, 672–683, https://doi.org/10.1056/NEJMoa031884 (2004).
Ferrara, N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 280, C1358–1366 (2001).
Chen, C. M. & Wang, L. F. High-dose vascular endothelial growth factor increases surfactant protein gene expressions in preterm rat lung. Early Hum Dev 83, 581–584, https://doi.org/10.1016/j.earlhumdev.2006.12.005 (2007).
Compernolle, V. et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8, 702–710, https://doi.org/10.1038/nm721 (2002).
Dao, D. T. et al. A paradoxical method to enhance compensatory lung growth: Utilizing a VEGF inhibitor. PLoS One 13, e0208579, https://doi.org/10.1371/journal.pone.0208579 (2018).
Lassus, P. et al. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 164, 1981–1987, https://doi.org/10.1164/ajrccm.164.10.2012036 (2001).
Tsao, P. N. et al. Vascular endothelial growth factor in preterm infants with respiratory distress syndrome. Pediatr Pulmonol 39, 461–465, https://doi.org/10.1002/ppul.20205 (2005).
Abdel-Hady, S., Abdel-Ghafar, E., Abdel-Rehim, I. & Abdel-Gawad, E. R. Vascular endothelial growth factor in preterm infants with respiratory distress syndrome. Egypt J Immunol 14, 43–49 (2007).
Kalay, S. et al. The role of VEGF and its soluble receptor VEGFR-1 in preterm newborns of preeclamptic mothers with RDS. J Matern Fetal Neonatal Med 26, 978–983, https://doi.org/10.3109/14767058.2013.766692 (2013).
Tsao, P. N. et al. Excess soluble fms-like tyrosine kinase 1 and low platelet counts in premature neonates of preeclamptic mothers. Pediatrics 116, 468–472, https://doi.org/10.1542/peds.2004-2240 (2005).
Lassus, P., Ristimaki, A., Ylikorkala, O., Viinikka, L. & Andersson, S. Vascular endothelial growth factor in human preterm lung. Am J Respir Crit Care Med 159, 1429–1433, https://doi.org/10.1164/ajrccm.159.5.9806073 (1999).
Staff, A. C., Braekke, K., Harsem, N. K., Lyberg, T. & Holthe, M. R. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 122, 33–39, https://doi.org/10.1016/j.ejogrb.2004.11.015 (2005).
Catarino, C. et al. Fetal and maternal angiogenic/anti-angiogenic factors in normal and preeclamptic pregnancy. Growth Factors 27, 345–351, https://doi.org/10.3109/08977190903184670 (2009).
Kwon, J. Y. et al. Decreased endothelial progenitor cells in umbilical cord blood in severe preeclampsia. Gynecol Obstet Invest 64, 103–108, https://doi.org/10.1159/000100081 (2007).
Ersch, J., Fauchere, J. C., Bucher, H. U., Hebisch, G. & Stallmach, T. The pulmonary paradox in premature infants: in-utero infected lungs do better than those with accelerated maturation. J Perinat Med 32, 84–89, https://doi.org/10.1515/JPM.2004.016 (2004).
Ferrazzani, S., Caruso, A., De Carolis, S., Martino, I. V. & Mancuso, S. Proteinuria and outcome of 444 pregnancies complicated by hypertension. Am J Obstet Gynecol 162, 366–371 (1990).
Schiff, E., Friedman, S. A., Mercer, B. M. & Sibai, B. M. Fetal lung maturity is not accelerated in preeclamptic pregnancies. Am J Obstet Gynecol 169, 1096–1101 (1993).
Tul, N. et al. Outcome of small for gestational age preterm singletons: a population-based cohort study. J Perinat Med 44, 941–944, https://doi.org/10.1515/jpm-2015-0321 (2016).
Dani, C. et al. Risk factors for the development of respiratory distress syndrome and transient tachypnoea in newborn infants. Italian Group of Neonatal Pneumology. Eur Respir J 14, 155–159 (1999).
Amorim, M. M., Santos, L. C. & Faundes, A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. Am J Obstet Gynecol 180, 1283–1288 (1999).
WHO. International statistical classification of diseases and related health problems, 10th revision, Fifth edition. World Health Organization (2016).
Bulletins-Obstetrics, A. C. O. P. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol 99, 159–167 (2002).
Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 854, 1–452 (1995).
Acknowledgements
We acknowledge Premature Baby Foundation of Taiwan sponsored the Taiwan Premature Infant Developmental Collaborative Study Group: Dr. Kuo-Inn Tsou at Cardinal Tien Hospital, Dr. Chyong-Hsin Hsu at Mackay Memorial Hospital, Dr. Wu-Shiun Hsieh at National Taiwan University Hospital, Dr. Shu-Chi Mu at Shin-Kong Wu Ho-Su Memorial Hospital in Taipei, Dr. Jui-Ying Lin at Chang Gung Memorial Hospital in Linkou, Dr. Hung-Chih Lin at China Medical University Hospital in Taichung, Dr. Chao-Ching Huang at National Cheng Kung University Hospital in Tainan, Dr. Kai-Sheng Hsieh at Veterans General Hospital in Kaohsiung. We thank research nurses and residents of the participating hospitals for their help with the registration and data collection. Financial support of this work by the National Taiwan University Hospital (NTUH. 108-T15) and Ministry of Science and Technology (MOST 108-2314-B-002-155-MY3) are gratefully acknowledged.
Author information
Authors and Affiliations
Consortia
Contributions
P.N.T. conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content; Y.H.W. conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; H.I.Y. designed the data collection instruments, performed all data analyses, and reviewed and revised the manuscript; H.C.C., C.Y.C., W.S.H. and K.I.T. conceptualized and designed the study, reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wen, YH., Yang, HI., Chou, HC. et al. Association of Maternal Preeclampsia with Neonatal Respiratory Distress Syndrome in Very-Low-Birth-Weight Infants. Sci Rep 9, 13212 (2019). https://doi.org/10.1038/s41598-019-49561-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49561-8
This article is cited by
-
The association between sex and neonatal respiratory distress syndrome
BMC Pediatrics (2024)
-
Global, Regional and National Trends in the Burden of Neonatal Respiratory Failure and essentials of its diagnosis and management from 1992 to 2022: a scoping review
European Journal of Pediatrics (2023)
-
Downregulation of TCL6 protected human trophoblast cells from LPS-induced inflammation and ferroptosis
Functional & Integrative Genomics (2023)
-
Lung recruitment improves the efficacy of intubation-surfactant-extubation treatment for respiratory distress syndrome in preterm neonates, a randomized controlled trial
BMC Pediatrics (2022)
-
Self-monitoring of blood pressure among women with hypertensive disorders of pregnancy: a systematic review
BMC Pregnancy and Childbirth (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.