Abstract
The northern tamarisk beetle Diorhabda carinulata (Desbrochers) was approved for release in the United States for classical biological control of a complex of invasive saltcedar species and their hybrids (Tamarix spp.). An aggregation pheromone used by D. carinulata to locate conspecifics is fundamental to colonization and reproductive success. A specialized matrix formulated for controlled release of this aggregation pheromone was developed as a lure to manipulate adult densities in the field. One application of the lure at onset of adult emergence for each generation provided long term attraction and retention of D. carinulata adults on treated Tamarix spp. plants. Treated plants exhibited greater levels of defoliation, dieback and canopy reduction. Application of a single, well-timed aggregation pheromone treatment per generation increased the efficacy of this classical weed biological control agent.
Similar content being viewed by others
Introduction
The genus Tamarix (Tamaricaceae) are invasive Eurasian woody trees or shrubs increasingly present and dominant in riparian areas of the western United States1,2,3,4. Multiple Tamarix species, collectively referred to as saltcedar or tamarisk, are present in the United States, with widespread hybridization between the species3. To simplify the discussion of this species complex, Tamarix species and their hybrids will hereafter be referred to as Tamarix. Since its introduction, Tamarix has significantly degraded native plant communities and wildlife habitat through the replacement of native plant assemblages with monocultures4. The loss of native plant assemblages from Tamarix invasion imperils multiple populations of threatened or endangered species2. The loss of ecosystem services and functions due to Tamarix invasion has been estimated between $133–185 million U.S. dollars annually5,6. The disruption of ecological processes caused by Tamarix ecosystem dominance was the impetus for the development of a classical biological control program for Tamarix, utilizing the northern tamarisk beetle Diorhabda carinulata Desbrochers (formerly D. elongata deserticola Brullé) (Coleoptera: Chrysomelidae)7,8. After extensive host-specificity testing, D. carinulata was found to be host specific to Tamarix and did not pose a risk to native plants, leading to the approval and introduction of D. carinulata to the United States7,8.
In North America, D. carinulata populations are multivoltine, with 1–3 generations per year9,10. Diorhabda carinulata overwinters in the adult stage and emerges in spring, coinciding with Tamarix bud break10. After spring emergence, adults feed and mate followed by the deposition of clutches of eggs onto Tamarix foliage9. Once the eggs hatch, the three larval stages feed within the Tamarix canopy then descend into the understory litter to form pupal cells10. After metamorphosis, the adults emerge from the litter and disperse both short and long distances11. Along with the dispersal behavior, the adults aggregate on Tamarix plants12. In response to shorter day length at the approach of autumn, D. carinulata adults enter a reproductive diapause but continue feeding to accumulate metabolic reserves, after which they burrow into the litter to overwinter13.
All life stages of D. carinulata feed exclusively on Tamarix foliage. Feeding by D. carinulata abrades the Tamarix foliar cuticle; widespread cuticular disruption by high densities of beetles results in desiccation of the foliage, leading to partial or complete host defoliation14. Defoliation by D. carinulata can dramatically increase the level of physiological stress experienced by affected plants and depletes reserves of stored carbon15,16,17. This diminishes foliage production and root mass in the years following a defoliation event16. Multiple years of intense herbivory by D. carinulata can result in Tamarix mortality16,18.
Techniques to manipulate densities of D. carinulata in the field have recently been investigated, specifically to increase the biocontrol efficacy of sparse beetle populations19. These manipulations involved the use of D. carinulata’s aggregation pheromone (2E,4Z)-2,4-heptadien-1-ol, which was identified by Cossé et al.12,19,20. Treatments incorporating semiochemicals applied through a specialized pheromone lure application technology (SPLAT®: ISCA Technologies, Riverside, CA, USA) made it possible to create or enhance aggregations of adults and larvae. Weekly application of 1-g doses of SPLAT impregnated with aggregation-causing semiochemicals (2.17% (2E,4Z)-2,4-heptadien-1-ol) resulted in persistent and higher densities of D. carinulata on treated plants. Higher densities resulted in intensified herbivory with increased rates of defoliation, plant dieback, and reduction in live canopy volume. These are desirable outcomes for a classical weed biological control program. Along with intensifying impact on Tamarix, the ability to aggregate populations of a biological control agent in the field is also useful for facilitating monitoring (e.g., to track presence/absence, persistence, life stage or abundance) by biological control practitioners12,20,21.
Recently published results show that the release of the aggregation pheromone attained from 1-g doses of formulated SPLAT maintained aggregations of D. carinulata for only 7–10 days19. The limited duration of lure activity was due to rapid volatilization of the aggregation pheromone from the small volume of the dollops used in the 1-g application. The restricted bioactivity of 1-g treatments significantly constrains the practical utility of this technology. Treatments based on a 1-g lure required weekly applications throughout the summer to maintain consistent aggregations of D. carinulata18. The activity of SPLAT-based pheromone treatments can be extended by adjusting the volume applied22. An increased application rate of pheromone impregnated SPLAT was therefore investigated using larger dollops. The objective of this study was to determine if three applications of 4-g doses of SPLAT targeting discrete adult emergence in this multivoltine beetle could result in season-long manipulation of D. carinulata densities. The rationale for evaluating targeted application of larger volumes of SPLAT-based semiochemical lures was to determine if longer term aggregations could be maintained, therefore reducing the time commitment required for practical deployment of this technique by land managers.
Results
Release rate analysis
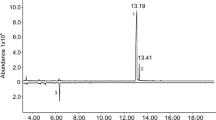
Release rates of D. carinulata aggregation pheromone (2E,4Z)-2,4-heptadien-1-ol from 4-g dollops of field-aged SPLAT over a 31-day period are displayed in Fig. S1. The release rate model y = 19384x−2.3 yielded an R2 of 0.93 and a decay constant of −2.3. On day 1, mean D. carinulata aggregation pheromone emitted from treated dollops was 17514 nghr−1. Pheromone emissions from the 4-g dollops rapidly decreased between the first and second day of volatile collection, but stabilized after day 15. Between day 15 and 31, the pheromone was released at a mean rate of 228 nghr−1.
Field results
Reproductive adults
In 2014, weekly sweeps of recently emerged over-wintering (P) adult D. carinulata on Tamarix fitted with treatment lures yielded a mean of 1.3 on plants receiving the BL (blank or control) treatment, compared to 3.5 on plants receiving the PH (aggregation pheromone) treatment (F1,185.2 = 21.32, P < 0.001) (Fig. 1a). The 2014 weekly sweeps of F1 adults resulted in overall greater mean densities than the sweeps of 2014 P generation adults, again with higher densities swept from plants treated with the pheromone lure, 7.3 from BL-treated plants vs. 14.3 from PH-treated plants (F1,232.1 = 20, P < 0.001) (Fig. 1c). There was a significant site effect for F1 adult numbers in 2014 (F1,232.1 = 10.7, P = 0.001 on 1, 232.1 d.f.); however, a site x treatment interaction was not found to be significant (F1,232.1 = 20, P = 0.58).
Weekly mean ± SE capture per three sweeps of adult Diorhabda carinulata on plants receiving 4-g dollops of pheromone impregnated SPLAT (PH) or blank SPLAT (BL). Densities captured 2014–15 of P generation overwintered adults (a,b), F1 reproductive adults (c,d), and F2 non-reproductive adults (e,f). Overall the pheromone effect was significant and greater for (a) (F1,185.2 = 21.32, P < 0.001), (b) (F1,186.3 = 12.3, P < 0.001), (c) (F1,232.1 = 20, P < 0.001), (d) (F1,208.3 = 20.1, P < 0.001), and (f) (F1,348 = 31, P < 0.001). There was no overall pheromone effect for (e) (F1,234.4 = 1.2, P = 0.28).
Mean weekly sweep captures of overwintered P adults in 2015 were lower than recorded in 2014, averaging 1.1 adults on BL-treated plants vs. 2.2 adults on PH-treated plants (F1,186.3 = 12.3, P < 0.001) (Fig. 1b). Counts of the 2015 F1 adults were also lower than reported for 2014 F1 adults, averaging 1.2 and 2.7 adults per three sweeps, respectively, on BL-treated vs. PH-treated plants (F1,208.3 = 20.1, P < 0.001) (Fig. 1d). There was no significant site effect for overwintered P or F1 adult sweep capture in 2015 (F1,188 = 1.6, P = 0.21; F 1,211.4 = 2.9, P = 0.09).
Non-reproductive adults
No pheromone-mediated difference in weekly sweep captures of F2 adults occurred in 2014 (F1,234.4 = 1.2, P = 0.28) (Fig. 1e). There was a significant site effect (F1,234.4 = 7, P = 0.008), but the interaction between site and treatment for F2 adults was not significant (F1,234.4 = 1, P = 0.32). In 2015, sweeps of PH-treated plants yielded significantly higher densities of F2 adults, averaging 6.6 adults compared to a mean of 2.7 adults on BL-treated plants (F1,348 = 31, P < 0.001) (Fig. 1f). There was a significant site effect in 2015 (F1,350.3 = 5.5, P = 0.02), and an ordinal interaction between site and treatment (F1,348 = 6.2, P = 0.01).
Larval densities
Increased densities of adult D. carinulata recorded on PH-treated plants were correlated with increased mean densities of larvae captured in weekly sweeps of the same plants (Fig. 2). In 2014, the mean number of larvae captured per three sweeps each week averaged 5.1 on PH-treated plants, compared to 3.7 on BL-treated plants (F1,467 = 7.2, P = 0.007) (Fig. 2a). There was no site effect for numbers of larvae in 2014 (F1,467 = 1.4, P = 0.23); however, there was a significant ordinal interaction between treatment and site (F1,467 = 3.9, P = 0.05). Densities of larvae captured in weekly sweeps during 2015 were lower overall, but the numbers were still greater on PH-treated compared to BL-treated plants, averaging 3.2 vs. 1.5, respectively (F1,514.1 = 45.3, P < 0.001) (Fig. 2b).
Weekly mean ± SE larval Diorhabda carinulata capture per three sweeps on plants receiving 4-g dollops of pheromone impregnated SPLAT (PH) or 4 g dollops of blank SPLAT (BL), in 2014 (a), and 2015 (b). Overall pheromone effect was significant and greater for (a) (F1,467 = 7.2, P = 0.007), and (b) (F1,514.1 = 45.3, P < 0.001).
Damage, dieback and canopy volume
In 2014, ratings of D. carinulata feeding damage for PH-treated plants differed significantly from damage ratings reported for BL-treated plants (F1,655 = 34.8, P < 0.001) (Fig. 3a). Although all monitored plants (i.e., PH and BL) experienced nearly complete defoliation during summer 2014, in-season regrowth after defoliation reported for BL-treated plants was significantly greater compared with regrowth after defoliation reported for PH-treated plants (Fig. 3a). Again in 2015, PH-treated plants had greater feeding damage than BL-treated plants (F1,799.1 = 76.5, P < 0.001) (Fig. 3b). No significant in-season regrowth was observed in 2015.
Increased feeding damage recorded on PH-treated plants was reflected in increased dieback observed in the following year, both in 2015 (F1,44 = 10, P = 0.003) and 2016 (F1,43 = 17.5, P < 0.001) (Fig. 4). Dieback was greater for both the PH-treated and BL-treated plants in 2015 compared to 2016, most likely due to the greater level of defoliation recorded across the study site in 2015 compared to 2016. Canopy volume did not significantly differ one year after defoliation. In the second year after defoliation, PH-treated plants had smaller canopy volumes compared with study plants receiving a second season of BL treatment (F1,140 = 6.3, P = 0.01) (Fig. 5).
Discussion
The use of synthetic aggregation pheromones to manipulate the spatial distribution of biological control agents has been suggested as a method to intensify damage on under-exploited weed infestations23.The results of this investigation confirm that the deployment of an aggregation pheromone is a feasible approach for manipulating the density and spatial distribution of D. carinulata to enhance biological control of Tamarix. Effective spatial manipulations and enhanced herbivory were achieved with only three targeted applications of the aggregation pheromone per year. The ability of biological control practitioners to alter densities of a classical biological control agent weeks after an application of an attractive lure could have broad impacts in the field of biological control.
Emissions of synthetic (2E,4Z)-2,4-heptadien-1-ol, the D. carinulata aggregation pheromone, from field deployed 4-g dollops of SPLAT were variable for the first 5 days after exposure to environmental conditions. Emission rates stabilized at 10 days and remained relatively constant over the following 21 days, until the study was terminated on day 31. On the first day, the hourly amount of synthetic aggregation pheromone emitted from 4-g dollops approached the amount estimated to be produced by 3,300 adult D. carinulata males in one hour, based on the reported emission rate of 5.2 ng/male/hour12. The average pheromone release rate from the 4-g dollops between 10 and 31 days was equivalent to estimated hourly pheromone emissions by 60 adult male D. carinulata12.
Differences in captures of reproductive adult beetles on Tamarix receiving the control vs. pheromone treatment indicates that a single application of a 4-g dollop of SPLAT impregnated with (2E,4Z)-2,4-heptadien-1-ol can maintain local aggregations of adult D. carinulata over the course of their generational life span (Fig. 1)10. The response of the non-reproductive F2 adults was not consistent across the two years of this study. No pheromone mediated changes in density were observed in 2014 but increased F2 adult densities were recorded in 2015 (Fig. 1e,f).
Increased rates of damage, dieback, and greater changes in canopy volume were correlated with higher D. carinulata densities. Damage ratings for monitored trees varied greatly across the years. In 2014, all of the monitored plants, regardless of the treatment received, were nearly completely defoliated by week 10, with observable damage occurring primarily after week 5. After week 10, the BL-treated plants had substantially higher rates of regrowth compared to the PH-treated plants, indicated by a decrease in mean damage rating for BL-treated plants (Fig. 3a). This suggests that the increased damage rating for PH-treated plants earlier in the season is indicative of the influence of the pheromone treatment on the timing or intensity of herbivory, which impairs the ability of PH-treated plants to regrow after defoliation.
Higher in-season feeding damage ratings reported for all study plants in 2014 were correlated with the greater rate of dieback on all study plants recorded in 2015. Differences in canopy volume were, in contrast to the more immediate influence of damage on dieback, only detected after plants experienced a second year of defoliation. However, the limited but targeted application of the D. carinulata aggregation pheromone resulted in a mean 73% reduction in canopy volume in only two years. This result was unexpected because Tamarix exposed to two years of defoliation by Diorhabda spp. typically experiences a 25–33% reduction in canopy volume24.
Application of D. carinulata aggregation pheromone based lures effectively allows for land managers to dictate where D. carinulata feeding damage will occur. This would make pheromone formulations a very useful tool in managing Tamarix stands that have not been sufficiently defoliated to cause canopy die back or mortality. Reports in which impact due to herbivory has been measured show a high degree of variation in die-back and mortality from site to site25. In one study mortality was measured at nearly 80% at a site that had been defoliated multiple times, while at a neighboring site two kilometers away, which had been more sporadically defoliated, Tamarix mortality was only 20%11. In such instances pheromone formulations could be used to attract and concentrate beetles at areas with low mortality or die back. There are areas in western Colorado where D. carinulata has been present for ten years, and where impact has been minimal25. Landowners would probably welcome the use of pheromone-based lures as an additional management tool to increase the impact of Diorhabda spp. on Tamarix. Further research and development will be needed to finalize the production of a commercially viable pheromone lure system for D. carinulata. We expect that this efficient low-environmental impact herbivore aggregation solution for Tamarix management will become commercially available and widely adopted, but the total cost for the treatment of an individual tree will depend on the level of adoption and commercialization of the technology. A similar system utilizing pheromone impregnated lures is available for approximately 11 U.S. dollars per protected tree (SPLAT Verb, ISCA® Technologies, Riverside, CA, USA).
The field application of the aggregation pheromone would likely have no adverse impacts or non-target effects. Low concentrations of the aggregation pheromone are present in the foliage of Tamarix, giving it a ubiquitous presence in stands of Tamarix20. The aggregation pheromone is specific to Diorhabda species, with low likelihood of its application interfering with other organism’s pheromone systems. Giving land managers a tool to direct D. carinulata around in the field would also allow for land managers to minimize indirect effects from the insects. If a detrimental indirect effect is being observed, land managers can now redirect D. carinulata to alternate areas to minimize that detrimental interaction. By drawing beetles away from some areas where Tamarix is thought to provide transient ecosystem services, the use of pheromones could have broad implications for conservation, as areas sensitive to the removal or damage of Tamarix could be avoided, while intensifying impacts in desired areas19,25. The application of this technology could be beneficial to the conservation of native habitat. For example, Tamarix commonly dominates landscapes, but there is usually a remnant population of native vegetation present within Tamarix infestations4,26. Applying the aggregation pheromone to increase D. carinulata herbivory on Tamarix plants surrounding remnant native plants could remove the competition exerted by Tamarix with minimal risk or disturbance to the native plants. Conditions facilitating recruitment of cottonwoods (Populus spp.) and willows (Salix spp.) are also commonly associated with the successful recruitment of Tamarix, resulting in seedling nurseries that are composed of both the native plants and Tamarix27,28. Selective removal of Tamarix seedlings from these mixed species nurseries, whether through herbicide application or mechanical removal, is time-consuming and all but impossible to accomplish, considering that Tamarix seedlings can reach densities as high as 170,000 individuals/m2 on mud flats29. If land managers used pheromone lures to increase D. carinulata aggregations on mixed native/Tamarix nurseries, the host specificity of the agent would confer a competitive advantage on non-Tamarix seedlings. Increased mortality of Tamarix during seedling establishment has the potential to reduce long term negative environmental impacts of Tamarix invasion, such as salt accumulation and altered fire regimes, on the viability and diversity of affected native plant communities30,31.
The ability to manipulate the spatial distribution of D. carinulata has biological control applications beyond the enhancement of feeding on Tamarix. By applying the aggregation pheromone, and manipulating the densities of D. carinulata in targeted areas, dispersal and establishment can be readily assessed. Specifically, the aggregation pheromone can be used to implement a monitoring procedure where plants are treated and act as ‘sentinels’ for the presence of D. carinulata32. Treated sentinel plants would allow for easy monitoring of D. carinulata, especially if combined with passive yellow sticky card or delta traps12,20.
Material and Methods
Field experiment location and insect source
Tamarix stands along the Bighorn River near Lovell, WY were used as the study area during the summer of 2014, 2015, and 2016. Locations were selected based on existing infestations of saltcedar and the presence of D. carinulata populations. The D. carinulata populations present in the study area are bivoltine and are the descendants of individuals originally collected near the town of Fukang, Xinjiang Province, in northwestern China10,33.
Lure and treatments
Two types of lures were prepared for field testing using ISCA® Technologies’ wax emulsion matrix SPLAT. The first type of lure, hereafter referred to as PH, consisted of SPLAT impregnated with (2E,4Z)-2,4-heptadien-1-ol, an aggregation pheromone produced by D. carinulata12. The pheromone was synthesized according to Petroski34. The second type of lure, hereafter referred to as BL, consisted of the base formulation of SPLAT with no active ingredient, which functioned as a control treatment. Semiochemical active ingredient (2E,4Z)-2,4-heptadien-1-ol was present at a concentration of 2.17% in PH treatment lures, and 0% in BL control lures.
The use of 4-g doses of SPLAT, referred to as dollops, facilitated one-time per generation application of SPLAT lure treatments. Treatments were applied to coincide with the emergence of (1) overwintered reproductive adults (P generation); (2) the F1 generation, which were also reproductive; and (3) the F2 generation, which are non-reproductive but actively feed until entering overwintering diapause. In 2014, the first application of SPLAT was June 16 (week 1), the second application of SPLAT was July 16 (week 5), and the final application of SPLAT was August 18 (week 10). In 2015, the first application of SPLAT was May 26 (week 1), the second application of SPLAT was July 13 (week 8), and the final application of SPLAT was August 18 (week 13).
Release rate analysis
To determine the release rate of the pheromone from 4-g dollops, individual dollops were applied to cattle ear tags and exposed to outdoor conditions at the Bozeman (Montana) Forestry Sciences Laboratory (USDA Forest Service, Rocky Mountain Research Station). Cattle ear tags (Y-Tex Corporation, Cody, WY, USA) were used as the application substrate for dollops because of their durability, portability, and general ease of handling. Volatile collections from these field aged dollops were made at 1, 3, 5, 10, 15, 25, and 31 days to characterize pheromone release rates. Dollops were temporarily transported from the outdoors to the laboratory for volatile collection, but were otherwise continuously exposed to outdoor conditions. Collections were made in glass volatile collection chambers for varying durations depending on the length of field exposure (15 min for samples aged 1, 3, 5, and 10 d; 30 min at 15 d; and 1 hr at 25 and 35 d). Volatile collection traps containing 30 mg of super-Q (Alltech Associates, Inc., Deerfield, IL, USA) adsorbent were fixed in place at the apical opening of the volatile collection chamber where purified air was delivered at a rate of 100 ml/min. Collected volatiles were eluted from the traps into vials using 200 µl methylene chloride (purity >99%) (Fischer Scientific, Pittsburg, PA, USA) and the samples were spiked with 10 µl of a 0.84 ngµl−1 solution of 1-octanol (purity >99%) (Sigma-Aldrich, St. Louis, MO, USA) in methylene chloride as an internal standard. Volatiles were analyzed using a gas chromatograph (Agilent 6890; Agilent Technologies, Santa Clara, CA) coupled to a mass selective detector (MSD, Agilent 5973 instrument). Quantification was made relative to the internal standard.
Field study
The existing arrays of Tamarix plants were randomly assigned treatments at two study sites located in a greasewood (Sarcobatus vermiculatus Hook) floodplain of the Bighorn River characterized as having average Tamarix densities of 0.65 plants/m2 35. The study sites were located approximately 1 km apart.
Twelve replicates of each treatment (PH and BL) were deployed on the study sites. Dollops of SPLAT were applied to cattle ear tags using an 80-ml syringe. Application of 5 ml of either the semiochemical or control formulations of SPLAT yielded 4-g dollops. Treated ear tags were attached with a loop of wire to the branches of treatment plants. Plants selected to receive a SPLAT treatment were based on the existing array of plants at the research locations, with a requirement of treated plants to be approximately 20 m apart. This distance has been experimentally confirmed to minimize possible interference between volatile treatments12,20. Plant size and age class was allowed to vary with the treatments, with plants canopies as small as 4 m3 and canopies as large as 150 m3 receiving treatments. The average canopy volume of BL-treated plants was 34.9 m3, while the average canopy volume of PH-treated plants was 35.7 m3.
Treated Tamarix plants were sampled weekly using a heavy duty canvas sweep net that had an opening diameter of 30.5 cm and a depth of 61 cm. Treated plants were swept three times with a 1.25 m upward arc36. After sweeping, the captured D. carinulata adults and larvae were counted and returned to the tree where they originated. Reproductive status of captured adults was assessed for subsequent analysis. Determination of reproductive status was based on the condition of the ovaries, ovarioles, and accessory glands discerned from dissected adults13. Adults used for dissections were collected adjacent to the research locations.
Damage due to D. carinulata was visually rated as the percentage of Tamarix foliar tissue damaged and was measured in increments of 5%37. Feeding by adults or larvae causes affected foliage to turn brown, wither, and frequently die. Plants that were completely brown are considered to be 100% damaged, even if the withered foliage is still attached to the plant. Tamarix can recover from defoliation by D. carinulata and can even regrow foliage within the same season as the defoliation event. If regrowth of the Tamarix plants was observed, the percentage regrowth was visually estimated and the percentage was added as non-damaged green foliage.
Percent dieback and canopy volume were determined once during the field seasons of 2015 and 2016 for each treated plant. Dieback was recorded as the percentage of branches with live foliage the previous year that did not produce foliage in the current year. Evaluation of dieback was made well after Tamarix bud burst to allow enough time for new growth to occur. Branch color, flexibility and the presence or absence of foliage were used to categorize individual branches as dead or alive. Canopy volume was recorded in the fall, to account for possible regrowth and recovery of the Tamarix plants. Canopy volume was estimated using a formula for a cube. Two perpendicular measurements were taken at the plant’s widest points to estimate canopy length and width, and another measurement was taken at the tallest point to estimate canopy height.
Statistical analysis
Data from the two research sites were pooled for analysis. A repeated measure ANOVA, using within-year sampling date as the repeated measure, and SPLAT formulation applied (PH vs. BL) as the treatment factor, was used to evaluate for potential differences in adult D. carinulata capture densities. Data were ln(x + 1) transformed to better meet the assumptions of normality. Data on D. carinulata adults were segregated for analysis according to reproductive or non-reproductive status of the sampled cohort.
Damage rating was similarly assessed using a repeated measures ANOVA, with sampling day as the repeated measure and SPLAT application as the treatment factor. Potential changes in canopy volume were evaluated using a repeated measure ANOVA with year as the repeated measure and SPLAT application as the treatment factor. Canopy volume was ln(x + 1) transformed to better match the assumptions of normality. Repeated measure ANOVAs used type III error and Satterthwaite approximation for degrees of freedom. Percentage dieback was analyzed using a one-way ANOVA. ANOVAs were conducted using the lmer function in the lmerTest package of R software version 3.1.238.
Disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture (USDA). USDA is an equal opportunity provider and employer.
Data Availability
All data analyzed during this study is included in Supplementary Information files.
References
Kovalev, O. V. Co-evolution of the tamarisks (Tamaricaceae) and pest arthropods (Insecta; Arachnida: Acarina), with special reference to biological control prospects. Trudy Zoologicheskogo Instituta 259, 1–109 (1995).
Dudley, T. L., DeLoach, C. J., Levich, J. E. & Carruthers, R. I. Saltcedar invasion of western riparian areas: impacts and new prospects for control in Transactions of the 65th North American Wildlife & Natural Resources Conference (eds McCabe, R. E. & Loos, S. E) 345–381 (Wildlife Management Institute, 2000).
Gaskin, J. F. & Schaal, B. A. Hybrid Tamarix widespread in US invasion and undetected in native Asian range. Proc. Natl. Acad. Sci. USA 99, 11256–11259 (2002).
Friedman, J. M. et al. Dominance of non-native riparian trees in western USA. Biol Invasions 7, 747–751 (2005).
Duncan, C. A. et al. Assessing the economic, environmental, and societal losses from invasive plants on rangeland and wildlands. Weed Technology 18, 1411–1416 (2004).
Zavaleta, E. The economic value of controlling an invasive shrub. AMBIO: a Journal of the Human Environment 29, 462–467 (2000).
Kauffman, W. Program for Biological Control of Saltcedar (Tamarix spp) in Thirteen States. USDA-APHIS Environmental Assessment, https://www.aphis.usda.gov/plant_health/ea/downloads/salteafonsi.pdf (2005).
U.S. Department of Agriculture Animal and Plant Health Inspection Service. Field Release of a Nonindigenous Leaf Beetle, Diorhabda elongata (Coleoptera: Chrysomelidae), for Biological Control of Deciduous Saltcedar, Tamarix ramosissima and T. parviflora (Tamaraceae). Environmental Assessment (1999).
Bean, D. W., Wang, T., Bartelt, R. J. & Zilkowski, B. W. Diapause in the leaf beetle Diorhabda elongata (Coleoptera: Chrysomelidae), a biological control agent for tamarisk (Tamarix spp.). Environmental Entomology 36, 531–540 (2007).
Lewis, P. A., DeLoach, C. J., Knutson, A. E., Tracy, J. L. & Robbins, T. O. Biology of Diorhabda elongata deserticola (Coleoptera: Chrysomelidae), an Asian leaf beetle for biological control of saltcedars (Tamarix spp.) in the United States. Biol. Control 27, 101–116, https://doi.org/10.1016/s1049-9644(03)00002-1 (2003).
Bean D., Dudley T. & Hultine K. Bring on the beetles in Tamarix: A Case Study of Ecological Change in the American West (eds Sher, A. & Quigley, M.) 377–403 (Oxford University Press, 2013).
Cossé, A. A., Bartelt, R. J., Zilkowski, B. W., Bean, D. W. & Petroski, R. J. The aggregation pheromone of Diorhabda elongata, a biological control agent of saltcedar (Tamarix spp.): Identification of two behaviorally active components. Journal of Chemical Ecology 31, 657–670 (2005).
Bean, D. W., Dudley, T. L. & Keller, J. C. Seasonal timing of diapause induction limits the effective range of Diorhabda elongata deserticola (Coleoptera: Chrysomelidae) as a biological control agent for tamarisk (Tamarix spp.). Environmental Entomology 36, 15–25 (2007).
Dudley, T. L. & Kazmer, D. J. Field assessment of the risk posed by Diorhabda elongata, a biocontrol agent for control of saltcedar (Tamarix spp.), to a nontarget plant, Frankenia salina. Biological Control 35, 265–275 (2005).
Snyder, K. A., Uselman, S. M., Jones, T. J. & Duke, S. Ecophysiological responses of salt cedar (Tamarix spp. L.) to the northern tamarisk beetle (Diorhabda carinulata Desbrochers) in a controlled environment. Biological Invasions 12, 3795–3808 (2010).
Hudgeons, J. L. et al. Defoliation by introduced Diorhabda elongata leaf beetles (Coleoptera: Chrysomelidae) reduces carbohydrate reserves and regrowth of Tamarix (Tamaricaceae). Biological Control 43, 213–221 (2007).
Hultine, K. R. et al. Sap flux-scaled transpiration by tamarisk (Tamarix spp.) before, during and after episodic defoliation by the saltcedar leaf beetle (Diorhabda carinulata). Agricult. Forest Meteorol. 150, 1467–1475 (2010).
Dudley, T. L. & Bean, D. W. Tamarisk biocontrol, endangered species risk and resolution of conflict through riparian restoration. BioControl 57, 331–347 (2012).
Gaffke, A. M. et al. Semiochemicals to enhance herbivory by Diorhabda carinulata aggregations in saltcedar (Tamarix spp.) infestations. Pest Manag. Sci. 74, 1494–1503 (2018).
Cossé, A. A., Bartelt, R. J., Zilkowski, B. W., Bean, D. W. & Andress, E. R. Behaviorally active green leaf volatiles for monitoring the leaf beetle, Diorhabda elongata, a biocontrol agent of saltcedar, Tamarix spp. Journal of Chemical Ecology 32, 2695–2708 (2006).
Yoccoz, N. G., Nichols, J. D. & Boulinier, T. Monitoring of biological diversity in space and time. Trends Ecol. Evolut. 16, 446–453 (2001).
Mafra-Neto, A. et al. Manipulation of Insect Behavior with Specialized Pheromone and Lure Application Technology (SPLAT®). Pest Management with Natural Products 1141, 31–58 (2013).
Wood M., Flores A., McGinnis L. & Peabody E. Lassoing wicked weeds of the West. AgResearch Magazine, https://agresearchmag.ars.usda.gov/ar/archive/2007/jul/weeds0707.pdf (2007).
Dudley, T. L. & DeLoach, C. J. Saltcedar (Tamarix spp.), endangered species, and biological weed control - Can they Mix? Weed Technology 18, 1542–1551 (2004).
Kennard, D. et al. Tamarix dieback and vegetation patterns following release of the northern tamarisk beetle (Diorhabda carinulata) in western Colorado. Biological Control 101, 114–122 (2016).
Shafroth, P. B. et al. Riparian restoration in the context of Tamarix control in Tamarix: A Case Study of Ecological Change in the American West (eds Sher, A. & Quigley, M.) 404–425 (Oxford University Press, 2013).
Fenner, P., Brady, W. W. & Patton, D. R. Observations on seeds and seedlings of Fremont cottonwood. Desert Plants 6, 55–58 (1984).
Sher, A. A., Marshall, D. L. & Taylor, J. P. Establishment patterns of native Populus and Salix in the presence of invasive nonnative. Tamarix. Ecol. Appl. 12, 760–772 (2002).
Warren, D. K. & Turner, R. M. Saltcedar Tamarix chinensis seed production seedling establishment and response to inundation. Journal of the Arizona Academy of Science 10, 135–144 (1975).
Salinas, M. J., Blanca, G. & Romero, A. T. Riparian vegetation and water chemistry in a basin under semiarid Mediterranean climate, Andarax River, Spain. Environmental Management 26, 539–552 (2000).
Busch, D. E. Effects of fire on southwestern riparian plant community structure. Southwestern Naturalist 40, 259–267 (1995).
Britton, K. O., White, P., Kramer, A. & Hudler, G. A new approach to stopping the spread of invasive insects and pathogens: early detection and rapid response via a global network of sentinel plantings. New Zealand Journal of Forestry Science 40, 109–114 (2010).
DeLoach, C. J. et al. Host specificity of the leaf beetle, Diorhabda elongata deserticola (Coleoptera: Chrysomelidae) from Asia, a biological control agent for saltcedars (Tamarix: Tamaricaceae) in the Western United States. Biological Control 27, 117–147 (2003).
Petroski, R. J. Straightforward preparation of (2E,4Z)-2,4-heptadien-1-ol and (2E,4Z)-2,4-heptadienal. Synthetic Communications 33, 3233–3241 (2003).
Ladenburger, C. G., Hild, A. L., Kazmer, D. J. & Munn, L. C. Soil salinity patterns in Tamarix invasions in the Bighorn Basin, Wyoming, USA. Journal of Arid Environments 65, 111–128 (2006).
Jamison, L. & Lanci, J. Tamarisk Leaf Beetle Monitoring Protocol. Colorado Department of Agriculture, Tamarisk Coalition, and University of California-Santa Barbara, https://riversedgewest.org/sites/default/files/files/2012_Tamarisk_Leaf_Beetle_Monitoring_Protocol_07_01_2014.pdf (2012).
Tamarisk Coalition. Tamarisk Monitoring Protocol. Colorado Department of Agriculture, https://riversedgewest.org/sites/default/files/files/Tamarisk_Impact_Monitoring_Protocol_2013_Palisade_Insectary.pdf (2013).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software 82, 1–26 (2017).
Acknowledgements
This work was supported by agreement 11-CA-11420004-054 and 16-CA-11420004-047 of the Forest Health Technology and Enterprise Team- Biological Control of Invasive Native and Non-Native Plants (FHTET-BCIP). We thank Jon Rico, Rodrigo Silva and Carmen Bernardi for formulating the aggregation pheromone into SPLAT. We thank Megan Hofland and Adam Cook for assistance. We also thank the Bighorn National Recreation Area (BICA-2014-SCI-0002) for providing research locations.
Author information
Authors and Affiliations
Contributions
S.S., T.D., D.B., R.P. and D.W. developed experimental objectives, and provided input on experimental design, implementation, data analysis, and manuscript preparation. J.R. developed methodologies for chemical synthesis and provided the pheromone. A.M. provided input on the formulation of the pheromone for field deployment. A.G. designed and implemented experiments, collected, processed and analyzed data, and prepared the manuscript for publication.
Corresponding author
Ethics declarations
Competing Interests
T.D. and J.R. are sole owners of Restoration Science LLC, which developed and provided the pheromone compound, as used in this research. This product is not currently commercially available. A.M. is the CEO of ISCA Technologies, the company that formulated the pheromone in their proprietary carrier matrix, SPLAT, for this research. This product formulation is not currently commercially available. A.G., S.S., D.B., R.P. and D.W. declare they have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gaffke, A.M., Sing, S.E., Dudley, T.L. et al. Field demonstration of a semiochemical treatment that enhances Diorhabda carinulata biological control of Tamarix spp.. Sci Rep 9, 13051 (2019). https://doi.org/10.1038/s41598-019-49459-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49459-5
This article is cited by
-
Attraction of the Air Potato Leaf Beetle, Lilioceris Cheni, (Coleoptera: Chrysomelidae) to leaf Volatiles of the Air Potato, Dioscorea bulbifera
Journal of Chemical Ecology (2023)
-
Salinity driven interactions between plant growth and a biological control agent
Biological Invasions (2021)
-
Establishing Diorhabda carinulata: Impact of Release Disturbances on Pheromone Emission and Influence of Pheromone Lures on Establishment
Journal of Chemical Ecology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.