Abstract
Heterogenous data about the prognostic impact of atrial fibrillation (AF) in patients with ventricular tachyarrhythmias exist. Therefore, this study evaluates this impact of AF in patients presenting with ventricular tachyarrhythmias. 1,993 consecutive patients presenting with ventricular tachyarrhythmias (i.e. ventricular tachycardia and fibrillation (VT, VF)) on admission at one institution were included (from 2002 until 2016). All medical data of index and follow-up hospitalizations were collected during the complete follow-up period for each patient. Statistics comprised univariable Kaplan-Meier and multivariable Cox regression analyses in the unmatched consecutive cohort and after propensity-score matching for harmonization. The primary prognostic endpoint was long-term all-cause mortality at 2.5 years. AF was present in 31% of patients presenting with index ventricular tachyarrhythmias on admission (70% paroxysmal, 9% persistent, 21% permanent). VT was more common (67% versus 59%; p = 0.001) than VF (33% versus 41%; p = 0.001) in AF compared to non-AF patients. Long-term all-cause mortality at 2.5 years occurred more often in AF compared to non-AF patients (mortality rates 40% versus 24%, log rank p = 0.001; HR = 1.825; 95% CI 1.548–2.153; p = 0.001), which may be attributed to higher rates of all-cause mortality at 30 days, in-hospital mortality and mortality after discharge (p < 0.05) (secondary endpoints). Mortality differences were observed irrespective of index ventricular tachyarrhythmia (VT or VF), LV dysfunction or presence of an ICD. In conclusion, this study identifies AF as an independent predictor of death in patients presenting consecutively with ventricular tachyarrhythmias.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) represents the most common arrhythmia worldwide, since about 33.5 million individuals were estimated to suffer from AF in 20101. AF is associated with increased comorbidity, such as stroke or heart failure, a 2-fold increase of mortality in both men and women and around 3% in anticoagulated AF patients1,2. In minor part, death may rarely be attributed to stroke, whereas death from progressive heart failure and sudden cardiac death (SCD) is more common in AF patients2.
SCD and ventricular tachyarrhythmias are predominantly seen in the presence of coronary artery disease (CAD) and acute myocardial infarction (AMI). Accordingly, about half of cardiac deaths after AMI are related to ventricular tachyarrhythmias, such as ventricular tachycardia (VT) or fibrillation (VF) and consecutive SCD3,4,5,6. Accumulating evidence suggests a mechanistic link in between AF and ventricular tachyarrhythmias, which may be explained by reduced ventricular refractoriness and pro-arrhythmic short-long-short sequences preceding the onset of ventricular tachyarrhythmias in the presence of AF rather than in sinus rhythm7.
According to the literature, several community-based studies demonstrated a higher incidence of future SCD in AF patients at long-term follow-up7,8,9,10. A sub-analysis of the Engage AF-TIMI 48 trial showed a rate of SCD estimated at 45% of cardiovascular deaths in pre-selected AF patients being investigated initially for the effectiveness of edoxaban compared to warfarin for stroke prevention9. Furthermore, the Oregon-SUD study found a higher rate of AF related to SCD in 652 SCD patients compared to age- and sex- matched CAD controls11. Notably the increasing SCD risk was no longer attributed to AF in the presence of heart failure11,12.
However, no data is currently available, whether the presence of AF may be associated independently with mortality in consecutive real-life patients presenting on admission with ventricular tachyarrhythmias. Therefore, this study evaluates the differences of short- and long-term survival of patients surviving ventricular tachyarrhythmias on admission depending on the presence or absence of AF.
Methods
Study patients
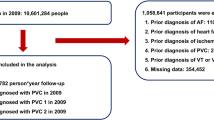
The present study is derived from the “Registry of malignant arrhythmias and sudden cardiac death” (RACE-IT), which included retrospectively all patients presenting with at least one episode of ventricular tachyarrhythmias and sudden cardiac arrest between 2002 and 2016 at one institution, as recently been outlined13. Ventricular tachyarrhythmias included ventricular tachycardia (VT) and fibrillation (VF) as defined by current European guidelines14. Sustained VT was defined by duration of more than 30 seconds or by causing hemodynamic collapse. Non-sustained VT was defined by duration of less than 30 seconds both with wide QRS complex (≥120 milliseconds) at a rate greater than 100 beats per minute14. Ventricular tachyarrhythmias were documented by 12-lead electrocardiogram (ECG), ECG tele- monitoring, ICD or by external defibrillator monitoring. Documented VF was treated by external defibrillation and in case of prolonged instability with additional intravenous anti-arrhythmic drugs during cardiopulmonary resuscitation (CPR).
This study is based on a retrospective data analysis/registry and has been approved by the local ethics commission II of the faculty of Medicine Mannheim, University of Heidelberg, where no informed consent was deemed necessary for this study (ethical approval number 2016612NMA) (ClinicalTrials.gov identifier: NCT02982473). All methods were carried out in accordance with relevant guidelines and regulations.
Definition of study groups, inclusion and exclusion criteria
For the present analysis only patients presenting with and surviving ventricular tachyarrhythmias at index hospital stay were included. Risk-stratification was performed according to the presence of AF according to European guidelines2. Documentation of AF was derived from ECG recording on admission and medical history being documented within the electronic hospital information system. Paroxysmal AF was defined as self-terminating in most cases within 48 hours and lately for up to 7 days, including AF episodes that are cardioverted within 7 days. Persistent AF lasts longer than 7 days including episodes terminated by cardioversion either by drugs or by direct current cardioversion after 7 days or more. Permanent AF was defined as accepted by the patient and physician without pursuing further rhythm control.
Patients with early cardiac death defined as occurring <24 hours after onset of ventricular tachyarrhythmias or an assumed unstable cardiac condition on index admission were excluded from the present study14. Each patient was counted only once for inclusion when presenting with the first episode of ventricular tachyarrhythmias.
Study endpoints
The primary endpoint was defined as long-term all-cause mortality at 2.5 years of follow-up. Secondary endpoints were all-cause mortality at 30-days, in-hospital death at index and all-cause mortality of surviving patients of index hospitalization (i.e. after discharge). Risk stratification was performed within subgroups of VT, VT, and AF subtypes, LV dysfunction and overall implantable cardioverter defibrillators (ICD).
All-cause mortality was documented using our electronic hospital information system and by directly contacting state resident registration offices (“bureau of mortality statistics”) across Germany. Identification of patients was verified by place of name, surname, day of birth and registered living address. In 48 patients, no data on patients’ survival could have been retrieved, as those patients were even not reachable by telephone, and therefore these patients were excluded from final analyses (corresponding lost to follow-up rate of 1.7%).
Statistical methods
The following analyses were applied stepwise to evaluate the prognostic impact of predefined variables for all-cause mortality: Firstly, within the entire cohort, uni-variable stratification was performed using the Kaplan-Meier method with comparisons between groups using uni-variable hazard ratios (HR) given together with 95% confidence intervals. Secondly, propensity score analyses were performed, since this study includes consecutively all patients with ventricular tachyarrhythmias without randomization15,16. Accordingly, a propensity score (probability for belonging to AF = yes) was calculated for each individual based on the same predefined variables (see below). Afterwards, matched pairs were created using the method of nearest neighbor matching with a caliper distance of 5%. This means: each pair consisted of one individual with AF = yes and one individual with AF = no, respectively, whose propensity scores differed by less than 5%. We found 496 pairs with mean propensity score 0.3722 +/− 0.1412 (AF = 0) and 0.4134 +/− 0.1379 (AF = 1). Uni-variable stratification was re-calculated according to Kaplan-Meier methods for each above-said subgroup within the propensity-matched cohort. Thirdly, multivariable Cox regression models were developed using the “forward selection” option, where only statistically significant variables (p < 0.05) were included and analyzed simultaneously. Multivariable Cox regressions were applied within the entire and propensity-matched cohorts.
Predefined variables being used for propensity score matching (step 2) and multivariable Cox-regressions (step 3) included: baseline parameters (age, gender), chronic diseases (diabetes, chronic kidney disease (glomerular filtration rate < 90 mL/min/1.73 m2)), acute comorbidities (acute myocardial infarction, ST segment elevation myocardical infarction (STEMI), Non ST segment elevation myocardical infarction (NSTEMI), left ventricular ejection fraction (LVEF) <35%, cardiogenic shock, cardiopulmonary resuscitation (CPR), presence of an ICD, presence of a shockable rhythm (i.e. VT/VF) at index, and presence of AF.
Long-term follow-up period for evaluation of the primary endpoint was set at the median survival of AF patients to guarantee complete survival of at least 50% of affected patients. Patients not meeting long-term follow-up were censored.
The result of a statistical test was considered as a statistical trend for p < 0.1 and significant for p < 0.05 and SAS, release 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistics.
Quantitative data are given as mean ± standard error of mean (SEM), median and interquartile range (IQR), and ranges depending on the distribution of the data and were compared using the Student’s t test for normally distributed data or the Mann-Whitney U test for nonparametric data. Deviations from a Gaussian distribution were tested by the Kolmogorov-Smirnov test. Spearman’s rank correlation for nonparametric data was used to test univariate correlations. Qualitative data are presented as absolute and relative frequencies and compared using the Chi² test or the Fisher’s exact test, as appropriate. Additionally, standardized mean differences (d) were applied in addition to p values for the comparisons of patient’s characteristics between females and males. (d) was assessed calculated with a logit model. Values of (d) < 0.2 were defined similarity between groups, whereas (d) > 0.2 defined relevant differences in patients’ characteristics between AF and non-AF patients17.
Results
Entire unmatched study cohort
Within the unmatched study population of 1,993 consecutive patients presenting with ventricular tachyarrhythmias on admission at our institution, a history of AF was present in 31% of patients. Most patients suffered from paroxysmal (70%), followed by permanent (21%) and persistent AF (9%). The rate of VT was significantly higher in AF patients (67% versus 59%; p = 0.001), whereas VF was more common in non-AF patients (41% versus 33%; p = 0.001) (Table 1, left columns). AF patients had a higher cardiovascular risk profile and a higher rate of prior heart failure, CAD, valvular heart disease, ICD, chronic kidney disease and obstructive pulmonary disease. At index presentation, rates of acute myocardial infarction, cardiogenic shock, non-ischemic cardiomyopathy, CAD and CPR were equally distributed in both groups. Non-AF patients underwent PCI more often at index mostly at the LAD. AF patients presented with higher rates of LVEF <35% alongside higher rates of overall ICD, beta-blockers, digitalis, amiodarone and anticoagulant therapies (Table 1, left columns).
Prognosis of AF and non-AF patients
The overall median long-term follow-up time was 3.8 years (IQR 257 days–7.6 years), whereas median survival time in AF-patients was 2.5 years (IQR 96 days–5.5 years) compared to a longer median of 4.7 years (IQR 1.2 years–8.3 years) in non-AF patients. The 2.5 years survival period derived from the affected AF patients was used for all outcome analyses.
AF patients presenting with ventricular tachyarrhythmias were associated with a higher rate of the primary endpoint of long-term all-cause mortality at 2.5 years compared to non-AF patients (40% versus 24%; log-rank p = 0.001; HR = 1.825; 95% CI 1.548–2.153; p = 0.001; Fig. 1, first panel; Table 2 left columns). Increasing rates of all-cause mortality in AF patients were already observed for secondary endpoints at 30 days (17% versus 12%; HR = 1.467; 95% CI 1.146–1.878; p = 0.002), at index hospitalization (19% versus 13%; HR = 1.467; 95% CI 1.146–1.878; p = 0.002), and in patients surviving index hospitalization (21% versus 12%; HR = 1.467; 95% CI 1.146–1.878; p = 0.002) (Table 2 left columns).
Increased mortality in patients with AF was observed also in VT (mortality rate 36% versus 18%; log rank p = 0.001; HR = 2.283; 95% CI 1.817–2.869; p = 0.001) (Fig. 1, second panel), and also in VF patients only (mortality rate 49% versus 33%; log rank p = 0.001; HR = 1.572; 95% CI 1.230–2.009; p = 0.001) (Fig. 1, third panel). Amongst AF patients, the presence of VF was associated with higher rates of the primary endpoint compared to VT (mortality rate 49% versus 36%; log rank p = 0.001; HR = 1.587; 95% CI 1.229–2.048; p = 0.001) (Fig. 1, fourth panel).
Additionally, long-term all-cause mortality was significantly higher in AF patients irrespective of VT or VF in the presence of both LVEF ≥35% or <35% (p < 0.002) (Fig. 2A (VT patients) & B (VF patients), left panel: LVEF ≥35%; right panel: LVEF <35%). AF patients were still associated with a higher rate of long-term mortality in the presence of an ICD both within VT and VF patients (p = 0.001) (Fig. 3, left/middle/right panels). Patients with permanent AF revealed a higher rate of the primary endpoint compared to persistent AF patients (48% versus 31%, log-rank p = 0.036) (Fig. 4).
In multivariable Cox regression models, AF patients presenting with ventricular tachyarrhythmias were at 1.3 times higher risk of the primary endpoint of long-term all-cause mortality at 2.5 years (HR 1.314; 95% CI 1.070–1.613; p = 0.009), besides age (HR 1.030), male gender (HR 1.597), diabetes (HR 1.321), STEMI (HR 0.542), chronic kidney disease (HR 2.112), LVEF <35% (HR 2.171), cardiogenic shock (HR 1.937), and CPR (HR 1.380) (Fig. 5).
Matched study cohort
Since the present study includes consecutively patients presenting with ventricular tachyarrhythmias on admission some confounding might be present due to the heterogeneous comorbidities, which emphasize the real-life character of the present study population. Therefore, propensity score matching was performed for harmonization revealing similar baseline characteristics in each group (AF vs. non-AF each n = 496) except for minor differences in age, AF types, prior AMI, valvular heart disease, prior but not overall ICD, CAD, digitalis, amiodarone and anticoagulant therapies (Table 1, left columns).
Notably after propensity score matching, AF patients were still associated with increasing rates of the primary prognostic endpoint of long-term all-cause mortality at 2.5 years (long-term mortality rate 37% versus 28%, log rank p = 0.036; HR = 1.440; 95% CI 1.155–1.794; p = 0.001; Fig. 6; Table 2 right columns). Statistical trends were still observed for all predefined secondary endpoints even after propensity score matching (Table 2 right columns).
Discussion
This real-world data suggests that a history of AF may be associated with an increased risk of long-term all-cause mortality in patients presenting with ventricular tachyarrhythmias. Mortality differences were already seen at 30 days, at index hospitalization and in patients surviving index hospitalization. They were irrespective of the presence of VT, VF, LV dysfunction and presence of an ICD. The prognostic disadvantage of AF was proven irrespective of LV dysfunction or presence of an ICD and was comparable to established risk factors such as cardiogenic shock, LVEF <35%, CPR, chronic kidney disease, diabetes and age, as well as after propensity-score matching.
This study consistently identifies the presence of AF as a robust predictor of adverse prognosis in patients presenting with ventricular tachyarrhythmias. On the one hand, AF patients are still endangered by an annual stroke rate of 1.5% impairing individual qualities of life due to varying physical disability. On the other hand, annual death rates of AF patients are estimated even higher (>3%) and are usually attributed to incident heart failure and SCD2. However, whether the presence of incident episodes of ventricular tachyarrhythmias in AF patients may impact prognosis has rarely been investigated. Most studies reported about higher rates of SCD or ventricular tachyarrhythmias in AF patients either in preselected ICD or population-based cohorts2.
The presence of AF has recently been shown to affect both clinically overt and subclinical alterations of ventricular structure and function. Potential pathologic mechanisms consist of the development of increasing ventricular rate, microvascular or endothelial dysfunction, systemic inflammation leading to consecutive impaired myocardial perfusion and heterogenous atrioventricular conduction18. In effect, increasing AF burden may be associated with impaired bidirectional atrio-ventricular supply-demand ischemia and progressive fibrosis sustaining adverse cardiac remodelling18,19. Beyond, atrial fibrillation may induce short-long-short sequences, premature ventricular complexes due to RR irregularity, QTc prolongation (>440 ms) and wide QRS (>130 ms) reflecting arrhythmogenic substrates for an increased vulnerability to ventricular tachyarrhythmias such as VT or VF during AF20,21,22,23,24,25.
Clinical studies showed evidence that the presence of AF was associated with increased rates of ventricular tachyarrhythmias and appropriate ICD shocks in ICD in the presence of LVEF <35%26. AF plus ventricular tachyarrhythmias may be detected simultaneously in about 8.9% of all detected ventricular tachyarrhythmias27. Presence of persistent AF was associated with progressive heart failure (LVEF <35%). Large-scaled population-based studies demonstrated a higher incidence of SCD at long-term follow-up in AF patients28,29. Furthermore, virtually all risk prediction scores for primary preventive ICD therapy found AF to be a strong risk factor in patients with LVEF <35%30,31. In contrast the adverse prognostic impact of AF was demonstrated in patients presenting with ventricular tachyarrhythmias even in the presence of LVEF >35%, as demonstrated in the present study.
The present results may evoke implications of how to assess patients with evidence of ventricular tachyarrhythmias and a history of AF. Risk stratification needs to be improved incorporating strategies to reduce the arrhythmogenic substrate both with respect to the AF burden and for ventricular tachyarrhythmias. This may in turn translate into a potential prognostic benefit for this specific subset of patients. Recent randomized controlled trials evaluating the impact of pulmonary vein isolation of AF demonstrated an improvement of quality of life and decrease of treatment-failure compared to optimal medical therapy32,33,34,35. Notably a prognostic benefit related to pulmonary vein isolation has only been demonstrated in patients with AF and concomitant heart failure with LVEF <35% with regard to lower rates of death from any cause or hospitalization for worsening heart failure36. The major finding of the present study showing that a history AF in patients presenting with ventricular tachyarrhythmias may negatively impact long-term prognosis reveals the urgent need for future prospective randomized controlled trials evaluating the prognostic impact of the following medical therapies in this sub-set of high-risk patients: (1) pulmonary vein isolation, (2) electrophysiological testing by exact electroanatomic mapping and consecutive VT ablation, (3) supply with an ICD, (4) the reduction of the ischemic burden by PCI of critical CAD and (5) a more aggressive therapy by antiarrhythmic drugs of class II or III. Until now, no clear recommendation can be given for these patients in daily routine care, unless these issues will not be evaluated as outlined.
Finally, the presence of AF needs to be accounted as another prognostic risk factor in patients with ventricular tachyarrhythmias. Classic risk factors such as LVEF <35% may reveal limited information, for instance in secondary preventive ICD recipients37, and since half of future SCD patients reveal a preserved ejection fraction irrespective of ischemic or non-ischemic origin38,39. Therefore the spectrum of potential risk factors for SCD has been extended towards myocardial scarring, reversible LV dysfunction, genetic determinants, ECG patterns and late gadolinium enhancement assessed by cardiac magnetic resonance imaging37. Except for VF patients with LVEF <35% reflecting progredient stages of heart failure, the presence of AF may sustain as another arrhythmic prognostic risk factor in patients presenting with ventricular tachyarrhythmias.
Study limitations
This study has several limitations related to its study design as a registry-based, single-centre, retrospective and observational analysis. Therefore, the results of this study are at the most hypothesis-generating. However, performing a prospective study with adequate power and randomization of AF and non-AF patients is hard to investigate in future. In order to minimize potential selection bias retrospectively, propensity score matching was applied. Even thereafter some cofounding due to slight differences of age and further unmeasured variables in both groups, e.g. factors related to the out-of-hospital setting, may still be present. Therefore, a stricter matching was performed (calibre distance of 0.001) without further differences of age and similar results. Mode of death was not documented mostly by registration offices. An independent clinical event committee was not applied, since all clinical data was documented reliably by individual cardiologists during routine clinical care being blinded to final data analyses. The additional prognostic impact of pulmonary vein isolation in AF patients was beyond the scope of the present study and needs to be evaluated in further prospective RCT. Whether AF itself is the cause or merely a symptom of underlying combination of disease processes in patients with ventricular tachyarrhythmias may not be drawn from the present results.
Conclusions
AF is associated with increased secondary mortality in patients presenting with ventricular tachyarrhythmias.
References
Chugh, S. S. et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129, 837–847, https://doi.org/10.1161/circulationaha.113.005119 (2014).
Kirchhof, P. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37, 2893–2962, https://doi.org/10.1093/eurheartj/ehw210 (2016).
Anyfantakis, Z. A. et al. Acute coronary angiographic findings in survivors of out-of-hospital cardiac arrest. Am Heart J 157, 312–318, https://doi.org/10.1016/j.ahj.2008.09.016 (2009).
Yousuf, O., Chrispin, J., Tomaselli, G. F. & Berger, R. D. Clinical management and prevention of sudden cardiac death. Circ Res 116, 2020–2040, https://doi.org/10.1161/CIRCRESAHA.116.304555 (2015).
Zheng, Z. J., Croft, J. B., Giles, W. H. & Mensah, G. A. Sudden cardiac death in the United States, 1989 to 1998. Circulation 104, 2158–2163 (2001).
Neumar, R. W. et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 118, 2452–2483, https://doi.org/10.1161/CIRCULATIONAHA.108.190652 (2008).
Chen, L. Y., Benditt, D. G. & Alonso, A. Atrial fibrillation and its association with sudden cardiac death. Circulation journal: official journal of the Japanese Circulation Society 78, 2588–2593 (2014).
Chen, L. Y. et al. Atrial fibrillation and the risk of sudden cardiac death: the atherosclerosis risk in communities study and cardiovascular health study. JAMA Intern Med 173, 29–35, https://doi.org/10.1001/2013.jamainternmed.744 (2013).
Eisen, A. et al. Sudden Cardiac Death in Patients With Atrial Fibrillation: Insights From the ENGAGE AF-TIMI 48 Trial. J Am Heart Assoc 5, https://doi.org/10.1161/JAHA.116.003735 (2016).
Koene, R. J. et al. Predictors of sudden cardiac death in atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. PLoS One 12, e0187659, https://doi.org/10.1371/journal.pone.0187659 (2017).
Reinier, K. et al. The association between atrial fibrillation and sudden cardiac death: the relevance of heart failure. JACC. Heart failure 2, 221–227, https://doi.org/10.1016/j.jchf.2013.12.006 (2014).
Bardai, A. et al. Atrial fibrillation is an independent risk factor for ventricular fibrillation: a large-scale population-based case-control study. Circ Arrhythm Electrophysiol 7, 1033–1039, https://doi.org/10.1161/CIRCEP.114.002094 (2014).
Behnes, M. et al. Coronary Chronic Total Occlusions Represent an Independent Predictor of Mortality in Ventricular Tachyarrhythmias. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology, https://doi.org/10.4244/eij-d-18-00496 (2019).
Priori, S. G. et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 36, 2793–2867, https://doi.org/10.1093/eurheartj/ehv316 (2015).
Austin, P. C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 46, 399–424, https://doi.org/10.1080/00273171.2011.568786 (2011).
Ferdinand, D., Otto, M. & Weiss, C. Get the most from your data: a propensity score model comparison on real-life data. Int J Gen Med 9, 123–131, https://doi.org/10.2147/IJGM.S104313 (2016).
Austin, P. C. Assessing covariate balance when using the generalized propensity score with quantitative or continuous exposures. Statistical methods in medical research, 962280218756159, https://doi.org/10.1177/0962280218756159 (2018).
Wijesurendra, R. S. & Casadei, B. Atrial fibrillation: effects beyond the atrium? Cardiovasc Res 105, 238–247, https://doi.org/10.1093/cvr/cvv001 (2015).
Behnes, M. et al. Transforming growth factor beta 1 (TGF-beta 1) in atrial fibrillation and acute congestive heart failure. Clin Res Cardiol 100, 335–342, https://doi.org/10.1007/s00392-010-0248-1 (2011).
Rashba, E. J. & Estes Iii, N. A. Is there a mechanistic link between atrial fibrillation and vulnerability to ventricular arrhythmias? J Cardiovasc Electrophysiol 22, 1253–1255, https://doi.org/10.1111/j.1540-8167.2011.02135.x (2011).
Akoum, N. W., Sanders, N. A., Wasmund, S. L. & Hamdan, M. H. Irregular ventricular activation results in QT prolongation and increased QT dispersion: a new insight into the mechanism of AF-induced ventricular arrhythmogenesis. J Cardiovasc Electrophysiol 22, 1249–1252, https://doi.org/10.1111/j.1540-8167.2011.02110.x (2011).
Borleffs, C. J. et al. Prognostic importance of atrial fibrillation in implantable cardioverter-defibrillator patients. J Am Coll Cardiol 55, 879–885, https://doi.org/10.1016/j.jacc.2009.09.053 (2010).
Chishaki, A. S., Li, F. J., Takeshita, A. & Chishaki, H. Different features of ventricular arrhythmias and the RR-interval dynamics in atrial fibrillation related to the patient’s clinical characteristics: an analysis using RR-interval plotting. J Electrocardiol 37, 207–217 (2004).
Keefe, D. L., Schwartz, J. & Somberg, J. C. The substrate and the trigger: the role of myocardial vulnerability in sudden cardiac death. Am Heart J 113, 218–225 (1987).
Somberg, J. C. et al. Enhancement of myocardial vulnerability by atrial fibrillation. Am J Ther 11, 33–43 (2004).
Lemola, K. et al. Ventricular proarrhythmic effects of atrial fibrillation are modulated by depolarization and repolarization anomalies in patients with left ventricular dysfunction. Pacing and clinical electrophysiology: PACE 32, 99–105, https://doi.org/10.1111/j.1540-8159.2009.02182.x (2009).
Stein, K. M. et al. Do atrial tachyarrhythmias beget ventricular tachyarrhythmias in defibrillator recipients? J Am Coll Cardiol 40, 335–340 (2002).
Naser, N. et al. The Cumulative Incidence of Stroke, Myocardial infarction, Heart Failure and Sudden Cardiac Death in Patients with Atrial Fibrillation. Med Arch 71, 316–319, https://doi.org/10.5455/medarh.2017.71.316-319 (2017).
Murakoshi, N. & Aonuma, K. Epidemiology of arrhythmias and sudden cardiac death in Asia. Circulation journal: official journal of the Japanese Circulation Society 77, 2419–2431 (2013).
Barsheshet, A. et al. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 59, 2075–2079, https://doi.org/10.1016/j.jacc.2012.02.036 (2012).
Goldenberg, I. et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 51, 288–296, https://doi.org/10.1016/j.jacc.2007.08.058 (2008).
Blomstrom-Lundqvist, C. et al. Effect of Catheter Ablation vs Antiarrhythmic Medication on Quality of Life in Patients With Atrial Fibrillation: The CAPTAF Randomized Clinical Trial. JAMA 321, 1059–1068, https://doi.org/10.1001/jama.2019.0335 (2019).
Mark, D. B. et al. Effect of Catheter Ablation vs Medical Therapy on Quality of Life Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA, https://doi.org/10.1001/jama.2019.0692 (2019).
Packer, D. L. et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA, https://doi.org/10.1001/jama.2019.0693 (2019).
Wilber, D. J. et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 303, 333–340, https://doi.org/10.1001/jama.2009.2029 (2010).
Marrouche, N. F. et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med 378, 417–427, https://doi.org/10.1056/NEJMoa1707855 (2018).
Zipse, M. M. & Tzou, W. S. Sudden cardiac death in nonischemic cardiomyopathy: Refining risk assessment. J Cardiovasc Electrophysiol 28, 1361–1366, https://doi.org/10.1111/jce.13284 (2017).
Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37, 2129–2200, https://doi.org/10.1093/eurheartj/ehw128 (2016).
Wellens, H. J. et al. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 35, 1642–1651, https://doi.org/10.1093/eurheartj/ehu176 (2014).
Author information
Authors and Affiliations
Contributions
M.Be.: substantially contributed to the conception and design of the work, data acquisition and analysis as well as interpretation of data for the work and drafted the work and revisited for critically important intellectual content. J.R., G.T., T.S., L.R., A.B., T.R., D.E., N.E., P.K., I.E.-B., S.L., C.N., K.M., M.A., T.B., D.F., C.W. and M.Bo.: substantially contributed to data acquisition and analysis as well as interpretation of data for the work and revisited for critically important intellectual content. I.A.: conceived the study, substantially contributed to the conception and design of the work, data acquisition and analysis as well as interpretation of data for the work and drafted the work and revisited for critically important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Behnes, M., Rusnak, J., Taton, G. et al. Atrial Fibrillation Is Associated with Increased Mortality in Patients Presenting with Ventricular Tachyarrhythmias. Sci Rep 9, 14291 (2019). https://doi.org/10.1038/s41598-019-49325-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49325-4
This article is cited by
-
Racial disparities in ventricular tachycardia in young adults: analysis of national trends
Journal of Interventional Cardiac Electrophysiology (2022)
-
Atrial fibrillation is associated with increased risk of lethal ventricular arrhythmias
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.