Abstract
Diachasmimorpha longicaudata (Ashmead, 1905) (Hymenoptera: Braconidae) is considered one of the main biological control agents of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). However, the application of toxic baits for the management of C. capitata might exert side effects on the parasitoid. The objective of this study was to evaluate the side effects of toxic bait formulations on D. longicaudata. The food attractants Anamed, 3% Biofruit, 1.5% CeraTrap, 1.25% Flyral, 3% Isca Samaritá, 3% Isca Samaritá Tradicional, and 7% sugarcane molasses mixed with an organophosphate insecticide [malathion, 2.0 grams of active ingredient (g a.i.) L−1] and the commercial formulation Gelsura (2.0 and 4.0 g a.i. L−1 alpha-cypermethrin) showed high toxicity to D. longicaudata adults (>90% mortality) after 96 h and were thus classified as harmful (Class 4). Similarly, 3% Isca Samaritá Tradicional and 7% sugarcane molasses in formulations with the insecticides spinosad and spinetoram (0.096 g a.i. L−1 or kg) were moderately harmful (Class 3). In contrast, the food attractants Anamed, 3% Biofruit, 1.5% CeraTrap, 1.25% Flyral, and 3% Isca Samaritá Tradicional in combination with spinosad and spinetoram and the formulation Success 0.02CB (0.096 g a.i. L−1 spinosad) were classified as harmless (<10% mortality up to 96 h, Class 1). Additionally, these formulations did not reduce the parasitism and emergence rate of the F1 generation of D. longicaudata in C. capitata larvae. Formulations of toxic baits based on spinosyn are suitable for the management of C. capitata together with the parasitoid D. longicaudata.
Similar content being viewed by others
Introduction
The Mediterranean fruit fly, Ceratitis capitata (Wiedemann, 1824) (Diptera: Tephritidae), is a polyphagous and cosmopolitan species with a high capacity for infesting and damaging 361 host species belonging to 63 botanical families worldwide1. C. capitata has significant fruit-damaging potential because females of this species lay their eggs in fruit and the larvae subsequently open galleries in these fruits. The use of toxic baits has become one of the main strategies for the management of species populations worldwide2,3,4,5. The ingestion of toxic baits containing a lethal agent (insecticide molecule) mixed with a food attractant by adult Tephritidae causes mortality6.
Due to their broad spectrum and rapid action on fruit flies, organophosphate insecticides were once the most common insecticides in formulations of toxic baits2,3. However, because the use of broad-spectrum insecticides such as organophosphates needs to be avoided3,6, products derived from the fermentation of the bacterium Saccharopolyspora spinosa (Mertz and Yao) for the development of spinosyn insecticides (spinosad and spinetoram)7 are viable alternatives to organophosphates in toxic bait formulations2,6,7,8,9,10,11,12.
In Brazil, spinosyn-based insecticides are available in a concentrated suspension (Tracer 480 SC, spinosad), as a wettable powder (Spindle, spinosad), and as water-dispersible granules (Delegate 250 WG, spinetoram). Both insecticides are used in the formulation of toxic baits as an admixture with a protein and sugar-based food attractant5. A ready-to-use formulation (Success 0.02CB) was recently introduced in the Brazilian market. This formulation contains spinosad as the lethal agent, is internationally known as GF-120 NF13 and is registered for the management of C. capitata in mango crop.
Despite its low toxicity to mammals and fish, the insecticide spinosad can exert deleterious effects on the parasitism and longevity of different species of parasitoids after its ingestion3,14,15 and also exerts effects on the demographic traits of populations of natural enemies of parasitoids16.
The larval-pupal endoparasitoid Diachasmimorpha longicaudata (Ashmead, 1905) (Hymenoptera: Braconidae) has excellent potential for use in management programs for C. capitata in Brazil17. Factors linked to high parasitism and host-seeking capacity characterize the species as promising for large-scale multiplication and inundative releases in the field, and these strategies have been used in the United States, Guatemala, and Mexico18,19. Another important aspect is the biology of D. longicaudata, which is influenced by the size and age of the host, because larger C. capitata larvae in duce the production of higher amounts of D. longicaudata females. The effects of toxic bait formulations used in fruit-growing areas in Brazil on populations of D. longicaudata are unknown. Thus, this study aimed to evaluate the side effects of toxic bait formulations on D. longicaudata to ensure that these would not compromise integrated management programs for fruit flies.
Results
Toxicity on D. longicaudata
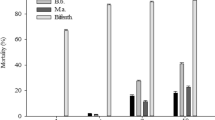
The food attractants Anamed, 3% Biofruit, 1.5% CeraTrap, 1.25% Flyral, and 7% sugarcane molasses showed low toxicity (<20% mortality) to D. longicaudata adults; the effects of these attractants were statistically similar (F6, 72 = 27.55, P < 0.0001) to those of the control (negative control, 15% mortality) after 96 h, and these attractants were thus classified as harmless (Tables 1 and 2). However, the food attractants 3% Isca Samaritá and 3% Isca Samaritá Tradicional resulted in more than 25% D. longicaudata mortality at 96 h, which was significantly different (P < 0.05) from the effects of the other food attractants, and these attractants were thus classified as slightly harmful to D. longicaudata adults (Tables 1 and 2). In toxic bait formulations, all food attractants mixed with the insecticide Malathion 1000 EC [2.0 gram of the active ingredient (g a.i.). L−1] showed higher toxicity (P < 0.05) to D. longicaudata adults during the first 24 h (mortality >85%) (48%) compared with the respective food attractant, and because 100% mortality was observed at 48 h, these formulations were classified as harmful (Class 4, Table 1). In contrast, the spinosyn-based insecticides (spinetoram and spinosad at a concentration of 0.096 g a.i. L−1 or kg) in an admixture with the food attractants Anamed, 3% Biofruit, 1.5% CeraTrap, 1.25% Flyral, and 3% Isca Samaritá Tradicional showed low toxicity at 96 h (<20% mortality), and their effects were statistically similar (P < 0.05) to those of the respective food attractants; thus, these insecticides were classified as harmless (Class 1, Table 3). However, 3% Isca Samaritá and 7% sugarcane molasses in an admixture with spinosad or spinetoram were classified as moderately harmful (Class 3) and harmful (Class 4), respectively, because their application resulted in more than 50% mortality at 24 h (Table 3). The ready-to-use toxic bait Success 0.02CB (0.96 g a.i. L−1 spinosad) caused low toxicity (up to 16.4% mortality at 96 h) to D. longicaudata adults and was classified as harmless (Class 1), and its effects were similar (P < 0.05) to those observed in insects fed a solution of water and 80% honey (Table 4). However, the exposure of D. longicaudata adults to the Gelsura formulation (2.0 and 4.0 g a.i. L−1 alpha-cypermethrin) resulted in higher than 90% mortality at 96 h, and this formulation was thus classified as harmful (Class 4).
Side effects on D. longicaudata
Because the food attractants Anamed, 3% Biofruit, 1.5% CeraTrap, 1.25% Flyral, and 3% Isca Samaritá Tradicional alone or in an admixture with the insecticide spinosad or spinetoram, 3% Isca Samaritá in an admixture with spinosad and the Success 0.02CB formulation showed low toxicity (<50% mortality) on D. longicaudata adults, the coefficients for the reductions in the parasitism or emergence of the parasitoid on C. capitata larvae were estimated. The results showed that the exposure of D. longicaudata adults to Anamed, 3% Biofruit, 1.5% CeraTrap, and 1.25 Flyral alone or in admixture with the insecticide spinosad or spinetoram (0.096 g a.i. L−1 or kg) for 24 h did not significantly (P < 0.05) reduce the parasitism and adult emergence rates of C. capitata larvae (<30% reductions) compared with the negative control (80% honey-water solution). Therefore, these insecticides were classified as harmless (Class 1) with respect to both evaluated responses (Table 5). In contrast, 3% Isca Samaritá Tradicional alone or in an admixture with spinosad or spinetoram exerted significant side effects in D. longicaudata adults. Specifically, both treatments induced reductions in parasitism (F16, 119 = 9.11, P < 0.0001) and insect emergence (F16, 125 = 4.06, P < 0.0001) compared with the negative control (Table 5), and these formulations were thus classified as slightly harmful (Class 2).
Discussion
Based on the isolated effects of the food attractants, 3% Isca Samaritá and 3% Isca Samaritá Tradicional were classified as moderately toxic to D. longicaudata adults. Additionally, these attractants negatively affected the rates of parasitism and emergence of the insects in F1 C. capitata larvae compared with a solution of water and 80% honey, which is considered the standard for laboratory rearing and multiplication of the species17. The same finding was not obtained with Anamed, 3% Biofruit, 1.5% CeraTrap, 1.25% Flyral, and 7% sugarcane molasses, which might be associated with the higher protein and carbohydrate composition of these attractants compared with that of 3% Isca Samaritá and 3% Isca Samaritá Tradicional. In this study, the amount of food attractant ingested by the insects during the exposure time (24 h) was not measured. However, D. longicaudata adults were attracted to and fed on all treatments offered.
High D. longicaudata mortality rates were measured after a short period (24 h) of feeding with the attractants 3% Isca Samaritá and 3% Isca Samaritá Tradicional and the mixtures without insecticide. This finding might indicate that these attractants do not provide the nutrients (carbohydrates) necessary for the survival of the individuals during the first days of life. According to previous studies, this period is considered crucial for the development and maturation of the ovaries, which is necessary for reproduction of the species20,21. In addition, although its detailed composition is not available from the manufacturer, the vegetable protein obtained from sugarcane can likely undergo a fermentation process after the addition of water, resulting in the generation of byproducts that are toxic to D. longicaudata, such as ethanol. Similar findings have been reported for Drosophila melanogaster Meigen, 1830 (Diptera: Drosophilidae) against Figitidae parasitoid wasps22,23.
The highest toxicity against D. longicaudata adults was obtained with the toxic baits formulated with the insecticide Malathion 1000 EC (2.0 g a.i. L−1) in an admixture with Anamed, 3% Biofruit, 1.5% CeraTrap, 1.25% Flyral, 3% Isca Samaritá, 3% Isca Samaritá Tradicional, and 7% sugarcane molasses (Class 4). D. longicaudata adults fed toxic baits containing an organophosphate insecticide showed high nervous hyperactivity “tremors” within a few hours of feeding. Due to its high toxicity to fruit fly species, organophosphate insecticides have commonly been used in the formulation of toxic baits3,11,24. Additionally, the ease of acquisition and the low cost of the active ingredients constitute advantages to fruit growers in rural properties that utilize toxic bait formulations5.
Toxic baits formulated with the organophosphate insecticide malathion were compared with the ready-to-use formulation Gelsura (2.0 and 4.0 g a.i. L−1 alpha-cypermethrin), which is under evaluation in Brazil for the management of Anastrepha fraterculus (Wiedemann, 1830) (Diptera: Tephritidae) and C. capitata5. Studies carried out in the Mediterranean region have demonstrated the potential of use of this formulation for the population suppression of Bactrocera oleae (Rossi, 1790) (Diptera: Tephritidae) in olive crops and C. capitata in citrus25. To date, scarce information on the toxicity and side effects of this formulation on natural enemies has been reported. The present study demonstrated that the toxic bait resulted in high mortality of D. longicaudata adults during the first 24 h after ingestion, leading to its classification as harmful (Class 4). This result might be associated with the knockdown effect caused by the presence of the pyrethroid insecticide alpha-cypermethrin at 0.2%, as observed in preliminary tests with A. fraterculus and C. capitata (unpublished data).
In contrast, toxic baits formulated with a food attractant plus spinosad (Tracer 480 SC) or spinetoram (Delegate 250 WG) at a concentration of 0.096 g a.i. L−1 or kg, which is equivalent to the concentration in the ready-to-use formulation Success 0.02CB, showed low toxicity (<10% mortality) on D. longicaudata. Studies conducted in other regions also showed that toxic baits containing the insecticide spinosad exert reduce effects on biological control agents. An evaluation of the effect of the GF-120 NF bait on D. longicaudata through cage bioassays with mango plants resulted in less than 40% mortality3.
Similarly, toxic bait formulations containing spinosad are less toxic to the parasitoids Fopius arisanus (Sonan, 1932) and Psyttalia fletcheri (Silvestri, 1916) (Hymenoptera: Braconidae) than toxic baits formulated with the insecticide malathion3. As verified in Hawaii, the field application of 11 sprayings of toxic baits containing the insecticide spinosad (GF-120 NF) had no effects on the F. arisanus population. However, the toxicity of spinosyn-based insecticides depends on the manner of application (ingestion or topical)15, the dose and time of exposure and the insect species being studied3,26.
In addition, toxic baits containing spinosyn-based insecticides (spinosad and spinetoram) and the ready-to-use formulation Success 0.02CB exerted no side effects on parasitism. Additionally, the mean number of offspring in the F1 generation after 7 consecutive days of parasitism in C. capitata larvae fed these formulations was similar to that obtained with the 80% honey solution17. The feeding of D. longicaudata adults with the toxic bait GF-120 NF in an admixture with honey for 24 h exerted no side effects on the progeny. However, the daily feeding of the toxic bait GF-120 NF and honey resulted in no significant reductions in the offspring number3.
The Integrated Control of Noxious Animals and Plants (IOBC) has promoted studies aiming to standardize selectivity tests and thereby reduce problems related to differences in methodology. Nevertheless, diverging results continue to be reported for many reasons27. The classification of a specific product (used at the same rate) can range from harmless to harmful depending on the developmental stage of the natural enemy, and differences (e.g., in body size and sex ratio; different tolerances have been found between males and females) can be detected between a specific species and the natural enemies of that species27. Thus, a better understanding of the diversity of biological control species in agroecosystems and of the different mechanisms through which pesticides can affect the effectiveness of these species is needed.
The new challenge in the study of the selectivity of pesticides for natural biocontrol agents is to go beyond the description of lethal/sublethal effects of pesticides on natural control agents. Similarly, the ecological structure within agroecosystems should be considered. The results obtained in this study are based on criteria directly obtained from the IOBC with respect to the side effects of insecticides on natural enemies. The above-described context can be very simple and might differ from what would occur in field conditions. As a result, the criteria provided by the IOBC should be improved to aid the classification of insecticides and toxic bait formulations with respect to their natural enemies27.
The results for the different toxic baits included in this study were obtained in the laboratory, where the D. longicaudata adults were caged and forced to feed on the material offered. Thus, the side effects on D. longicaudata in the field might be less damaging. This possibility is associated with the low biological persistence of toxic baits when applied in the field because the active ingredient is degraded by the presence of light or constant rainfall3,10,11. In addition, parasitoids can avoid contact with toxic baits and look for other food sources, as has been observed with F. arisanus28,29. Based on this finding, it is also important to evaluate other bioassay methodologies for assessing the effects on parasitoids, as proposed in a previous study30. Given these results, fruit flies can be managed by combining chemical control through the application of spinosyn-based toxic baits with biological control using D. longicaudata for the suppression of pest populations. However, new studies should be performed under semi-field and field conditions to obtain a better understanding of the effect of the tested formulations on the parasitoids.
Materials and Methods
Insect rearing
C. capitata and D. longicaudata adults were obtained from the Entomology Laboratory of Embrapa Temperate Climate, Pelotas, RS, Brazil, and were maintained in air-conditioned rooms (temperature of 25 ± 2 °C, 70 ± 10% relative air humidity, and 12-h photoperiod). C. capitata adults were obtained from mango fruits (Mangifera indica L.) collected in the municipality of Pelotas, Rio Grande do Sul, Brazil (31°38′20″ S and 52°30′43″ W), infested with C. capitata larvae and were established and maintained (20 generations) in the laboratory on an artificial diet prior to their use in the bioassay31. In the laboratory, the adults were kept in plastic cages (57-cm long × 39-cm wide × 37-cm high; Sanremo, Bettanin Industrial S/A) and were provided distilled water supplied via capillarity from hydrophilic cotton and an artificial diet based on refined sugar, wheat germ, and beer yeast (Bionis YE NS + MF) (3: 1: 1 ratio), which was supplied in a Gerbox germination box (11-cm long × 11-cm wide × 3.5-cm high)31. The methodology proposed in a previous study29 was used for the preparation of the rearing and egg collection cages. The collected eggs were transferred to Erlenmeyer glass containers (500 mL) and aerated for 24 h. After this period, the eggs (≈9,200) were deposited on a strip of filter paper (10 cm2) using a micropipette (30 μL) and placed in plastic containers (17-cm wide × 27.6-cm long × 7-cm high) over a layer of artificial diet (300 mL) for larval development32. After nine days, the artificial diet was removed, and the larvae were collected using a sieve (0.22-mm mesh) and packed in plastic containers (300 mL) on a layer of moistened fine vermiculite (1 cm), where pupation and emergence of the adults occurred.
D. longicaudata individuals were obtained from field collections of C. capitata larvae in the municipality of Pelotas, RS, Brazil. In the laboratory, the insects were maintained for approximately 30 generations prior to their use in the bioassay and maintained in plastic cages (45-cm long × 30-cm wide × 30-cm high)17. The adults were fed an 80% honey aqueous solution supplied via capillarity using a strip of sponge (Spontex) placed inside a Petri dish (3 cm in diameter). Seven days after emergence, third instar larvae from reared C. capitata were exposed to parasitism for 1 h (30 larvae per female D. longicaudata). After this period, the larvae were removed and maintained on a layer of fine vermiculite (1 cm) in plastic containers (200 mL), and the top of each container was closed with a lid to enable pupation and the emergence of adult parasitoids. The D. longicaudata adults used in the bioassays were collected from C. capitata maintained under laboratory conditions for approximately 20 generations.
Bioassays and toxic bait formulations
To evaluate the toxicity and side effects of the toxic bait formulations on longicaudata adults, ingestion bioassays were performed in an air-conditioned room (temperature of 25 ± 2 °C, 70 ± 10% relative humidity, and 12-h photoperiod). The tested food attractants were Anamed, 3% Biofruit, 1.5% CeraTrap, 1.25% Flyral, 3% Isca Samaritá, 3% Isca Samaritá Tradicional and 7% sugarcane molasses (Table 1). The concentrations of these food attractants were defined based on the manufacturer’s recommendations and practical experience. The following insecticides were used to formulate toxic baits based on admixtures containing the above-mentioned food attractants: Malathion 1000 EC (1.0 g a.i. L−1 malathion; Cheminova Ltda., São Paulo, Brazil), Tracer 480 SC (480 g a.i. L−1 spinosad) and Delegate 250 WG (250 g a.i. kg spinetoram; Dow AgroSciences Industrial Ltda., São Paulo, Brazil). These products are registered for the management of fruit flies in Brazil.
The ready-to-use formulations used in this study were Success 0.02CB [0.24 g a.i. L−1 spinosad; Dow AgroSciences Industrial Ltda., São Paulo, Brazil; diluted in water at a ratio of 1:1.5 volume/volume (v/v), i.e., 1 part of the commercial product to 1.5 parts of water] and Gelsura [6.0 g a.i. L−1 alpha-cypermethrin, a polymer matrix containing the active ingredient alpha-cypermethrin; BASF SA, São Paulo, Brazil; diluted 1:2 and 2:1 (parts of the commercial product:parts of water)]. A solution containing 80% honey and water was used as the negative control for both treatments.
A completely randomized experimental design with 32 treatments and 10 replicates per treatment (food attractant + insecticide, only food attractant, or 80% honey and water solution) and 10 D. longicaudata (n = 100) couples per treatment was used in this study.
Toxicity toward D. longicaudata adults
Three-day-old insects from the rearing cage were starved for 12 h. At the end of this period, 10 couples were placed inside a cage consisting of a plastic container (500 mL) inverted on an acrylic plate (12 cm in diameter). The top of the case was cut out and covered with a fine mesh net to allow ventilation. Subsequently, one drop (10 mm) of each treatment was placed with a micropipette (100 μL) on a plastic plate (2.5 cm in diameter) composed of plastic paraffin film and parafilm paper (Bemis Company, Inc., USA). The insects were allowed to feed on the treatments for a period of 24 h. Subsequently, the insects were fed an 80% honey aqueous solution supplied via capillarity as previously described. The numbers of insects that were still alive or had died after 24, 48, 72, and 96 h of exposure were recorded. The insects that showed no reaction to touching with a fine-tipped brush were considered dead. To isolate the effect of each food attractant on D. longicaudata adults, the mortality of each toxic bait formulation (food attractant + insecticide) was corrected to that obtained with the corresponding food attractant using the formula developed by Henderson and Tilton33. Similarly, the mortality caused by the food attractant was corrected with that obtained with the negative control (water and 80% honey solution). Based on the mortality (M) data at 96 h, the treatments were classified according to the criteria defined by IOBC/WPRS as follows: Class 1 = harmless (M < 25%), Class 2 = slightly harmful (25% ≤ M ≤ 50%), Class 3 = moderately harmful (51% ≤ M ≤ 75%), and Class 4 = harmful (M > 75%)34.
Side effects on D. longicaudata adults
The treatments that caused less than 50% mortality in a population of D. longicaudata adults (Classes 1 and 2) were used. Adults (10 couples per cage, 3 days of age) were fasted for a period of 12 h and used for the assessment of adult toxicity as described. After 24 h of treatment, the surviving adults were fed a mixture of water and 80% honey until the end of the bioassay. Starting on the seventh day after emergence, third instar larvae of C. capitata (30 larvae per female) were offered the treatment daily for seven consecutive days17. After 1 h of daily parasitism, the larvae were removed and stored in plastic containers (100 mL) containing a layer of fine vermiculite (1 cm) until adult emergence. After emergence of the first insect (C. capitata or D. longicaudata), the puparia were evaluated daily. At the end of the bioassay, pupae that remained intact were dissected to assess the presence of nonemergent flies or parasitoids and tthus determine the true parasitism rate. The reductions in the parasitism capacity (%) and emerged insects (%) obtained with each treatment were determined in comparison with the negative control and calculated using the formula RP = [(1 − T/C) * 100], where T is the mean parasitism or mean emergence with the treatment (the toxic bait formulation or the food attractant alone) and C is the mean parasitism or emerged insects obtained with the negative control (water + honey solution)35. Based on the obtained reductions in the parasitism (% RP) and emergence (% RE) of D. longicaudata, the treatments were classified according to the IOBC as follows: harmless (RP or RE < 30%), slightly harmful (30 ≤ RP or RE ≤ 79%), moderately harmful (80 ≤ RP or RE ≤ 99%), and harmful (RP or RE > 99%).
Statistical analysis
The data were initially subjected to residual analysis to confirm the assumption of normality obtained with the Shapiro-Wilk test and to an analysis of the variance homogeneity based on the Bartlett test using the PROC UNIVARIATE procedure in SAS 9.136. The survival rates of the D. longicaudata adults that did not present a normal distribution were subjected to Box-Cox transformation prior to the analyses. Subsequently, all the data were subjected to a two-way analysis of variance using PROC GLM36. The differences between the treatments were determined by the least-squares means (PDIFF option in PROC GLM) followed by Tukey’s adjustment based on a 5% significance threshold using SAS 9.1 software36. To evaluate the side effects on D. longicaudata adults, the data on parasitism (%) and emergence (%) were evaluated for normality using the Shapiro-Wilk test and homoscedasticity using the Hartley and Bartlett test and then subjected to analysis of variance (ANOVA option in PROC GLM) using the F test (P < 0.05)36. When statistically significant, the means were compared by Tukey’s test at the 5% significance level (P < 0.05).
Data archiving
This article does not report new empirical data or software.
References
Zucchi, R. A. Fruit flies in Brazil - Hosts and parasitoids of the Mediterranean fruit fly. Contém informações institucionais, técnicas, notícias e publicações Available at, http://www.lea.esalq.usp.br/ceratitis. (accessed 13th July 2019) (2012).
Chueca, P. et al. Spinosad bait treatments as alternative to malathion to control the mediterranean fruit fly Ceratitis capitata (Diptera: Tephritidae) in the Mediterranean Basin. Journal of Pest Science 32, 407–411 (2007).
Ruiz, L. Lethal and sublethal effects of spinosad-based GF-120 bait on the tephritid parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Biological Control 44, 296–304 (2008).
Navarro-Llopis, V., Primo, J. & Vacas, S. Efficacy of attract-and-kill devices for the control of Ceratitis capitata. Pest Management Science 69, 478–482 (2013).
Botton, M. et al. Moscas-das-frutas na fruticultura de clima temperado: Situação atual e perspectivas de controle através do emprego de novas formulações de iscas tóxicas e da captura massal. Agropecuária Catarinense 29, 103–107 (2016).
Vargas, R. I., Miller, N. W. & Prokopy, R. J. Attraction and feeding responses of mediterranean fruit fly and a natural enemy to protein baits laced with two novel toxins, phloxine B and spinosad. Entomologia Experimentalis et Applicata 102, 273–282 (2002).
Galm, U. & Sparks, T. C. Natural product derived insecticides: discovery and development of spinetoram. Journal of Industrial Microbiology and Biotechnology 43, 185–193 (2016).
Raga, A. & Sato, M. E. Effect of spinosad bait against Ceratitis capitata (Wied.) and Anastrepha fraterculus (Wied.) (Diptera: Tephritidae) in laboratory. Neotropical Entomology 34, 815–822 (2005).
Piñero, J. C., Mau, R. F. L. & Vargas, R. I. Managing oriental fruit fly (Diptera: Tephritidae), with spinosad-based protein bait sprays and sanitation in papaya orchards in Hawaii. Journal of Economic Entomology 102, 280–289 (2009).
Flores, S., Gomez, L. E. & Montoya, P. Residual control and lethal concentrations of GF-120 (Spinosad) for Anastrepha spp. (Diptera: Tephritidae). Journal of Economic Entomology 104, 1885–1891 (2011).
Harter, W. R. et al. Toxicities and residual effects of toxic baits containing spinosad or malathion to control the adult Anastrepha fraterculus (Diptera: Tephritidae). Florida Entomologist 98, 202–208 (2015).
Gazit, Y. & Akiva, R. Toxicity of Malathion and Spinosad to Bactrocera zonata and Ceratitis capitata (Diptera: Tephritidae). Florida Entomologist 100, 385–389 (2017).
Prokopy, R. J. et al. How effective is GF-120 fruit fly bait spray applied to border area sorghum plants for control of melon flies (Diptera: Tephritidae)? Florida Entomologist 87, 354–360 (2004).
Desneux, N., Decourtye, A. & Delpuech, J. M. The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology 52, 81–106 (2007).
Biondi, A., Desneux, N., Siscaro, G. & Zappalà, L. Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 87, 803–812 (2012).
Biondi, A., Zappalà, L., Stark, J. D. & Desneux, N. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS One 8, e76548 (2013).
Meirelles, R. F., Redaelli, L. R. & Ourique, S. B. Comparative biology of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) reared on Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae). Florida Entomologist 96, 412–418 (2013).
Montoya, P. Field superparasitism by Diachasmimorpha longicaudata attacking Anastrepha spp. larvae on mango fruits. Biological Control 64, 160–165 (2013).
Vargas, R. I. et al. Spatial dynamics of two oriental fruit fly (Diptera: Tephritidae) parasitoids, Fopius arisanus and Diachasmimorpha longicaudata (Hymenoptera: Braconidae), in a guava orchard in Hawaii. Environmental Entomology 42, 888–901 (2013).
Sivinski, J., Aluja, M. & Holler, T. Food sources for adult Diachasmimorpha longicaudata, a parasitoid of tephritid fruit flies: effects on longevity and fecundity. Entomologia Experimentalis et Applicata 118, 193–202 (2006).
Benelli, G. et al. The impact of adult diet on parasitoid reproductive performance. Journal of Pest Science 90, 1–17 (2017).
Milan, N. F., Kacsoh, B. Z. & Schlenke, T. A. Alcohol consumption as self-medication against blood-borne parasites in the fruitfly. Current Biology 22, 488–493 (2012).
Lynch, Z. R., Schlenke, T. A., Morran, L. T. & Roode, J. C. Ethanol confers differential protection against generalist and specialist parasitoids of Drosophila melanogaster. PLoS One 12, e0180182 (2017).
Manrakhan, A., Kotze, C., Daneel, J. H., Stephen, P. R. & Beck, R. R. Investigating a replacement for malathion in bait sprays for fruit fly control in South African citrus orchards. Crop Protection 43, 45–53 (2013).
Ruiz, C. B. Experiencias en el control de “Batrocera oleae, Ceratitis capitata” y otras plagas emergentes, en la zona mediterránea, mediante técnicas de “Atract and Kill”. Phytoma España, pp50 (2013).
Takahashi, Y., Kojimoto, T., Nagaoka, H., Takagi, Y. & Oikawa, M. Tests for evaluating the side effects of chlorothalonil (TPN) and spinosad on the parasitic wasp (Aphidius colemani). Journal of Pest Science 30, 11–16 (2005).
Bueno, A. F., Carvalho, G. A., Dos Santos, A. C., Sosa-Gomez, D. R. & Silva, D. M. Pesticide selectivity to natural enemies: challenges and constraints for research and field recommendation. Ciencia Rural 47, e20160829 (2017).
Vargas, R. I. et al. Comparative demography of six fruit fly (Diptera: Tephritidae) parasitoids (Hymenoptera: Braconidae). Biological Control 25, 30–40 (2001).
McQuate, J. T., Peck, S. L., Barr, P. G. & Sylva, C. D. Comparative evaluation of spinosad and phloxine b as toxicants in protein baits for suppression of three fruit fly (Diptera: Tephritidae) species. Journal of Economic Entomology 98, 1170–1178 (2005).
Abbes, K. et al. Combined Non-Target Effects of Insecticide and High Temperature on the Parasitoid Bracon nigricans. PLoS One 10, e0138411 (2015).
Nunes, A. M. et al. Dietas artificiais para a criação de larvas e adultos da mosca-das-frutas sul-americana. Pesquisa Agropecuária Brasielira 48, 1309–1314 (2013).
Gonçalves, R. S. et al. Biology and fertility life table of Aganaspis pelleranoi (Hymenoptera: Figitidae) in larvae of Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae). Annals of the Entomological Society of America 106, 791–798 (2013).
Henderson, C. F. & Tilton, E. W. Tests with acaricides against the brow wheat mite. Journal of Economic Entomology 48, 157–161 (1955).
Sterk, Gl et al. Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRS-Working Group “Pesticides and Beneficial Organisms”. BioControl 44, 99–117 (1999).
Hassan, S.A. et al. A laboratory method to evaluate the side effects of plant protection products on Trichogramma cacoeciae Marchal (Hymenoptera: Trichogrammatidae). In: Candolfi, M. P.et al. (eds) Guidelines to evaluate side-effects of plant protection products to non-target arthropods. IOBC/WPRS, Reinheim. pp 107–119 (2000).
SAS Institute. SAS System - SAS/STAT. computer program, version 9.2. By SAS Institute, Cary, NC. 84p (2011)
Acknowledgements
This work was financially supported by the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES) for financial support and scholarships.
Author information
Authors and Affiliations
Contributions
D.B., J.W., A.N., C.A.B., D.E.N. and M.B. conceived and designed the research. D.B., L.B., R.Jr., R.C.B.T., F.G. and C.N. conducted experiments. D.B. and M.B. analyzed the data. D.B., J.W., A.N., C.A.B., L.B. and R.Jr. wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bernardi, D., Nondillo, A., Baronio, C.A. et al. Side effects of toxic bait formulations on Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Sci Rep 9, 12550 (2019). https://doi.org/10.1038/s41598-019-49106-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49106-z
This article is cited by
-
Can spinosad be effective for the integrated management of Anastrepha ludens (Tephritidae) in soil and fallen fruit, and be compatible with the parasitoid Diachasmimorpha longicaudata (Braconidae)?
Phytoparasitica (2021)
-
Lethal and Sublethal Effects of Annona spp. Derivatives on Bemisia tabaci MEAM 1 (Hemiptera: Aleyrodidae) in Tomato
Neotropical Entomology (2021)
-
Lethal and sublethal toxicities of acetogenin-based bioinsecticides on Ceratitis capitata and the parasitoid Diachasmimorpha longicaudata
Phytoparasitica (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.