Abstract
We report synchrotron-based operando soft X-ray microscopic photoelectron spectroscopy under charge-discharge control of single crystalline LiCoO2 (LCO) particles as an active electrode material for an all solid-state lithium-ion battery (LIB). Photoelectron mapping and the photoelectron spectrum of a selected microscopic region are obtained by a customized operando cell for LIBs. During the charge process, a more effective Li extraction from a side facet of the single crystalline LCO particle than from the central part is observed, which ensures the reliability of the system as an operando microscopic photoelectron analyzer that can track changes in the electronic structure of a selected part of the active particle. Based on these assessments, the no drastic change in the Co 2p XPS spectra during charge-discharge of LCO supports that the charge-polarization may occur at the oxygen side by strong hybridization between Co 3d and O 2p orbitals. The success of tracking the electronic-structure change at each facet of a single crystalline electrode material during charge-discharge is a major step toward the fabrication of innovative active electrode materials for LIBs.
Similar content being viewed by others
Introduction
Development of high-performance lithium-ion batteries (LIBs) with large energy density, high power performance, high charge-discharge cycle stability, and high safety is strongly demanded to promote the use of clean energy devices, such as electric vehicles, to build a sustainable low-carbon society1. In the development of negative electrode materials for LIBs, strategies for the materials design have been established and metal lithium or silicon-lithium alloys have attracted much attention as high capacity materials2,3. However, strategies for such innovative materials to be used at the positive electrode have not been established because there are a lot of unknown charge-discharge mechanisms that occur at the positive electrode4,5,6,7,8. To overcome this situation, researchers have undertaken cutting-edge analytical techniques to further understand these positive electrode materials and to create a strategy for materials design. Various kinds of analytical techniques, like two-dimensional nuclear magnetic resonance spectroscopy9,10,11, annular bright-field and high-angle annular dark field scanning transmission electron microscopy12,13, and micro-Raman spectroscopy14,15,16, have been widely used in the research of battery materials, and these methods have unveiled a lot of information on the microscopic structures in LIBs, such as the partial structure of organic compounds at the solid-electrolyte interface, distributions of each element like lithium, metal, and oxygen, and cross-sectional mapping information in a large area of the coated electrode films.
For the positive electrode materials of LIBs, lithium insertion and extraction change the crystal structure of the host crystals. The crystal-structural change is triggered by the changes in the electronic structure of the host material during the Li insertion/extraction process17. In addition, lithium ions diffuse into the host crystals along a direction that is dependent on the crystal structure. For example, diffusion of lithium ions in LiFePO4 (olivine-type), LiCoO2 (LCO, layered rock salt), and LiMn2O4 (spinel-type) is one-dimensional, two-dimensional, and three-dimensional, respectively18,19,20. To understand the above-mentioned complex phenomena, analytical techniques using synchrotron radiation X-rays have enabled a major advance: hard X-rays having a short wavelength can be used to clarify the crystal structure21,22 and soft X-ray spectroscopies, which cover the 3d transition-metal L and oxygen K edges, are advantageous to obtain element-specific electronic-structure information on the host material23,24,25,26,27,28. Concerning the measurement area on the electrode materials, the recent development of X-ray optics has enabled a focusing of the X-ray beam down to the several tens of nanometer scale29,30,31. This has enabled us to observe various micro areas of the microcrystals of electrode materials and understand the stability of each micro region6,32,33. More important has been the rapid growth of the state-of-the-art operando X-ray measurements, where X-ray progress can be performed during voltage/current operation of the battery6,23,27,34,35,36. All of the characteristics of synchrotron X-ray spectroscopies are essential for accurate observation and detailed understanding of the diffusion and domain wall kinetics of the intercalation electrode materials and even more complex charge-discharge mechanisms, including redox potential, capacity, and cycle stability of electrode materials.
Here, we report the operando analysis of X-ray photoelectron microscopy by using an all-solid-state lithium-ion battery cell originally developed for the 3DnanoESCA station at the University of Tokyo Synchrotron Radiation Outstation beamline BL07LSU in SPring-829. A single crystalline hexagonal plate of LCO, with a size of around 10 µm, was fabricated by a flux method as the target material. LCO is suitable as a model material for the novel analysis method because LCO is a typical material for use at the positive electrode of LIBs. We achieved lithium insertion and extraction of LCO and obtained the mapping image of photoelectrons for selected energy regions at each area under potential control.
Results and Discussion
Figure 1 displays the operando photoelectron microspectroscopy system for the all solid-state LIBs. A photoelectron map could be obtained by scanning a soft X-ray beam focused on a sample using a Fresnel zone plate (FZP) and detecting the photoelectrons at each beam spot. An operando cell for the all solid-state LIBs was prepared for photoelectron microspectroscopy in a high vacuum chamber.
As the working electrode, an Au mesh with a carbon micro-grid was used for electrochemical measurements and photoelectron mapping. An Al sheet was welded to the edge of the Au mesh to establish an electrical connection to one of the five terminals of the cell holder as the cathode current collector. An LCO slurry was dropped onto the micro-grid and the dried slurry was sandwiched with a solid electrolyte. We used dilute slurry, i.e. small amount of LCO particles, for the clear observation of each isolated single crystalline particle on the microgrid. Before installation to the high vacuum chamber, a high magnification optical image was obtained to identify the X-Y coordinates of the selected single crystalline LCO particles. A Cu current anode collector, metal Li as anode material, PEO/LiTFSI solid (dry polymer) electrolyte, Li7La3Zr2O12 (LLZ) ceramics electrolyte, PEO/LiTFSI dry polymer electrolyte, and the Au mesh with LCO were all fixed by ceramics jigs. The adhesive property of PEO/LiTFSI dry polymer created a good interface between the dry polymer and the metal Li, LLZ, and LCO to decrease the interfacial resistance. The carbon micro-grid played the role of a conduction path from the Au mesh to the single crystalline LCO particles.
During the limited time of the synchrotron radiation X-ray analysis, the operando cell was heated by a ceramics heater to approximately 55 °C to improve the ionic conductivity of the dry polymer and LLZ, which realized a high CV sweeping rate of the cell. Terminals A and B were used to supply the current for the heating. The operando cell was set on the cell holder in a glove box and transferred to 3DnanoESCA without air exposure.
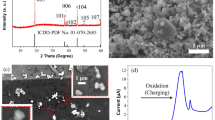
To discuss the relationship between Li diffusion and redox reaction of Co in the host crystal, we collected the SEM image and XRD pattern of the single crystalline LCO particles. Considering the spatial resolution of the 3DnanoESCA station, the particle size of the single crystals was tuned to approximately 10 µm to distinguish the photoemission signal from each facet. Figure 2(a) shows the SEM image of the single crystalline LCO particles. The plate morphology of a triangular facet in the (101) plane37 with a size of approximately 10 µm was observed. Therefore, adequate single crystalline LiCoO2 for operando measurement by the 3DnanoESCA station was fabricated by the flux method. Figure 2(b) indicates the XRD pattern of the fabricated sample and a pattern from the ICDD-PDF data of LCO (No. 01-070-2685). The XRD pattern was in excellent agreement with the ICDD pattern. The obtained lattice constants of a = 2.816 Å and c = 14.053 Å were almost identical to those of a = 2.816 Å and c = 14.054 Å in the ICDD-PDF data of LCO.
Cyclic voltammetry was conducted for Li extraction and insertion of the single crystalline LCO particles. Figure 3(a) shows the Li extraction up to 4.5 V (vs. Li/Li+) and Li insertion down to 2.8 V (vs. Li/Li+). A peak around 3.5–4 V was caused by an oxidation reaction of LCO with the extraction reaction of Li. During the photoemission measurements, the potential was intentionally controlled by repeating a potentiostatic mode at 4.5 V (or 2.8 V) for 3 sec and an open circuit voltage (OCV) mode for 57 sec. This repeated cycling of the modes avoided self-discharge and relaxation reactions, which might result in a significant change of the potential under equilibrium conditions after extraction/insertion of Li.
Figure 3(b) shows OCV curves after the potentiostatic mode at 4.5 V and 2.8 V for 3 sec at the 1st, 20th, 100th, 200th, and 300th repetition. Immediately after sweeping the voltage to 4.5 V and 2.8 V, we had to wait for the Li extraction and insertion to finish after over 30 repetitions of the potentiostatic mode for 3 sec and OCV mode for 57 sec before each operando photoemission measurement, because the high rate of CV sweeping gave rise to a delayed extraction and insertion of Li. The small change of the voltage in the OCV curve (e.g., from 4.5 V to approx. 4.2 V) ensured the oxidation of LCO. After the 300th repetition for the 4.5 V condition, the potential was swept down to 2.8 V to monitor the Li insertion reaction and reduction of the LCO particles. A reduction peak was not observed due to the small amount of LCO particles. Nevertheless, the OCV curves after the potentiostatic mode at 2.8 V for 3 sec indicated an almost constant potential at around 3 V up to the 20th repetition, which confirmed the reduction reaction of LCO by Li insertion. After the 100th repetition, the potential decreased down to approximately 2.6 V, which was the initial OCV before sweeping the voltage to 4.5 V, possibly because the repetition of many times returned the cell potential to the initial condition.
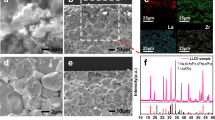
Figure 4(a) shows the 2D image of the LCO particle obtained with an optical microscope (Keyence VHX-1000 and VH-Z500R) and Fig. 4(b) shows the corresponding 2D mapping of O 1 s photoemission integrated in the binding energy region of 522.5–546.5 eV. Images from a low magnification to high magnification were obtained from both microscopes. The micro-grid was overlapped on the single crystalline LCO particle in the 2D image obtained with the optical microscope while it was almost invisible in the O 1s photoemission image. At the center of the LCO particle, marked by a black circle in Fig. 4(b), wide scan (Fig. 4(c)) and Li 1s/Co 3p narrow scan (Fig. 4(d)) XPS spectra were obtained at the initial condition, after charge up to 4.5 V, and after discharge down to 2.8 V. While strong O 1s and C 1s sharp peaks were detected, the narrow scan XPS spectra clearly identified the Co 3p peak and a small contribution of the Li 1s peak38. The Co 3p peak profile could be fitted with three Gaussian curves for Co 3p1/2, Co 3p3/2 and satellite peaks38,39. Noted that the three peaks had identical energy and no drastic change of relative intensity ratios among the initial, charge and discharge conditions. It was considered that strong hybridization between Co 3d and O 2p orbitals40 made holes created by the charge reaction that were localized at the oxygen side, because changes in the O K-edge X-ray absorption spectra (XAS) for LCO upon the charge process were greater than those in the Co L2,3-edge XAS in previous ex situ XAS studies40,41. Therefore, the O 2p orbital should play an important role in the redox reaction rather than the Co 3d orbital. We could not obtain detailed information from the narrow scan O 2p and 1s spectra owing to the significant overlap of background noise by the oxygen components in the dry polymer, which were excited by a stray X-rays from outside the active area of the FZP.
(a) 2D image by optical microscope and (b) 2D mapping of O 1s photoemission using the operando cell. Black circle indicates the measured area. (c) Wide and (d) Li 1s/Co 3p XPS spectra of LCO at initial condition, after charge and discharge condition. (#1: peak position of Li 1s38, #2: peak position of Co 3p 3/238,39, #3: peak position of Co 3p1/238,39, #4: peak position of Co 3p satellite38).
In this operando measurement, we confirmed no drastic change of electronic structure of Co and the importance of strong hybridization of the Co 3d and O 2p orbitals. Precise discussion should be provided in the future development of the operando measurement system using all solid-state LIBs with oxygen free solid state electrolytes like sulphide.
Figure 5(a) shows the 2D mapping of Co 3p and Li 1s photoemission spectra integrated over the energy region from 47.5 to 71.5 eV of a single crystalline LCO particle after Li extraction by a charge reaction. The intensity of the left side of the LCO particle was higher than that of the plate area and other sides. This was because of the geometrical relationships between the photoelectron analyzer and incident beam spot on the sample. The Co 3p and Li 1s spectra integrated over the strong intensity region (red square) of the left side and weak intensity region (black circle) are shown in Fig. 5(b). Li/Co atomic ratios for the two regions can be obtained by integrating the Gaussian fits for each Co 3p and Li 1s core level and were corrected with the photoionization cross sections of Li 1s (0.0023 Mb) and Co 3p (0.061 Mb) for an incident energy of 1041 eV42. The Li/Co ratios were calculated to be 1.2 and 2.8 for the red square and black circle, respectively. It was considered that the lower lithium ratio of the edge region than that of the flat-center region meant that Li in the edge region was easily extracted. These results indicated that our microscopic photoelectron spectroscopy system could clearly distinguish the electronic structure of a selected part of each active single crystalline LCO particle even under operando condition. In addition, the spectra at each partition exhibit similar morphology when monitored by the Co 3p region, in spite of different ratios of Li to Co, as shown in Fig. 5(b). This result implies that the Co 3p spectrum is not drastically changed by the Li insertion and extraction. From these results, photoelectron mapping by the 3DnanoESCA station is useful to derive position-dependent information of the chemical and electronic states for each element.
Conclusions
An operando measurement system for LIB using synchrotron radiation soft X-rays exhibits photoelectron spectra at selected micro regions and photoelectron mapping of active materials during the charge-discharge condition. Development of an all solid-state-LIB with high rate properties allows for the experiment using synchrotron radiation soft X-rays with limited time. From the operando analysis, important information on the charge-discharge mechanism of LCO is obtained. Generally, the redox reaction of LCO is discussed by using the formal charge of Co3+ ⇆ Co4+ 43,44 related by Li extraction/insertion. However, the Co spectra at the initial condition, and after charge and discharge conditions, are not changed in this operando measurement. It is considered that the charge-polarization might occur at the oxygen side based on the strong hybridization between Co 3d and O 2p orbitals. Thus, the importance of operando analysis of oxygen has been identified. Hereafter, in order to discuss the state of charge in our operando measurement system, a novel configuration with a working electrode made of a single crystalline particle as an active material is necessary. Moreover, we will develop this operando system for the analysis of electronic structure information at each facet of the single crystal and for visualization of the change in electronic structure by Li diffusion and redox reaction of elements in the host crystals.
Method
Single crystalline LCO was fabricated by a flux method. NaCl (3.0 g), LiOH·H2O (0.006 mol), and Co(NO3)2·6H2O (0.005 mol) were placed into a Al2O3 crucible with a lid. The crucible was placed into a muffle furnace and heated at 1000 °C for 5 h in air. Finally, the samples were washed with deionized water and dried under vacuum condition.
LCO and super P Li, as an electro-conductive additive, and n-methyl-2-pyrrolidone were mixed by sonication. The slurry was dropped and dried on the Au mesh with a carbon micro-grid, which was connected to an Al current collector. The electrode was used as a working electrode of the operando cell, as shown in Fig. 1(b). A key point for construction of the operando cell is fabrication of a flat working electrode to create low interfacial resistivity for lithium ion transport and to produce an Au mesh with carbon microgrid without mechanical damage. For not only electrochemical but also mechanical reasons, to construct the operando cell, we needed a hard and flat solid-state electrolyte. Thus, we used the Li7La3Zr2O12 (LLZ) plate electrolyte. The interfacial resistivity between LCO and LLZ is high due to the point contact. Therefore, dry polymer electrolytes are used to decrease the interfacial resistivity.
Preparation of the operando cell and setting on the five-terminal folder was conducted in an Ar-filled glovebox and transferred to the 3DnanoESCA station using a transfer vessel without air exposure. Two terminals of the holder were used for the ceramics heater, as shown in Fig. 1(a). For removing water in the dry polymer of the operando cell, the cell was heated to approximately 55 °C in the high vacuum chamber of the 3DnanoESCA station. After that, heating was conducted during measurement to increase the ionic conductivity of both the solid-state electrolytes of LLZ and dry polymer.
For photoelectron microspectroscopy measurements on the 3DnanoESCA station at BL07LSU in SPring-8, an incident X-ray (1000 eV) was focused by a Fresnel zone plate and an order-sorting pinhole aperture of 80 μm was used. Photoelectrons were detected by a modified angle-resolved photoelectron spectrometer (VG Scienta R3000-EWAL) with a pass energy of 200 eV. In the measurement of the initial condition, the terminal of a working electrode (LCO electrode) was grounded. After the measurement of the initial condition, terminal C (counter electrode of Li metal) and terminal D (working electrode) were connected to a potentiostat-galvanostat (solartron 1287) for the electrochemical measurements. A mapping image of the scanning photoelectron microscopy was obtained by the O 1 s core level spectra. Selected specific micro-area photoelectron spectra were measured at the initial condition, and after charge and discharge based on this O 1s mapping image. Finally, the mapping images of the energy region of Li 1s and Co 3p core levels were obtained in the same LCO particle.
References
Etacheri, V., Marom, R., Elazari, R., Salitra, G. & Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 4, 3243–3262 (2011).
Li, Z., Huang, J., Yann Liaw, B., Metzler, V. & Zhang, J. A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 254, 168–182 (2014).
Goriparti, S. et al. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 257, 421–443 (2014).
Park, M., Zhang, X., Chung, M., Less, G. B. & Sastry, A. M. A review of conduction phenomena in Li-ion batteries. J. Power Sources 195, 7904–7929 (2010).
Vetter, J. et al. Ageing mechanisms in lithium-ion batteries. J. Power Sources 147, 269–281 (2005).
Lim, J. et al. Origin and hysteresis of lithium compositional spatiodynamics within battery primary particles. Science 353, 566–571 (2016).
Van der Ven, A., Bhattacharya, J. & Belak, A. A. Understanding Li Diffusion in Li-Intercalation Compounds. Acc. Chem. Res. 46, 1216–1225 (2013).
Saubanère, M., McCalla, E., Tarascon, J. M. & Doublet, M. L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 9, 984–991 (2016).
Wagemaker, M., Kentjens, A. P. M. & Mulder, F. M. Equilibrium lithium transport between nanocrystalline phases in intercalated TiO2 anatase. Nature 418, 397–399 (2002).
Verhoeven, V. W. J. et al. Lithium dynamics in LiMn2O4 probed directly by two-dimensional 7Li NMR. Phys. Rev. Lett. 86, 4314–4317 (2001).
Wagemaker, M., van Eck, E. R. H., Kentgens, A. P. M. & Mulder, F. M. Li-Ion Diffusion in the Equilibrium Nanomorphology of Spinel Li4+xTi5O12. J. Phys. Chem. B 113, 224–230 (2009).
Lu, X. et al. New insight into the atomic structure of electrochemically delithiated O3-Li(1-x)CoO2 (0 ≤ x ≤ 0.5) nanoparticles. Nano letters 12, 6192–6197 (2012).
Wang, Y. Q. et al. Rutile-TiO2 nanocoating for a high-rate Li4Ti5O12 anode of a lithium-ion battery. J. Am. Chem. Soc. 134, 7874–7879 (2012).
Kumar, A. et al. Nanometer-scale mapping of irreversible electrochemical nucleation processes on solid Li-ion electrolytes. Sci. Rep. 3, 1621 (2013).
Pannala, S. et al. Multiscale modeling and characterization for performance and safety of lithium-ion batteries. J. Appl. Phys. 118, 072017 (2015).
Nanda, J. et al. Local state-of-charge mapping of lithium-ion battery electrodes. Adv. Funct. Mater. 21, 3282–3290 (2011).
Okamoto, Y. et al. Electronic structure and polar catastrophe at the surface of LixCoO2 studied by angle-resolved photoemission spectroscopy. Phys. Rev. B 96, 125147 (2017).
Nitta, N., Wu, F., Lee, J. T. & Yushin, G. Li-ion battery materials: Present and future. Mater. Today 18, 252–264 (2015).
Yamakawa, S., Yamasaki, H., Koyama, T. & Asahi, R. Numerical study of Li diffusion in polycrystalline LiCoO2. J. Power Sources 223, 199–205 (2013).
Sugiyama, J. et al. Li Diffusion in LixCoO2 Probed by Muon-Spin Spectroscopy. Phys. Rev. Lett. 103, 147601 (2009).
Huang, W., Marcelli, A. & Xia, D. Application of Synchrotron Radiation Technologies to Electrode Materials for Li- and Na-Ion Batteries. Adv. Energy Mater. 7, 1700460 (2017).
Bak, S.-M., Shadike, Z., Lin, R., Yu, X. & Yang, X.-Q. In situ/operando synchrotron-based X-ray techniques for lithium-ion battery research. NPG Asia Mater. 10, 563–580 (2018).
Asakura, D. et al. Operando soft x-ray emission spectroscopy of LiMn2O4 thin film involving Li-ion extraction/insertion reaction. Electrochem. commun. 50, 93–96 (2015).
Asakura, D. et al. Correlation between the O 2p Orbital and Redox Reaction in LiMn0.6Fe0.4PO4 Nanowires Studied by Soft X-ray Absorption. ChemPhysChem 17, 4110–4115 (2016).
Asakura, D., Nanba, Y., Makinose, Y., Matsuda, H. & Hosono, E. Investigation of the relationship between the cycle performance and the electronic structure in LiAlxMn2−xO4 (x = 0 and 0.2) using soft X-ray spectroscopy. Phys. Chem. Chem. Phys. 19, 16507–16511 (2017).
Liu, X. et al. Phase transformation and lithiation effect on electronic structure of LixFePO4: An in-depth study by soft X-ray and simulations. J. Am. Chem. Soc. 134, 13708–13715 (2012).
Liu, X. et al. Distinct charge dynamics in battery electrodes revealed by in situ and operando soft X-ray spectroscopy. Nat. Commun. 4, 2568 (2013).
Lin, F. et al. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 5, 3529 (2014).
Horiba, K. et al. Scanning photoelectron microscope for nanoscale three-dimensional spatial-resolved electron spectroscopy for chemical analysis. Rev. Sci. Instrum. 82, 113701 (2011).
Nagamura, N. et al. Direct observation of charge transfer region at interfaces in graphene devices. Appl. Phys. Lett. 102, 241604 (2013).
Holt, M., Harder, R., Winarski, R. & Rose, V. Nanoscale Hard X-Ray Microscopy Methods for Materials Studies. Annu. Rev. Mater. Res. 43, 183–211 (2013).
Ebner, M., Marone, F., Stampanoni, M. & Wood, V. Visualization and Quantification of Electrochemical and Mechanical Degradation in Li Ion Batteries. Science 342, 716–720 (2013).
Arango, Y. C. et al. Quantum Transport and Nano Angle-resolved Photoemission Spectroscopy on the Topological Surface States of Single Sb2Te3 Nanowires. Sci. Rep. 6, 29493 (2016).
Nelson Weker, J., Wise, A. M., Lim, K., Shyam, B. & Toney, M. F. Operando Spectroscopic Microscopy of LiCoO2 Cathodes Outside Standard Operating Potentials. Electrochim. Acta 247, 977–982 (2017).
Finegan, D. P. et al. In-operando high-speed tomography of lithium-ion batteries during thermal runaway. Nat. Commun. 6, 6924 (2015).
Petersburg, C. F., Daniel, R. C., Jaye, C., Fischer, D. A. & Alamgir, F. M. Soft X-ray characterization technique for Li batteries under operating conditions. J. Synchrotron Radiat. 16, 610–615 (2009).
Kim, Y., Lee, H. & Kang, S. First-principles and experimental investigation of the morphology of layer-structured LiNiO2 and LiCoO2. J. Mater. Chem. 22, 12874–12881 (2012).
Dahéron, L. et al. Electron Transfer Mechanisms upon Lithium Deintercalation from LiCoO2 to CoO Investigated by XPS. Chem. Mater. 20, 583–590 (2008).
Harilal, M. et al. Pseudocapacitive charge storage in single-step-synthesized CoO-MnO2-MnCo2O4 hybrid nanowires in aqueous alkaline electrolytes. J. Phys. Chem. C. 121, 21171–21183 (2017).
Yoon, W.-S. et al. Oxygen Contribution on Li-Ion Intercalation-Deintercalation in LiCoO2 Investigated by O K-Edge and Co L-Edge X-ray Absorption Spectroscopy. J. Phys. Chem. B. 106, 2526–2532 (2002).
Uchimoto, Y., Sawada, H. & Yao, T. Changes in electronic structure by Li ion deintercalation in LiCoO2 from cobalt L -edge and oxygen K -edge XANES. J. Synchrotron Radiat. 8, 872–873 (2001).
Yeh, J. J. & Lindau, I. Atomic subshell photoionization cross sections and asymmetry parameters: 1 ≤ Z ≤ 103. At. Data Nucl. Data Tables. 32, 1–155 (1985).
Bruce, P. G. In Solid state electrochemistry 5, 313 (Cambridge university press, 1997).
West, A. R. In Solid state chemistry and its applications 427 (John Wiley & Sons, 2014).
Acknowledgements
This work is partially conducted on the basis of the International joint research program for innovative energy technology by Ministry of Economy, Trade and Industry, Japan. A part of this work was supported by TIA collaborative research program “Kakehashi” and JST, PRESTO Grant Number JPMJPR17NB, Japan. Experiments at SPring-8 BL07LSU were performed jointly by the Synchrotron Radiation Research Organization and the University of Tokyo (Proposal Nos 2015B7402, 2016A7402, 2016B7402, 2017A7402, 2017B7402, 2018A7563, 2018A7567).
Author information
Authors and Affiliations
Contributions
E.H. and Y.H. conceived, designed, and coordinated the study. E.H. fabricated LCO. K.H., E.H., Y.H., D.A., N.N. and K.A. designed five terminal holder and established transfer system without air exposure. H.K. fabricated the dry polymer. E.H., H.K. and T.S. prepared the operando cell. K.A., N.N., D.A., E.H., Y.H. and M.O. conducted the experiment and analyzed the data by 3DnanoESCA under the operando condition. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akada, K., Sudayama, T., Asakura, D. et al. Microscopic photoelectron analysis of single crystalline LiCoO2 particles during the charge-discharge in an all solid-state lithium ion battery. Sci Rep 9, 12452 (2019). https://doi.org/10.1038/s41598-019-48842-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48842-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.