Abstract
A series of ZnO/Fe2O3/g-C3N4 photocatalysts were synthetized by impregnation of g-C3N4 with Zn(NO3)2·6H2O, and Fe(NO3)2·9H2O followed by calcination. The morphology, chemical composition, and structure of the resulted materials were carefully analyzed by various characterization techniques. The photocatalytic performance of ZnO/Fe2O3/g-C3N4 composites was evaluated based on the H2 evolution from water splitting reaction. The results showed that the ZnO/Fe2O3/g-C3N4 composite can effectively produce more H2 than pure g-C3N4 when irradiated under visible-light. H2 production rate over 3-ZnO/Fe2O3/g-C3N4 composite was of 25 μmol·h−1, which is 4 times higher than that obtained in the presence of pure g-C3N4, clearly showing a significant improvement of the photocatalytic activity of the prepared nanocomposite. This result was attributed to the formation of a heterojunction between g-C3N4 and ZnO/Fe2O3, which delayed the recombination of holes-electrons pairs and resulted in a remarkable increase in photocatalytic performance.
Similar content being viewed by others
Introduction
Hydrogen is considered an ideal, green energy carrier. Therefore, there is a continuously growing interest for the development of hydrogen production technologies as effective ways to solve energy crisis and environmental pollution1,2. Since Honda reported3 photocatalytic production of over TiO2 under sunlight, many research groups have synthesized semiconductor-based photocatalytic materials, such as oxides4, sulfides5, polymeric materials (e.g., CMP6,7, COF8, and CTF9,10) for the production of hydrogen by photocatalysis11,12. Compared with other photocatalytic materials, graphitic carbon nitride (g-C3N4) is non-toxic and can absorb visible-light. This low-priced material as photocatalyst is widely used for water splitting13, photocatalytic removal of organic pollutants14 and so on15,16,17. However, this promising material has a drawback related to the relatively rapid recombination of photogenerated hole-electron pairs, which limits its applications as photocatalyst under irradiation with visible light18. To address this issue, many strategies were applied, among them the modification of the structure and morphology of g-C3N4 or doping with heteroelements, which lead to noticeable improvements. The incorporation of metal cations in the g-C3N4 matrix induces structural changes of g-C3N4, leading to enhance photocatalytic performance of the final material, owing to the decrease in the combination rate of the photogenerated electrons-holes pairs. In addition, these structural changes may favor the expansion of the photocatalytic response of g-C3N4 to the visible-light range. It is worth underlying that these two achievements are both of great significance for all applications of g-C3N4. Qin et al.19 reported that C (carbon)-doped ZnO/g-C3N4 used for photocatalytic removal of MB and MO under visible-light exhibited higher photocatalytic performance than P25 (TiO2), ZnO, and g-C3N4. Yang20 et al. prepared Cu2O/g-C3N4 nanoparticles for the photocatalytic generation of H2. The rate of H2 evolution was of 33.2 μmol·h–1·g–1 over 5% Cu2O/g-C3N4 nanocomposite. This outstanding result was explained by the rapid separation and shift of photogenerated electron-hole pairs in the interface. Uma et al.21 reported that ZnO/g-C3N4 thin film is an effective photocatalytic material for removal of dyes, whose efficiency increased in comparison with that of ZnO. Yuan22 et al. reported that g-C3N4/CeO2/ZnO nanocomposites exhibited higher photocatalytic performance than that of bare g-C3N4, g-C3N4/CeO2, and ZnO for Methylene Blue (MB) degradation. This research offers the new rational method for environment purification and energy conversion under visible-light. Wang23 et al. synthetized core/shell ZnO@g-C3N4 photocatalysts by a reflux method for the photocatalytic removal of RhB. It was noticed that the photocatalytic performance of ZnO@g-C3N4 was superior to that of bare ZnO and g-C3N4. Some recent literatures have revealed some applications for ZnO/Fe2O3. For example, Ni24 et al. report that Ti/ZnO-Fe2O3 composite could restored of methanol by CO2 by photoelectrocatalyst. Di25 et al. founded that ZnO/Fe2O3/g-C3N4 could degraded sulfonamides. Li26 et al. report that RM-C (red mud modified ZnO-Fe2O3) as photo-Fenton catalyst could weaken the toxicity of Orange II under visible light. Up to now, there is no literature report for hydrogen production efficiency of ZnO/Fe2O3/g-C3N4.
In this paper, Fe(NO3)2 ·9H2O, Zn(NO3)2 ·6H2O, and g-C3N4 were used as starting materials to compound ZnO/Fe2O3/g-C3N4 composite by impregnation. The resulted sample was analyzed in relation to its composition, structure and morphology, whereas the photocatalytic activity was estimated based on the ability to produce H2 from the water splitting reaction under visible-light irradiation. A possible reaction mechanism of water splitting over the prepared sample was proposed and discussed.
Results and Discussion
Figure 1 shows the synthesis route of ZnO/Fe2O3/g-C3N4 composites. During the synthesis, Fe3+ and Zn2+ as the central ions may be form coordinating covalent bond with the N atom of g-C3N4 to form a coordination compound. As a p-type semiconductor, Fe2O3 can make good use of solar energy and combine with n-type semiconductor ZnO to complement each other. It was effectively constructed a p-n heterojunction and used the difference in conduction band potential to spontaneously migrate photogenerated electrons between interfaces to generate useful holes. Thereby that was generated efficient separation of holes and electrons for reducing the recombination of photogenerated electrons-holes, and finally improved photocatalytic activity.
The results of elemental analysis (EA) of bare g-C3N4 and 3-ZnO/Fe2O3/g-C3N4 composite are displayed in Table S1.The nitrogen and carbon contents were measured by elemental analysis, where as zinc and iron were analyzed by ICP-MS. The contents of N and C in pure g-C3N4 are 59.25 and 34.66%, respectively. For 3-ZnO/Fe2O3/g-C3N4, the contents of C and N are 33.71 and 58.64%, respectively while the content of Zn and Fe are 1.6 and 0.6 wt.%, respectively. This indicated that the addition of ZnO and Fe2O3 did not break the structure of g-C3N4 after the impregnation and calcination reactions, and ZnO and Fe2O3 were already loaded onto g-C3N4.
TGA analysis for bare g-C3N4 and 3-ZnO/Fe2O3/g-C3N4 composite were performed from 25 to 600 °C under N2 (5 °C min−1), and the recorded curves are displayed in Fig. S1. The shape of the TGA curve corresponding to 3-ZnO/Fe2O3/g-C3N4 composite indicates a thermal stability of this sample. The 3-ZnO/Fe2O3/g-C3N4 composite showed an obvious weight loss about at 400 °C with a total mass loss of around 70 wt.% at 650 °C in N2 atmosphere. After that, the modification in the TGA curve indicates the decomposition of the composites. Therefore, it can be affirmed that the prepared materials are relatively stable at 600 °C.
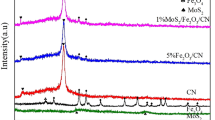
The XRD patterns of bare g-C3N4 and ZnO/Fe2O3/g-C3N4 samples are illustrated in Fig. 2(a). For the pure g-C3N4, two peaks at 13.5 and 27.4° were identified in the XRD pattern and attributed to (100) and (002) crystal planes, respectively (ICDD file 87–1526). The XRD patterns of 11-ZnO/Fe2O3/g-C3N4 and ZnO/Fe2O3 display diffraction peaks at 30.4, 35.6, 57.2, and 62.7°, which correspond to (220), (311), (440), and (511) crystal planes of Fe2O3 (ICDD file 79–0007) confirming the presence of Fe2O3 in the composite material27. The peaks identified at 22.5, 34.8, and 36.8° in both diffractograms were indexed as (100), (002), and (101) crystal planes of ZnO (JCPDs card 79–0206), indicating that the composite also contains ZnO phase28. As the content of ZnO/Fe2O3 increases in the composite samples, the strength of the peaks corresponding to pure g-C3N4 becomes weaker. The characteristic peaks of ZnO and Fe2O3 were not observed in the pattern of 1~7-ZnO/Fe2O3/g-C3N4 samples, because the contents of ZnO and Fe2O3 were too low. When the content of ZnO/Fe2O3 (2:1) is higher than 10%, the characteristic diffraction peaks of ZnO/Fe2O3 were identified in the diffractogram. No other new crystal phases were appeared in the XRD pattern of 11-ZnO/Fe2O3/g-C3N4. The XRD results show that zinc and iron were successfully supported on g-C3N4 as ZnO and Fe2O3, respectively. In addition, the preparation method and thermal treatment applied to the samples to convert the nitrate precursors in the corresponding oxides did not break the crystal structure of pure g-C3N4.
Figure 2(b) displays the FT-IR spectra of pure g-C3N4 and ZnO/Fe2O3/g-C3N4 samples. The bands at 1620–1500 cm−1 are attributed to the C-N-C vibrations of the benzene ring, where as those at 3450 and 1390 cm−1 are due to the vibrations and bending stretching of N-H and C-N, respectively. In that spectra of ZnO/Fe2O3/g-C3N4 composites, the bands identified between1620 and 1500 cm−1 correspond to stretching C-N-C vibration modes of the benzene ring. The bands at 1390 and 3450 cm−1 are attributed to the bending and stretching vibrations of C-N and N-H, respectively. The peaks at 551–652 cm−1 are assigned to the vibration of Zn-O and Fe-O bonds in ZnO and Fe2O3 networks. In the spectrum of ZnO/Fe2O3, the bands between 551–650 cm−1 correspond to the vibrations of Zn-O and Fe-O. Hence, the infrared results are in line with those of XRD indicating the formation of Fe2O3 and ZnO on the surface of g-C3N4.
Figure 3(a) shows the XPS spectra of bare g-C3N4 and 3-ZnO/Fe2O3/g-C3N4. Pure g-C3N4 mainly contains C, N, elements while 3-ZnO/Fe2O3/g-C3N4 composite contains Zn and Fe in addition to C, O, and N. The high resolution spectrum of Zn 2p revealed two binding energies at 1044.6 and 1020.4 eV attributed to Zn 2p1/2 and Zn 2p3/2 in ZnO, respectively. In addition, the peaks of Zn 2p at 1019.9 and 1022.3 eV were identified as Zn2+-O-Fe3+ and Zn-N in Fig. 3(c), respectively29,30,31. The binding energies of Fe 2p1/2 and Fe 2p3/2 were identified at 724.0 and 710.7 eV, respectively, which are in good accord with the standard spectra of Fe 2p1/2 and Fe 2p3/2 in Fe2O3.The peaks of Fe 2p at 713.6, 715.4 and 718.8 eV were attributed to Fe3+-O-Zn2+, Fe(OH)3, and N-Fe-OH in Fig. 3(d), respectively32. The high-resolution spectrum of O1s revealed four binding energies at 529.7, 531.8, 532.6 and 534.6 eV attributed to Fe3+-O-Zn2+, α-Fe2O3, ZnO, and H2O in Fig. S2(b), respectively. These results additionally confirm the load of g-C3N4 surface with ZnO and Fe2O3.
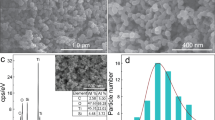
The structure and morphology of g-C3N4 and 3-ZnO/Fe2O3/g-C3N4 were analyzed SEM and TEM. Representative SEM images are shown in Fig. 4(a,b). The pure g-C3N4 displays a sheet-like structure. The composite containing ZnO/Fe2O3 displays a layered structure with irregularities and small particles on the surface, which are attributed to the oxide phases generated by calcination of nitrates. As shown by TEM images in Fig. S3(a), the pure g-C3N4 has a flat thin flake structure. For the composite material, Fig. S3(b) shows a TEM image in which the heterojunction formed between oxide phases and the g-C3N4 is well delimited. It is recognized that such heterojunctions between g-C3N4 and oxide phase (e.g. ZnO/Fe2O3) may enhance the photocatalytic activity of the compound material. The high-resolution TEM images (HRTEM) of 11-ZnO/Fe2O3/g-C3N4 show obvious lattice fringes of ZnO particles, an interplanar spacing of that is 0.247 nm (Fig. 4d), corresponding to the (111) facet, in well agreement with the results of XRD. Similarly, the obvious lattice fringes of Fe2O3 nanoparticles, an interplanar spacing of that is 0.492 nm correspond to the (102) facet, as also observed by XRD. The EDS results revealed that the composite material contains C, N, O, Zn, and Fe elements, indicating that ZnO/Fe2O3 has been loaded onto g-C3N4.
The BET of g-C3N4 and 3-ZnO/Fe2O3/g-C3N4 were obtained by the N2 adsorption&desorption isotherms performed at 77.3 K. As displayed in Fig. S4(a), the g-C3N4 sample displays a type isotherm with H3 type hysteresis, characteristic to a solid with the accumulation of flaky particles. The specific area of 3-ZnO/Fe2O3/g-C3N4 sample, calculated by applying the BET equation, is 23.5 m2/g, which is higher in comparison with that of bare g-C3N4 (11 m2 g−1). The specific surface area of ZnO/Fe2O3 sample is 1.21 m2/g (Fig. S5). The pore volume of g-C3N4 is higher than that of 3-ZnO/Fe2O3/g-C3N4. The increase of specific surface area along with lower pore volume indicates that ZnO/Fe2O3 is loaded to g-C3N4, which is also beneficial to increase photocatalytic activity33.
Figure 5(a) shows the UV-Vis spectra of pure g-C3N4 and ZnO/Fe2O3/g-C3N4 samples. The spectrum of g-C3N4 displays an absorption band in visible light at 440 nm. However, the absorption wavelength of ZnO/Fe2O3/g-C3N4 composites increased with the increase of ZnO/Fe2O3 content comparing to that observed for g-C3N4. Moreover, a red shift of the absorption bands of ZnO/Fe2O3/g-C3N4 was noticed as a result of the coordination bonds formed between Zn and N, Fe and N. These results show that the loading of ZnO/Fe2O3 on g-C3N4 composite changed the electronic structure of the graphitic support, which became able to absorb light at higher wavelengths in the visible range, property favorable for the photo-generation of the electron-hole pairs. According to the band gap energy calculated by the Kubelka-Munk function in Fig. S6, the corresponding band gap energy of g-C3N4 is about 2.82 eV while that of 3-ZnO/Fe2O3 is about 2.44 eV. The band gap energy of ZnO and Fe2O3 are 3.2 and 2.2 eV, respectively. The doping of ZnO/Fe2O3 changed the band structure of the photocatalyst and reduced the band gap energy comparing to that of g-C3N4, and even improved the absorption wavelength of visible light. Fluorescence spectrum of g-C3N4 and ZnO/Fe2O3/g-C3N4 samples were also registered aiming to provide information about transport and recombination of photogenerated hole-electron pairs at 365 nm, and they are shown in Fig. 5(b). The results display that g-C3N4 exhibits a strong fluorescence signal at 440 nm due to the electron transition of n-π* in g-C3N4, and the photogenerated electron is recombined with the holes. However, when the content of ZnO/Fe2O3 increased, the fluorescence intensity of the composite decreased, indicating a lower photogenerated electron binding rate34.
The photocatalytic performance, evaluated as H2 production form water splitting reaction, of all composites obtained under λ > 400 nm irradiation is shown in Fig. 6(a). The H2 production over ZnO/Fe2O3/g-C3N4 composite is 25 μmol·h−1, which is almost 4 times higher than that of pure g-C3N4 (6.5 μmol·h−1). This excellent performance is explained by an efficient separation of the electron-hole pairs as a result of the heterojunction generated by loading ZnO/Fe2O3 on g-C3N4 support. The photogenerated charge separation was determined by electrochemical impedance spectroscopy (EIS). The EIS spectra of g-C3N4 and 3-ZnO/Fe2O3/g-C3N4 composite are illustrated in Fig. 6(b). To note, the radius of 3-ZnO/Fe2O3/g-C3N4 composite is smaller than that of pure g-C3N4, indicating that the 3-ZnO/Fe2O3/g-C3N4 composite has a relatively low charge transport resistance, which can improve the charge separation and migration efficiency of electron-hole35. Photocurrent-time curves of g-C3N4 and 3-ZnO/Fe2O3/g-C3N4 composite are displayed in Fig. 6(c). It can be observed that the curves, obtained by alternatingly cutting off the visible light irradiation display several on-off cycles, the photocurrent of the two samples being significantly enhanced under irradiation. When the visible light irradiation was cut off, the current intensity rapidly decreased to zero, confirming the generation of the photocurrent by visible light irradiation. The fluorescent decay spectra also indicated that the 3-ZnO/Fe2O3/g-C3N4 composite has a shorter lifetime of 13.23 ns than that of g-C3N4 (17.62 ns) (Fig. 6d, Table S2), implying the low inactivation probability of photo-induced electrons and leading to high photocatalytic activity. The photocatalytic recyclability and stability of photocatalyst are important for the applications36. Moreover, the enhancement of the photocurrent in the ZnO/Fe2O3/g-C3N4 composite is attribute to the heterojunction formation between g-C3N4 and ZnO/Fe2O3, which promotes a valid separation of photo-generated holes and electrons. The stability and recyclability of 3-ZnO/Fe2O3/g-C3N4 composite were determined by three cycles under the same conditions (Fig. 6(e)). After three cycles, the photocatalyst 3-ZnO/Fe2O3/g-C3N4 had stability properties. The photocatalytic activity showed a slight decrease during the cycling process. It may be due to a decrease in the concentration of the substrate or a weaker response of the reactive sites to the photon. The apparent quantum yield (AQY) were shown in Fig. 6(f). The highest AQY of the 3-ZnO/Fe2O3/g-C3N4 photocatalyst is 0.4% at 380 nm, and then the AQY decreases with increasing the wavelength of the incident light, which is in keeping with the UV-Vis absorption spectra of the 3-ZnO/Fe2O3/g-C3N4 composite showing a maximum absorption peak at about 380 nm, proving that the evolution of H2 is indeed enhanced by the light absorption of the photocatalyst37.
(a) H2 evolution of the samples with 3 wt% Pt under visible-light (λ > 400 nm); (b) EIS plots and (c)Transient photocurrent curves and (d) Fluorescence decay spectra of g-C3N4 and 3-ZnO/Fe2O3/g-C3N4 composite; (e) Stability test of 3-ZnO/Fe2O3/g-C3N4 composite with 3 wt% under visible light (λ > 400 nm); (f) The AQY and UV-Vis absorption spectrum of 3-ZnO/Fe2O3/g-C3N4 composite.
The results obtained for the hydrogen production allowed the establishment of a potential mechanism that governs the production of H2 from water splitting reaction over the prepared ZnO/Fe2O3/g-C3N4 composites. The possible mechanism is schematically presented in Fig. 7. The conduction band (CB) and valence band (VB) of g-C3N4 are −0.91 (vs NHE) and 1.91 eV, respectively, as measured by electrochemical CV curve (Ev = Ec + 2.82) (Fig. S9). The CB and VB of ZnO/Fe2O3 are −0.86 eV (vs NHE) and 1.58 eV, respectively (Ev = Ec + 2.44). The electron transport is generated on the CB g-C3N4 of onto the CB of ZnO/Fe2O3, and g-C3N4 transfers electrons to the promoter Pt, where H+ get electron-reduced to H2, and hole transport is generated on the VB of g-C3N4. On the VB of ZnO/Fe2O3, TEOA gets a positive charge and becomes TEOA+.
Conclusion
In this paper, ZnO/Fe2O3/g-C3N4 composite photocatalysts were synthesized by impregnation followed by calcination and their photocatalytic performance was evaluated in H2 production from water splitting. The chemical composition in bulk and surface, crystalline phases, morphology, electronic states, band gap values, texture, interfacial electronic interactions, as well as charge transport and recombination were established by various characterization techniques, such as ICP-MS, XPS, XRD, DR UV-Vis, SEM, TEM, N2 physisorption, and EIS, respectively. The experimental results obtained for the photocatalytic tests showed remarkably improved activity for 3-ZnO/Fe2O3/g-C3N4 composite with compared to pure g-C3N4. Hence, the H2 evolution under visible light for the composite was of 25 μmol·h−1 while an activity of 6.5 μmol·h−1 was obtained for pure g-C3N4. This increased activity originated from the heterojunction formed between g-C3N4 and ZnO/Fe2O3, which enabled a high separation rate and mobility of the photogenerated electrons and holes. Therefore, this work proposed a feasible strategy for improving the photocatalytic activity of g-C3N4 by loading ZnO and Fe2O3, which can be useful for the design and preparation of other new and highly active composite photocatalysts for H2 production from water splitting.
Materials
Melamine (C3H6N6, 99%), triethanolamine (C6H15NO3, TEOA, AR), zinc nitrate hexahydrate (Zn(NO3)·6H2O, 98%), iron nitrate nonahydrate (Fe(NO3)2·9H2O, 98%), ethanol (CH3CH2OH, AR), tetrabutylammonium hexafluorophosphate (NBu4PF6, AR), sodium sulfate (Na2SO4, AR) were supplied from Sinopharm Chemical Reagent Co. Ltd (China).
Synthesis of the ZnO/Fe2O3/g-C3N4 photocatalysts
3 g of melamine was immediately heated at 550 °C and then hold for 4 h (5 °C min−1) under nitrogen flow to obtain the pure g-C3N4 powders. The synthesis procedure of the ZnO/Fe2O3/g-C3N4 photocatalysts are provided as below. Different mass rations of zinc nitrate hexahydrate and iron nitrate nonahydrate were dissolved in 80 mL ethanol. After, 500 mg of g-C3N4 was added into the solution and ultrasonicated for 24 h. The suspension was evaporated the solvent, and the gotten solid was heated in a tube furnace at 400 °C for 4 h under nitrogen flow. The products were labeled as x-ZnO/Fe2O3/g-C3N4, where x = (1, 3, 5, 7, 15 wt%) shows the ZnO/Fe2O3 content in the photocatalysts. The pure ZnO/Fe2O3 was get by directly calcination of the zinc nitrate hexahydrate and iron nitrate nonahydrate.
Characterization of the ZnO/Fe2O3/g-C3N4 photocatalysts
FT-IR (Fourier transform infrared, Tensor 27 FT-IR spectrometer) spectra of all samples were obtained using KBr disks. TGA were evaluated in STA449F5 from 30 to 800 °C under N2 atmosphere at a heating rate of 10 °C min−1. The Powder X-ray diffraction (PXRD) measurement was carried out on X-ray diffractometer (D/Max-3c). Morphology of the samples were conducted by using SEM (FEI, Quanta 200) and TEM (JEOL, JEM-2100). Surface areas and pore size distributions were recorded by BET on an ASAP 2420–4 (Micromeritics) at 77.3 K. UV-Vis reflectance spectra (DRS, UV-Lambda 950 PerkinElmer, US) were applied to characterize band gap and absorption length in the region 200–800 nm based on BaSO4. X-ray photoelectron spectroscopy (XPS) measurement was performed on a HI5700ESCA instrument with monochromatic Al Ka (1486.6 eV) X-ray radiation. PL of the samples were conducted using a Shimadzu F-7000 PC at an excitation wavelength of 365 nm. The Zn and Fe contents were measured by inductively coupled ICP-MS (Leeman).
Photocatalytic reaction
The generation of H2 was carried out over a photocatalyst in a quartz glass container. The photocatalyst (100 mg) was dispersed in 100 mL of solution, which contains 20 vol % TEOA as a sacrificial agent for H2 production. All photocatalytic experiments were performed in a quartz glass reactor that allowed the circulation of water at room temperature. The suspension was thoroughly removed air and irradiated by a 300 W Xe lamp (PLS-SEX300/300UV, Beijing Perfect Light Co.) equipped with a UV cut off filter (λ > 400 nm). The generated H2 was measured by an online Agilent 7890 gas chromatograph (TCD), using N2 as carrier gas for H2.
Change history
02 April 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-024-58322-1
References
Yu, X. B., Tang, Z. W., Sun, D. L., Ouyang, L. Z. & Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 88, 1–48 (2017).
Yasuhiro Tachibana, L. Va. J. R. D. Artificial photosynthesis for solar water-splitting. Nature Photon. 6, 511–518 (2012).
Fujishima, A. & Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 238, 37 (1972).
Ma, D. D. et al. Rational design of CdS@ZnO core-shell structure via atomic layer deposition for drastically enhanced photocatalytic H2 evolution with excellent photostability. Nano. Energy 39, 183–191 (2017).
Qiu, F. et al. Photocatalytic Hydrogen Generation by CdSe/CdS Nanoparticles. Nano. Lett. 16, 5347–5352 (2016).
Sprick, R. S. et al. Tunable organic photocatalysts for visible-light-driven hydrogen evolution. J. Am. Chem. Soc. 137, 3265–3270 (2015).
Wang, L. et al. Conjugated Microporous Polymer Nanosheets for Overall Water Splitting Using Visible Light. Adv. Mater. 29, 1702428 (2017).
Medina, D. D., Sick, T. & Bein, T. Photoactive and Conducting Covalent Organic Frameworks. Adv. Energy Mater. 7, 1700387 (2017).
Meier, C. B. et al. Structure-property relationships for covalent triazine-based frameworks: The effect of spacer length on photocatalytic hydrogen evolution from water. Polym. 126, 283–290 (2017).
Bi, J. et al. Covalent Triazine-Based Frameworks as Visible Light Photocatalysts for the Splitting of Water. Macromol. Rapid Commun. 36, 1799–1805 (2015).
Zhu, S. J. et al. Mesoporous Ni-Doped-Bi2O3 Microspheres for Enhanced Solar-Driven Photocatalysis: A Combined Experimental and Theoretical Investigation. J. Phys. Chem. C 121, 9394–9401 (2017).
Li, J. Y. et al. Probing the ring-opening pathways for efficient photocatalytic toluene decomposition. J. Mater. Chem. A 7, 3366–3374 (2019).
Wang, X. et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8, 76 (2008).
Suryawanshi, A. et al. Doubling of photocatalytic H2 evolution from g-C3N4 via its nanocomposite formation with multiwall carbon nanotubes: Electronic and morphological effects. Int. J. Hydrogen Energy 37, 9584–9589 (2012).
Chen, P. et al. Directional electron delivery and enhanced reactants activation enable efficient photocatalytic air purification on amorphous carbon nitride Co-functionalized with O/La. Appl. Catal. B: Environ. 242, 19–30 (2019).
Cui, W. et al. Enhancing ROS generation and suppressing toxic Intermediate production in photocatalytic NO oxidation on O/Ba co-functionalized amorphous carbon nitride. Appl. Catal. B: Environ. 237, 938–946 (2018).
Li, J. Y. et al. The spatially oriented charge flow and photocatalysis mechanism on Internal van der waals heterostructures. ACS. Catalysis 8, 8376–8385 (2018).
Cao, S. & Yu, J. g-C3N4-Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 5, 2101–2107 (2014).
Qin, J. Q. et al. Two-dimensional porous sheet-like carbon-doped ZnO/g-C3N4 nanocomposite with high visible-light photocatalytic performance. Mater. Lett. 189, 156–159 (2017).
Yin, C. J., Sun, S.-N. S. S. & Yang, S. Y. An in situ mediator-free route to fabricate Cu2O/g-C3N4 type-II heterojunctions for enhanced visible-light photocatalytic H2 generation. Appl. Surf. Sci. 434, 1224–1231 (2018).
Uma, R., Ravichandran, K., Sriram., S. & Sakthivel, B. Cost-effective fabrication of ZnO/g-C3N4 composite thin films for enhanced photocatalytic activity against three different dyes (MB, MG and RhB). Mater. Chem. Phys. 201, 147–155 (2017).
Yuan, Y. et al. Construction of g-C3N4/CeO2/ZnO ternary photocatalysts with enhanced photocatalytic performance. J. Phys. Chem. Solids 106, 1–9 (2017).
Wang, L. et al. In-situ growth of g-C3N4 layer on ZnO nanoparticles with enhanced photocatalytic performances under visible light irradiation. Mater. Lett. 188, 347–350 (2018).
Xia, S. J. et al. Ti/ZnO-Fe2O3 composite: synthesis, characterization and application as a highly efficient photoelectrocatalyst for methanol from CO2 reduction. Appl. Catal. B, Environ. 187, 122–133 (2016).
Di, G. L. et al. Visible-light degradation of sulfonamides by Z-scheme ZnO/g-C3N4 heterojunctions with amorphous Fe2O3 as electron mediator. J. Colloid Interface Sci. 538, 256–266 (2019).
Li, Y. S. et al. Green synthesis of red mud based ZnO-Fe2O3 composite used for photo-Fenton reaction under visible light. J. Clean. Prod. 207, 717–727 (2019).
Zhu, B. C., Xu, Q. L., Jiang, C. J., Cheng, B. & Yu, J. G. Constructing 2D/2D Fe2O3/g-C3N4 Direct Z-Scheme Photocatalysts with Enhanced H2 Generation Performance. Sol. RRL 2, 1800006 (2018).
Li, L., Sun, S.-Q., Wang, Y.-X. & Wang, C.-Y. Facile synthesis of ZnO/g-C3N4 composites with honeycomb-like structure by H2 bubble templates and their enhanced visible light photocatalytic performance. J. Photochem. Photobiol. A: Chemis. 335, 16–24 (2018).
Sankaranarayanan, T. M., Thirunavukkarasu, K., Pandurangan, A., Shanthi, V. & Sivasanker, S. The role of surface Zn2+ ions in the transesterification of vegetable oils over ZnO supported on Al2O3 and Fe2O3. Catal. Sci. Technol. 4, 851–860 (2014).
Haider, M. B. XPS Depth Profile Analysis of Zn3N2 Thin Films Grown at Different N2/Ar Gas Flow Rates by RF Magnetron Sputtering. Nanoscale Res. Lett. 12, 5 (2017).
Zhang, H. et al. Effect of surface carbon contamination on the chemical states of N-doped ZnO thin films. Appl. Phys. A 124, 147 (2018).
Huang, Z. H., Xie, N. H., Zhang, M., Xu, B. Q. & Nonpyrolyzed, F.-N. Coordination-Based Iron Triazolate Framework: An Efficient and Stable Electrocatalyst for Oxygen Reduction Reaction. Chem. Sus. Chem. 11, 1–9 (2018).
Cao, S.-W. et al. Solar-to-fuels conversion over In2O3/g-C3N4 hybrid photocatalysts. Appl. Catal. B: Environ. 147, 940–946 (2014).
Zang, Y. P., Li, P. L., Li, X. G., Lin, R. & Li, G. S. Synergistic collaboration of g-C3N4/SnO2 composites for enhanced visible-light photocatalytic activity. Chem. Eng. J. 246, 277–286 (2014).
Shi, X., Fujitsuka, M., Kim, S. & Majima, T. Faster Electron Injection and More Active Sites for Efficient Photocatalytic H2 Evolution in g-C3N4/MoS2 Hybrid. Small 14, 1703277 (2018).
Mao, N. & Jiang, J.-X. MgO/g-C3N4 nanocomposites as efficient water splitting photocatalysts under visible light irradiation. Appl. Surf. Sci. 476, 144–50 (2019).
Wen, Z. Q. et al. Morphology-controlled MnO2-Graphene oxides-diatomaceous earth 3-dimensional (3D) composites for high-performance supercapacitors. Dalton Trans. 45, 936–942 (2013).
Author information
Authors and Affiliations
Contributions
N. Mao performed the research work and assisted in the experiments. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The author declares no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mao, N. RETRACTED ARTICLE: Investigating the Heteronjunction between ZnO/Fe2O3 and g-C3N4 for an Enhanced Photocatalytic H2 production under visible-light irradiation. Sci Rep 9, 12383 (2019). https://doi.org/10.1038/s41598-019-48730-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48730-z
This article is cited by
-
Integration of plasmonic AgPd alloy nanoparticles with single-layer graphitic carbon nitride as Mott-Schottky junction toward photo-promoted H2 evolution
Scientific Reports (2022)
-
Three-dimensional Zn-based alloys for dendrite-free aqueous Zn battery in dual-cation electrolytes
Nature Communications (2022)
-
Fabrication of highly efficient g-C3N4/ZnO/Fe2O3 ternary composite with enhanced photocatalytic activity under visible light irradiation
Journal of Materials Science: Materials in Electronics (2022)
-
Development of ZnO/MOGAC nanocomposites for enhanced photocatalytic removal of PO43− and NO3- ions from wastewater under various light irradiations
Biomass Conversion and Biorefinery (2022)
-
Facile green synthesis of CuO- ZnO nanocomposites from Argyreia nervosa leaves extract for photocatalytic degradation of Rhodamine B dye
Biomass Conversion and Biorefinery (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.