Abstract
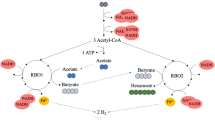
The caproate-producing bacterium, Megasphaera hexanoica, metabolizes fructose to produce C2~C8 carbon-chain carboxylic acids using various electron acceptors. In particular, odd-chain carboxylic acids (OCCAs) such as valerate (C5) and heptanoate (C7), were produced at relatively high concentrations upon propionate supplementation. Using a statistical experimental design method, the optimal culture medium was established for the selective production of OCCAs among the total produced acids. In a medium containing 2.42 g L−1 sodium acetate and 18.91 g L−1 sodium propionate, M. hexanoica produced 9.48 g L−1 valerate, 2.48 g L−1 heptanoate, and 0.12 g L−1 caproate. To clarify the metabolism of the exogenous added propionate for OCCAs production, 13C tracer experiments were performed by supplementing the culture broth with [1,2,3-13C3] propionate. The metabolites analysis based on mass spectrometry showed that the propionate was only used to produce valerate and heptanoate without being participated in other metabolic pathways. Furthermore, the carbon elongation pathway in M. hexanoica was explained by the finding that the incorporation of propionate and acetate in the produced valerate occurred in only one orientation.
Similar content being viewed by others
Introduction
Volatile fatty acids (VFAs) have various applications, such as microbial nitrogen removal, the production of biofuels, the generation of electricity using microbial fuel cells, the synthesis of biosurfactants and biopolymers, and usages in the food, textile, pharmaceutical, leather, and plastics industries1,2,3,4,5,6,7. VFAs that are secreted extracellularly can be classified into short-chain carboxylic acids (SCCAs), such as acetic acid, propionic acid, and butyric acid, and medium-chain carboxylic acids (MCCAs) with chains of 5~8 carbons (valeric acid, caproic acid, heptanoic acid, and caprylic acid). During anaerobic fermentation, SCCAs are elongated to MCCAs by the addition of 2-carbon (C2) units. Several bacteria have been reported to produce MCCAs by the reverse β-oxidation pathway (r-BOP)8,9.
Generally, the value of VFAs increases with the increasing carbon number. The market prices of different VFAs increase in the following order: acetic acid (400 ~ 700 USD/ton), propionic acid (1,900 ~ 2,000 USD/ton), butyric acid (2,000 ~ 2,200 USD/ton), and valeric acid (2,500 ~ 3,000 USD/ton). This pricing pattern can be attributed both to the wider industrial application of longer-chain carboxylic acids and to the greater difficulty of their production than that of SCCAs7. Therefore, there are many research reports on the r-BOP in which SCCAs are elongated to MCCAs, which have a higher economic value and are easier to separate and purify10,11,12,13. To convert SCCAs into MCCAs via condensation with acetyl-CoA by the r-BOP, energy-rich reduced compounds are needed to provide energy and reduce equivalents. Acetyl-CoA adds two carbons in each subsequent round of fatty acid synthesis. Consequently, acetate (C2) can be elongated to the butyrate (C4) and further elongated to caproate (C6) and caprylate (C8). Odd-carbon chains can also be produced in the presence of high cellular propionyl-CoA levels due to their incorporation in place of acetyl-CoA in the initial step of fatty acid synthesis. In this case, propionate (C3) will be elongated to valerate (C5) and further elongated to heptanoate (C7). Since acetyl-CoA is a common natural biogenic precursor for the biosynthesis of numerous metabolites, while propionyl-CoA is toxic at high concentrations, propionyl-CoA is rare and non-native to most organisms14. Presumably, most organisms have evolved to prevent the intracellular accumulation of propionyl-CoA14. Therefore, various studies have been conducted on even-chain MCCA production, but the production of odd-chain MCCAs, which are industrially useful as plasticizers and herbicides, as well as in the fragrance industry, has not been actively investigated15,16,17. Odd-chain carboxylic acid (OCCA) production has previously been achieved, but the concentration of OCCAs was low, and their productivities were lower than those of even-chain carboxylic acids (ECCAs)18,19.
Various chain elongation processes have been proposed to obtain OCCAs, such as valerate and heptanoate. A reactor microbiome consisting of a continuously stirred tank reactor (CSTR) supplied with propionate and ethanol produced 4.6 g L−1 valerate, 4.9 g L−1 caproate and 3.2 g L−1 heptanoate18. Another CSTR that utilized the initial sludge inoculum that was obtained from a full-scale anaerobic digester treating potato waste had a main elongated product of 3.1 g L−1 valerate along with 1.2 g L−1 caproate and traces of heptanoate from the propionate−ethanol mixture19. A pure culture of Clostridium kluyveri, which was also supplied with propionate and ethanol, produced 7.4 g L−1 valerate in a batch reactor after 18 days20, in addition to 0.8 g L−1 caproate and 0.3 g L−1 heptanoate. In contrast to mixed-culture fermentation, although the productivity was very low, the pure culture of C. kluyveri showed high selectivity for valerate. In another example, Megasphaera hexanoica, which was isolated by our group, produced various MCCAs, including both even- and odd-chain carboxylic acids (C5~C8) with high productivities and titers when C2, C3, and C4 carboxylic acids were added to the medium as electron acceptors9,21. In a medium containing acetate and propionate with fructose, M. hexanoica generated 5.7 g L−1 valerate, 1.5 g L−1 caproate, and 2.7 g L−1 heptanoate within just 1 day, showing a high selectivity of 85% for OCCAs. These physiological characteristics of M. hexanoica are expected to enable a process to generate OCCAs with high selectivity and productivity. Since anaerobic digestion of organic wastes has recently gained attention as a cost-effective and environmentally friendly alternative to acetate and propionate production through the prevailing acidogenic metabolic pathways in the digester, for the industrial implementation of OCCA production by M. hexanoica, the acetate and propionate from the anaerobic digestion process can be attractive and promising SCCAs.
Jeon et al. showed that the production of ECCAs was influenced by the concentrations of acetate and butyrate, and the response surface method (RSM) experiments were conducted to get an optimized medium composition, including acetate and butyrate for Caproiciproducens galactitolivorans, as well as M. hexanoica22,23. Adding butyrate into the medium could stimulate the production of ECCAs. Therefore, experiments tracing the 13C isotope were conducted to explain the direct conversion of the supplemented acids, such as acetate and propionate, into OCCAs.
The objective of this study was to achieve efficient production of OCCAs, such as valerate and heptanoate, with a high selectivity and production rate throughout the r-BOP with supplemented SCCAs by M. hexanoica. Then, the carbon elongation mechanism for the production of OCCAs by M. hexanoica was investigated in tracking experiments that involve 13C-labelled propionic acid. The findings from this study demonstrate that through the application of selective operating conditions for OCCA production, optimized compositions of supplemented SCCAs and extractive fermentation can be reproducibly driven towards the simultaneous stable production of high levels of MCCAs, especially specific OCCAs with higher levels of SCCAs conversion.
Results
OCCA production by M. hexanoica with supplemented propionate
M. hexanoica has been reported to produce various OCCAs, including valeric acid and heptanoic acid, to 4.1 g L−1 and 2.0 g L−1 in a medium that contains supplemented propionate9. Along with the change of propionate by the supplemented amount, the adjustment of acetate concentration was selected as a significant factor, because acetate has been reported to be an electron acceptor that increases cell growth and metabolite production in Megasphaera species24. Jeon et al. showed that the addition of acetate along with propionate increased the concentration of valeric acid and heptanoic acid by 1.3~1.4 times9. However, as shown in the Supplementary Figure S1, the addition of excess acetate reduced the selectivity of OCCAs. Therefore, the optimum concentration of acetate with propionate needs to be determined. Until now, no optimized conditions for OCCA production with supplemented SCCAs had been developed, and the factors that affect the propionate-based carbon elongation mechanism via r-BOP had not been investigated in depth.
Verification of OCCA production at the optimized medium compositions
To investigate the production of OCCAs from the supplemented SCCAs (acetate and propionate), the ranges of two independent variables (i.e., the concentrations of sodium acetate and sodium propionate) were decided according to the results of a series of experiments; namely, the one-fact-at-a-time test (OFAT), the fractional factorial experimental design (FFD), and the steepest ascent method experimental design (SAM)22,25,26. From these analyses, the concentration ranges of sodium acetate and sodium propionate were set to 2.29–2.51 g L−1 and 16.46–23.54 g L−1, respectively. To clarify the optimized concentration of supplemented acetate and propionate, an investigation using response surface methodology (RSM) with central composite design (CCD) was conducted. The valerate production was selected as the response due to the different cycles of the runs. The experimental design matrix was presented in Table 1. Thirteen experiments were performed in duplicate. A regression model was fitted to the production of valeric acid and was obtained in the form of the following equation:
where X2 represents the coded value of sodium propionate.
Using an analysis of variance (ANOVA) to evaluate the appropriateness of the model, the predicted model was examined by an F-test and the coefficient of determination, R2. The F and p values of the model were 13.11 and 0.0019, respectively (Table 2). R2 and the adjusted R2 were 0.90 and 0.83, respectively, which indicated that the regression model is appropriate for describing the results that are presented in Table 2. As shown in Equation (1), the p values of X2 and X22 were significant, i.e., p < 0.05 (Table 2), but X1, X12, and X1X2 had p > 0.05, which indicated that they were not significant. This result means that the concentration of sodium acetate did not significantly influence valeric acid production; therefore, sodium acetate (X1) was excluded from the regression model equation. From the optimum point for valeric acid production, which is shown in Fig. 1a, the optimized medium compositions were 2.42 g L−1 of sodium acetate and 18.91 g L−1 of sodium propionate, respectively. In the optimized compositions, the maximized concentration of valeric acid was calculated to be 13.15 g L−1 (Fig. 1a). In addition, as shown in Fig. 1b, heptanoic acid production was also calculated in the 3D contour plot, with an optimum point for heptanoic acid production (see Supplementary Information, Tables S1, S2 and Eq. S1). When the sodium acetate and sodium propionate were 2.44 g L−1 and 17.64 g L−1, respectively, the model predicted 3.21 g L−1 of heptanoic acid. The optimum conditions for heptanoic acid overlapped with the optimum range for valeric acid production, which indicated that heptanoic acid production was closely related to valeric acid production.
To verify the optimum conditions for OCCA production, the optimum conditions were evaluated by a time course experiment in a batch reactor. The results showed that the predicted value was similar to the experimental values (Fig. 2a). M. hexanoica produced 9.48 g L−1 valeric acid and 2.48 g L−1 heptanoic acid when grown in the optimized medium composition for 48 h. Interestingly, the acetate concentration was unchanged during fermentation because the produced acetate by M. hexanoica using fructose was utilized on the OCCAs production with supplemented propionate, and the amounts of even-carbon MCCAs, such as caproic acid and butyric acid, were extremely low. The produced amounts of OCCAs were 99% of the total produced MCCAs, with a highly selective production of OCCAs. The productivities of valerate in this study were higher than that of any other previous results and were almost two times higher due to extractive fermentation with a fed-batch culture (Table 3).
M. hexanoica has been reported to produce even-carbon MCCAs, mainly caproic acid, up to 9.7 g L−1 with fructose as the sole carbon source9. Even in our case, M. hexanoica consumed 20 g L−1 fructose as a carbon source, but most of the produced MCCAs were extremely biased towards OCCAs. Therefore, an important question was whether the addition of propionic acid stimulated OCCA production or it was self-incorporated. In particular, Fig. 2b shows that the supplemented propionate was utilized to produce OCCAs with high selectivity in comparison with the consumed and produced molar concentrations of each compound.
Investigation of selective OCCA production with carbon elongation mechanisms by r-BOP using supplemented 13C propionate
Propionate labelled with 13C for all carbons was supplemented to trace the position of the propionate incorporation into produced MCCAs and to investigate carbon elongation through r-BOP under the optimal condition mentioned above. Predecessor tracking of the 13C isotope was conducted to eliminate the possibility of the decomposition of propionic acid. As shown in Fig. 3, through the acrylate pathway, acetyl-CoA can be generated with one CO2 per molecule from extracellular propionate. The mass spectra of all fatty acids, including even-carbon fatty acids, were scrutinized. However, 13C isotopes were observed only in OCCAs, such as valeric acid and heptanoic acid (see Supplementary Information, Fig. S2). In addition, the analysis of gas components, such as CO2 and H2 by GC-MS, did not detect CO2 containing the 13C isotope in the gas phase (see Supplementary Information, Fig. S3).
The mass spectrum for the produced valeric acid was shown in Fig. 4. In Fig. 4a, the first ion, m/z 60 (peak 1), was observed, which indicated that the m/z 60 ion contained a stable isotope and was derived from carbohydrates, such as fructose. However, the m/z 73 ion (12C3H6O2+) and the m/z 87 ion (12C4H8O2+) were shifted to m/z 74 (peak 2, 13C12C2H6O2+) and to m/z 89 (peak 3, 13C212C2H8O2+), respectively, which indicated 13C labelling at the 3rd and 4th carbons of the produced valeric acid from the supplemented 13C-labelled propionic acid (Fig. 4b). The 13C isotope was also incorporated at the 5th carbon of valeric acid, since the mass fragment at m/z 104 appeared faintly. This result indicated that extracellular propionic acid was incorporated entirely into >C5 OCCAs, without decomposition by any catabolic pathway, and the 13C isotopes were observed at the 3rd, 4th and 5th carbons of valeric acid (Fig. 4b). In the genomic analysis of M. hexanoica (data not shown), there is no metabolic pathway for propionate utilization (including the acrylic pathway) in M. hexanoica. Thus, the extracellular propionic acid was only used as an electron acceptor via the r-BOX pathway for OCCAs production.
Mass spectrum of valeric acid obtained by GC-TOF/MS and the predicted structure of produced valeric acid. (a) Mass spectrum of valeric acid in a culture broth of Megasphaera hexanoica with [1,2,3-13C3] propionate. (b) Predicted structure of the resulting valeric acid with 13C-labelled moieties at the 3rd to 5th carbons. (c) Metabolic pathways of fractional 13C-labelled intermediate metabolites produced from [1,2,3-13C3] propionate input by Megasphaera hexanoica. Labelled 13C atoms, red circles; 12C atoms, blue circles.
Discussion
The supplemented SCCAs generally can be elongated to MCCAs through two different metabolic pathways, the keto-acid pathway (KAP) and the r-BOP. The engineered r-BOP showed better performance than KAP in synthesizing the MCCAs of varying carbon lengths from the supplemented SCCAs. Although the r-BOP can convert the SCCAs to MCCAs at a faster rate with low energy consumption, previous reports showed two bottleneck steps of the r-BOP, such as acyl-CoA synthetase (or transferase) and thiolase activities27. In the engineered r-BOP for pentanol (C5-alcohol) synthesis in engineered E. coli, pentanol was observed as only a minor component in the product mixture, and propionate supplementation increased the pentanol production28. To enhance the selectivity and to accelerate the productivity of the desired MCCA products, the information about bottleneck nodes in r-BOP should be updated, as this would facilitate further metabolic engineering.
Recently, MCCAs as secondary products of anaerobic fermentation via the r-BOP have been reported7 in anaerobic bioreactors that were inoculated with open cultures and fed with diverse carbon sources, such as ethanol, organic wastes, and sugars10,29,30. The microbiome in the anaerobic bioreactors showed that Megasphaera species play an important role as MCCAs producers30,31. In particular, Megasphaera elsdenii24 and Megasphaera indica32 showed excellent capability to produce MCCAs through chain elongation of the r-BOP using supplemented SCCAs. Recently, the production of valeric acid from the supplemented propionate by M. elsdenii33 demonstrated that Megasphaera species can produce not only ECCAs but also OCCAs. In this study, detailed carbon elongation mechanisms for the OCCA production of our newly isolated M. hexanoica was investigated using 13C labelled propionic acid.
In our previous study, M. hexanoica produced 5.7 g L−1 of valeric acid in medium supplemented with 100 mM acetate and propionate for OCCA production9. Bornstein and Barker reported selective OCCA production for up to 91% of the total MCCAs produced by C. kluyveri from the supplemented ethanol and propionate20. However, the batch culture was operated for 18 days until 7.4 g L−1 of valeric acid was obtained (Table 3). Likewise, Steinbüsch et al. reported heptanoate production from ethanol and propionate via carbon elongation with the r-BOP18. However, the selectivity of OCCAs was relatively low (Table 3), because the formation of acetyl-CoA from the supplemented ethanol was preferred to the propionyl-CoA formation from supplemented propionate18. In this study, using RSM, the optimum condition for highly selective OCCA production was obtained. Under the optimized condition, M. hexanoica produced 9.48 g L−1 of valeric acid and 2.48 g L−1 of heptanoic acid, while the selectivity of OCCAs was 99% (Table 3). In particular, the valeric acid concentration was up to 12.4 g L−1 with 0.51 g L−1 h−1 of productivity from the extractive fermentation with fed-batch culture. To our knowledge, this resulted in the highest production and productivity of valeric acid using anaerobic fermentation.
The molar concentration of consumed propionic acid was compared to the molar concentration of produced OCCAs: 117.0 ± 12.3 mM propanoic acid was consumed, and 109.5 ± 11.3 mM OCCAs (90.5 ± 8.9 mM of valeric acid and 19.0 ± 2.4 mM of heptanoic acid) was produced (Fig. 2b). As a result, the molar concentrations of acid consumed and produced were similar, which implied that the added propionic acid was incorporated into OCCAs. Thus, to understand what mechanism is used to convert propionate into valerate in M. hexanoica, tracking experiments using 13C labelled propionic acid ([1,2,3-13C3] propionate) were conducted. The tracking experiment using 13C isotope was an excellent, effective analysis tool of ABE fermentation34,35 and the acrylate pathway of M. elsdenii36.
Performing the tracking experiment using [1,2,3-13C3] propionate, it was revealed that the propionate incorporated into valerate without decomposition and the 13C isotopes were observed at the 3rd, 4th and 5th carbons of the valeric acid. The incorporation mechanism of propionic acid seemed to coincide with a carbon elongation mechanism using the r-BOP. The r-BOP tends to increase the carbon number of carboxylic acids by two carbons. The postulated incorporation mechanism, as shown in Fig. 4c, is that one valeric acid could be condensed from propionyl-CoA and acetyl-CoA. Similarly, valeryl-CoA could be condensed with one acetyl-CoA molecule given to heptanoyl-CoA. Although the heptanoic acid produced by the 13C-labelled propionic acid would not be predicted as a single isotope sequence in the GC-TOF/MS spectrum, if the reaction proceeded according to the r-BOP, then the heptanoic acid incorporating the added propionic acid, as shown in Fig. 4c, would have 13C located at the 5th, 6th and 7th carbons.
The r-BOP in this study started with the extracellular propionate entry into the cell. Some anaerobic bacteria, such as Clostridium actobutylicum ATCC 824, take extracellular carboxylic acids into the cell by phosphotransbutyrylase (Ptb) and butyrate kinase (Buk)37. The reaction mediated by Ptb-Buk produces or consumes ATP. On the other hand, acetyl-CoA transferase (ACT) mediates the uptake and secretion reaction without ATP. With regard to the secretion, ACT was predicted as an enzyme that catalyses the reaction to form caproate from caproyl-CoA in the putative pathway of caproic acid production in Ruminococcaceae bacterium CPB6 and M. elsdenii38,39. The enzyme was expected to catalyse in the reverse direction. In a similar reaction, propionyl-CoA transferase (PCT) from M. elsdenii mediates the reaction to form propionyl-CoA from the extracellular propionate40. Tseng et al. produced odd-chain fuels and chemicals via genetic engineering Escherichia coli inserted a pct gene from M. elsdenii28. It is predicted that ACT plays the role of introducing the extracellular carboxylic acid into the cell in M. hexanoica, as well. Indeed, a caproic acid-producing E. coli strain was constructed by expressing genes encoding ACT from M. hexanoica41. Thus, the carboxylic acids entering into the cell were converted and used in acyl-CoAs form throughout the r-BOP for acyl-CoA chain elongation. Thiolase (Thl) is involved in the condensation reaction between acetyl-CoA with acyl-CoA modules. In conventional genetic engineering to produce butyric acid or butanol, thiolase genes, such as AtoB from E. coli, were used to condense two acetyl-CoA molecules42. BktB from Ralstonia eutropha H16, another type of thiolase, is involved in the biosynthesis of longer chain polymers. BktB catalyses not only a condensation reaction between two acetyl-CoA molecules to produce acetoacetyl-CoA, but it also catalyses a condensation reaction between acetyl-CoA and propionyl-CoA to produce valeryl-CoA43. Furthermore, BktB could catalyse a condensation reaction between acetyl-CoA and butyryl-CoA to form 3-ketocaproyl-CoA, which can be used to produce caproate or n-hexanol (C6-alcohol)44. Although BktB showed a slow conversion rate of propionyl-CoA and butyryl-CoA into longer carbon substances, the protein crystal structure analysis of BktB revealed that the residues of amino acids around its active site form the proper structure for longer carbon chain products45.
In this study, M. hexanoica showed excellent selectivity and productivity of OCCA production using supplemented SCCAs. Through a tracking experiment using 13C isotope, the metabolic pathway of producing OCCAs in M. hexanoica was proposed as the r-BOP, including ACT and Thl as key enzymes. Thus, its excellent selectivity and productivity might be closely related to the excellent catalytic potential of ACT and Thl in M. hexanoica. More detailed information on ACT and Thl of M. hexanoica will be available after the completion of our investigation, which is current being conducting.
Methods
Media and culture conditions
All bacterial cultures were performed in an anaerobic environment. Megasphaera hexanoica was cultivated in a mPYF medium, which contained the following components dissolved in distilled water to a final volume of 1 L: yeast extract, 10 g; peptone, 5 g; tryptone, 5 g; beef extract, 5 g; fructose, 20 g; K2HPO4, 2 g; cysteine HCl·H2O, 0.5 g; hemin solution, 10 mL; and salt solution, 40 mL. The salt solution was prepared in distilled water to a final volume of 1 L: CaCl2·2H2O, 0.25 g; MgSO4·7H2O, 0.5 g; K2HPO4, 1 g; KH2PO4, 1 g; NaHCO3, 10 g; and NaCl, 2 g. To prepare the hemin solution, 50 mg of hemin (Sigma Aldrich) was dissolved in 1 mL of 1 N NaOH and then was diluted in distilled water to a final volume of 100 mL.
For the RSM experiments, sodium acetate and sodium propionate were used as factors and valeric acid was used as a response to maximize the production of valeric acid. Sodium acetate and sodium propionate were added to liquid broth before autoclaving. The liquid broth was prepared as 20 mL of medium in a 60 mL serum bottle under argon purging.
For extractive fermentation, a mixture of solvent solution of 10% (v/v) alamine 336 (Cognis) in oleyl alcohol (Ecogreen, DHW) was used. The solvent solution was washed by distilled water to remove trace elements and unknown chemicals that may inhibit the growth of M. hexanoica dissolved in an aqueous medium. Then, the mixture was autoclaved at 121 °C for 15 min before being used for the product extraction. The experiment was conducted in a 3-L fermenter (Fermentec FMT ST, Korea) with 1 L solvent solution. The seeding (5%, v/v) in the mid-exponential phase was inoculated into 1 L medium supplemented with optimum concentration of acetate and propionate. It was cultured for one day without extractive solvent to prevent the toxic effects of the solvent against bacterial cells. After one day of cultivation, the solvent solution was injected by a peristaltic pump onto the cultured broth. The biphasic status of the solvent and the aqueous culture of the broth was maintained during the extractive fermentation in the fed-batch culture, even though the fermenter was stirred at 80 rpm. As needed, the concentrated medium (10× nutrients along with 1× salts and concentrated fructose), was fed into the reactor to bring the fructose concentration up to 30 g/L and to provide nutrients for the culture.
For the isotope analysis, liquid broth was prepared as 2 mL media in a 16 × 125 mm Hungate tube under argon purging. Propionic acid with 13C at all three carbons (CLM-647, Cambridge Isotope Laboratories, Inc) was used at the optimal concentration as determined by the RSM experiments.
The pH of the medium was adjusted to 6.5 using 1 N HCl. Bacteria were cultured in a shaking incubator with rotation at 150 rpm and 37 °C. For this purpose, 5% (v/v) seed culture in mPYF was inoculated into a fresh mPYF medium. All experiments were performed in duplicate, and the results are shown as an average of the duplicate experiments.
Experimental design
The data from the fractional factorial experimental design were used for further analysis with the method of steepest ascent. The method of steepest ascent is a procedure for sequentially moving along the steepest ascent path, which was used to obtain the concentration ranges of variables for a central composite experimental design. A two-factor CCD consisting of 13 experimental runs with five replications at the central point was used to optimize the independent variables. The experimental results obtained with the central composite model were statistically analysed for reliability by ANOVA in R (version 3.2.3), by using the package ‘rsm’ to standardize the acetate and propionate the treatment axes46,47.
Analytical methods
The medium optimization experiments were conducted in duplicate, and the means are shown with standard deviations presented by error bars. The fatty acids in the broth were analysed by a gas chromatogram (GC; Agilent 7890, USA) equipped with a flame ionization detector, according to a previously described procedure48.
For isotope analysis in the gas phase, GC-MS was carried out with an Agilent 5975MS coupled with an Agilent 6890GC equipped with an HP-1MS column (60 m × 0.25 mm; film thickness 0.25 µm). The inlet temperature was 150 °C. The initial oven temperature was 40 °C, which was then increased to 0.5 °C/min to 45 °C, held for 4 min, and then was increased at 30 °C/min to 200 °C. The MS transfer line was held at 250 °C.
The GC-MS was performed on a TOF-MS (Leco Pegasus III) coupled with an Agilent 6890GC equipped with an HP-FFAP column (50 m × 0.2 mm; film thickness 0.33 µm) for isotope analysis in the liquid phase. The inlet temperature was 160 °C. The initial oven temperature of 130 °C was increased at 2 °C/min to 180 °C, then at 10 °C/min to 210 °C, and held for 2 min. The MS transfer line was maintained at 240 °C.
References
Cervantes, F. J., De la Rosa, D. A. & Gómez, J. Nitrogen removal from wastewaters at low C/N ratios with ammonium and acetate as electron donors. Bioresource Technology 79, 165–170, https://doi.org/10.1016/S0960-8524(01)00046-3 (2001).
Henstra, A. M., Sipma, J., Rinzema, A. & Stams, A. J. M. Microbiology of synthesis gas fermentation for biofuel production. Current Opinion in Biotechnology 18, 200–206, https://doi.org/10.1016/j.copbio.2007.03.008 (2007).
Borole, A. P., Hamilton, C. Y., Vishnivetskaya, T., Leak, D. & Andras, C. Improving power production in acetate-fed microbial fuel cells via enrichment of exoelectrogenic organisms in flow-through systems. Biochemical Engineering Journal 48, 71–80, https://doi.org/10.1016/j.bej.2009.08.008 (2009).
Rahman, P. K. & Gakpe, E. Production, characterisation and applications of biosurfactants-Review. Biotechnology (2008).
Avella, M., Martuscelli, E. & Raimo, M. Review Properties of blends and composites based on poly (3-hydroxy) butyrate (PHB) and poly (3-hydroxybutyrate-hydroxyvalerate)(PHBV) copolymers. Journal of Materials Science 35, 523–545 (2000).
Gavrilescu, M. & Chisti, Y. Biotechnology—a sustainable alternative for chemical industry. Biotechnology Advances 23, 471–499, https://doi.org/10.1016/j.biotechadv.2005.03.004 (2005).
Agler, M. T., Wrenn, B. A., Zinder, S. H. & Angenent, L. T. Waste to bioproduct conversion with undefined mixed cultures: the carboxylate platform. Trends in Biotechnology 29, 70–78, https://doi.org/10.1016/j.tibtech.2010.11.006 (2011).
Seedorf, H. et al. The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proceedings of the National Academy of Sciences 105, 2128–2133, 10.1073/pnas.0711093105 (2008).
Jeon, B. S., Choi, O., Um, Y. & Sang, B. I. Production of medium-chain carboxylic acids by Megasphaera sp. MH with supplemental electron acceptors. Biotechnol Biofuels 9, https://doi.org/10.1186/s13068-016-0549-3 (2016).
Grootscholten, T. I. M. Kinsky dal Borgo, F., Hamelers, H. V. M. & Buisman, C. J. N. Promoting chain elongation in mixed culture acidification reactors by addition of ethanol. Biomass and Bioenergy 48, 10–16, https://doi.org/10.1016/j.biombioe.2012.11.019 (2013).
Spirito, C. M., Richter, H., Rabaey, K., Stams, A. J. M. & Angenent, L. T. Chain elongation in anaerobic reactor microbiomes to recover resources from waste. Current Opinion in Biotechnology 27, 115–122, https://doi.org/10.1016/j.copbio.2014.01.003 (2014).
Vasudevan, D., Richter, H. & Angenent, L. T. Upgrading dilute ethanol from syngas fermentation to n-caproate with reactor microbiomes. Bioresource Technology 151, 378–382, https://doi.org/10.1016/j.biortech.2013.09.105 (2014).
Angenent, L. T. et al. Chain Elongation with Reactor Microbiomes: Open-Culture Biotechnology To Produce Biochemicals. Environ Sci Technol 50, 2796–2810, https://doi.org/10.1021/acs.est.5b04847 (2016).
Gonzalez-Garcia, R. et al. Microbial Propionic Acid Production. Fermentation 3, 21 (2017).
Woolford, M. K. Microbiological screening of the straight chain fatty acids (c1‐c12) as potential silage additives. Journal of the Science of Food and Agriculture 26, 219–228, https://doi.org/10.1002/jsfa.2740260213 (1975).
Liebergesell, M. et al. Formation of poly(3-hydroxyalkanoates) by phototrophic and chemolithotrophic bacteria. Archives of Microbiology 155, 415–421, https://doi.org/10.1007/bf00244955 (1991).
Renz, M. Ketonization of Carboxylic Acids by Decarboxylation: Mechanism and Scope. European Journal of Organic Chemistry 2005, 979–988, https://doi.org/10.1002/ejoc.200400546 (2005).
Grootscholten, T. I., Steinbusch, K. J., Hamelers, H. V. & Buisman, C. J. High rate heptanoate production from propionate and ethanol using chain elongation. Bioresource Technology 136, 715–718, https://doi.org/10.1016/j.biortech.2013.02.085 (2013).
Coma, M. et al. Product Diversity Linked to Substrate Usage in Chain Elongation by Mixed-Culture Fermentation. Environ Sci Technol 50, 6467–6476, https://doi.org/10.1021/acs.est.5b06021 (2016).
Bornstein, B. T. & Barker, H. A. The energy metabolism of Clostridium kluyveri and the synthesis of fatty acids. The Journal of biological chemistry 172, 659–669 (1948).
Jeon, B. S., Kim, S. & Sang, B.-I. Megasphaera hexanoica sp. nov., a medium-chain carboxylic acid-producing bacterium isolated from a cow rumen. International Journal of Systematic and Evolutionary Microbiology 67, 2114–2120, https://doi.org/10.1099/ijsem.0.001888 (2017).
Jeon, B. S. et al. In situ extractive fermentation for the production of hexanoic acid from galactitol by Clostridium sp BS-1. Enzyme Microb Tech 53, 143–151, https://doi.org/10.1016/j.enzmictec.2013.02.008 (2013).
Kim, B.-C. et al. Caproiciproducens galactitolivorans gen. nov., sp. nov., a bacterium capable of producing caproic acid from galactitol, isolated from a wastewater treatment plant. International journal of systematic and evolutionary microbiology 65, 4902–4908 (2015).
Hino, T., Miyazaki, K. & Kuroda, S. Role of extracellular acetate in the fermentation of glucose by ruminal bacterium, Megasphaera elsdenii. The Journal of General and Applied Microbiology 37, 121–129, https://doi.org/10.2323/jgam.37.121 (1991).
Czitrom, V. One-factor-at-a-time versus designed experiments. Am Stat 53, 126–131, https://doi.org/10.2307/2685731 (1999).
Box, G. E. P. & Wilson, K. B. In Breakthroughs in Statistics: Methodology and Distribution (eds Samuel Kotz & Norman L. Johnson) 270–310 (Springer New York, 1992).
Wu, J., Zhang, X., Xia, X. & Dong, M. A systematic optimization of medium chain fatty acid biosynthesis via the reverse beta-oxidation cycle in Escherichia coli. Metabolic engineering 41, 115–124 (2017).
Tseng, H.-C. & Prather, K. L. J. Controlled biosynthesis of odd-chain fuels and chemicals via engineered modular metabolic pathways. Proceedings of the National Academy of Sciences, https://doi.org/10.1073/pnas.1209002109 (2012).
Kucek, L. A., Nguyen, M. & Angenent, L. T. Conversion of L-lactate into n-caproate by a continuously fed reactor microbiome. Water Res 93, 163–171, https://doi.org/10.1016/j.watres.2016.02.018 (2016).
Andersen, S. J. et al. Electrolytic extraction drives volatile fatty acid chain elongation through lactic acid and replaces chemical pH control in thin stillage fermentation. Biotechnol Biofuels 8, 221 (2015).
Andersen, S. J. et al. a Clostridium group iV species Dominates and suppresses a Mixed culture Fermentation by Tolerance to Medium chain Fatty acids Products. Frontiers in bioengineering and biotechnology 5 (2017).
Lanjekar, V. B., Marathe, N. P., Venkata Ramana, V., Shouche, Y. S. & Ranade, D. R. Megasphaera indica sp. nov., an obligate anaerobic bacteria isolated from human faeces. International Journal of Systematic and Evolutionary Microbiology 64, 2250–2256, https://doi.org/10.1099/ijs.0.059816-0 (2014).
Yoshikawa, S. et al. Valerate production by Megasphaera elsdenii isolated from pig feces. Journal of bioscience and bioengineering 125, 519–524 (2018).
Oshiro, M., Hanada, K., Tashiro, Y. & Sonomoto, K. Efficient conversion of lactic acid to butanol with pH-stat continuous lactic acid and glucose feeding method by Clostridium saccharoperbutylacetonicum. Applied microbiology and biotechnology 87, 1177–1185 (2010).
Gao, M. et al. Metabolic analysis of butanol production from acetate in Clostridium saccharoperbutylacetonicum N1-4 using 13C tracer experiments. Rsc Advances 5, 8486–8495 (2015).
Counotte, G., Prins, R. & Janssen, R. Role of Megasphaera elsdenii in the fermentation of DL-[2-13C] lactate in the rumen of dairy cattle. Applied and Environmental Microbiology 42, 649–655 (1981).
Liu, S.-J. & Steinbüchel, A. Exploitation of butyrate kinase and phosphotransbutyrylase from Clostridium acetobutylicum for the in vitro biosynthesis of poly (hydroxyalkanoic acid). Applied microbiology and biotechnology 53, 545–552 (2000).
Zhu, X. et al. Production of high-concentration n-caproic acid from lactate through fermentation using a newly isolated Ruminococcaceae bacterium CPB6. Biotechnol Biofuels 10, 102, https://doi.org/10.1186/s13068-017-0788-y (2017).
Khan, M. A. Regulation of volatile fatty acid synthesis in Megasphaera elsdenii and hexanoic acid utilisation by Pseudomonas putida, Victoria University (2006).
Taguchi, S. et al. A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proceedings of the National Academy of Sciences 105, 17323–17327, https://doi.org/10.1073/pnas.0805653105 (2008).
Kim, S. G. et al. Optimization of hexanoic acid production in recombinant Escherichia coli by precise flux rebalancing. Bioresource Technology 247, 1253–1257, https://doi.org/10.1016/j.biortech.2017.10.014 (2018).
Lan, E. I. & Liao, J. C. Metabolic engineering of cyanobacteria for 1-butanol production from carbon dioxide. Metabolic Engineering 13, 353–363, https://doi.org/10.1016/j.ymben.2011.04.004 (2011).
Slater, S. et al. Multiple β-ketothiolases mediate poly(β-hydroxyalkanoate) copolymer synthesis in Ralstonia eutropha. Journal of Bacteriology 180, 1979–1987 (1998).
Dekishima, Y., Lan, E. I., Shen, C. R., Cho, K. M. & Liao, J. C. Extending carbon chain length of 1-butanol pathway for 1-hexanol synthesis from glucose by engineered Escherichia coli. Journal of the American Chemical Society 133, 11399–11401, https://doi.org/10.1021/ja203814d (2011).
Kim, E.-J., Son, H. F., Kim, S., Ahn, J.-W. & Kim, K.-J. Crystal structure and biochemical characterization of beta-keto thiolase B from polyhydroxyalkanoate-producing bacterium Ralstonia eutropha H16. Biochemical and Biophysical Research Communications 444, 365–369, https://doi.org/10.1016/j.bbrc.2014.01.055 (2014).
Lenth, R. V. Response-Surface Methods in R, Using rsm. J Stat Softw 32, 1–17 (2009).
Lenth, R. V. Surface Plots in the rsm Package. Education 20, 30 (2010).
Jeon, B. S. et al. Performance analysis of a proton exchange membrane fuel cell (PEMFC) integrated with a trickling bed bioreactor for biological high-rate hydrogen production. Energ Fuel 22, 83–86, https://doi.org/10.1021/ef700270y (2008).
Acknowledgements
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20173010092510) and by Hanyang University (HY-201100000000233-N).
Author information
Authors and Affiliations
Contributions
Kim, H. and Jeon, B.S. carried out the experiment. Kim, H. wrote the manuscript with support from Jeon, B.S. Sang, B.I. supervised the project.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, H., Jeon, B.S. & Sang, BI. An Efficient New Process for the Selective Production of Odd-Chain Carboxylic Acids by Simple Carbon Elongation Using Megasphaera hexanoica. Sci Rep 9, 11999 (2019). https://doi.org/10.1038/s41598-019-48591-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48591-6
This article is cited by
-
Anaerobic production of valeric acid from crude glycerol via chain elongation
International Journal of Environmental Science and Technology (2020)
-
Efficient, Simple Production of Corresponding Alcohols from Supplemented C2-C8 Carboxylic Acids in Escherichia coli Using Acyl-CoA Transferase from Megasphaera hexanoica
Biotechnology and Bioprocess Engineering (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.