Abstract
Cannabis and alcohol co-use is common, and the trend may increase further given the current popularity of cannabis legalization. However, the metabolic consequences of such co-use are unclear. Here, we investigated how co-administration of alcohol and ∆9-tetrahydrocannabinol (THC), the main psychoactive constituent of cannabis, affects body weight and visceral adiposity, and glucose and insulin homeostasis in rats. For 16 consecutive days during adolescence, male rats drank saccharin or alcohol after receiving subcutaneous oil or THC injections in Experiment 1 and voluntarily consumed alcohol, THC edible, or both drugs in Experiment 2. Experiment 1 showed that following abstinence, drug co-exposure reduced visceral fat and the amount of insulin required to clear glucose during an oral glucose tolerance test (OGTT). In Experiment 2, rats received a high-fat diet (HFD) after 3-week abstinence. Although adolescent drug use did not interact with the HFD to worsen hyperglycemia and hyperinsulinemia during an OGTT, HFD-fed rats that co-used alcohol and THC had the lowest insulin levels 75 min after an insulin injection, suggesting an altered rate of insulin secretion and degradation. These results suggest that THC and alcohol co-exposure can distinctly alter the physiology of glucose and insulin homeostasis in a rodent model.
Similar content being viewed by others
Introduction
The wide prevalence and burdensome nature of obesity and metabolic syndrome echoes the need for more studies to shed light on the predisposing and protective factors at play. Previous studies have established that early-life nutritional deficits or surfeits can influence neurological and metabolic health throughout the lifespan1, as does active or passive exposure to exogenous drugs like alcohol and cannabinoids2,3. Prolonged consumption of diets rich in calories, salt, saturated fatty acids, and refined sugars but low in fiber (“Western diet”) coupled with a sedentary lifestyle are notable contributors to the overweight/obese phenotype4. The contribution of exogenous drugs, especially alcohol and marijuana co-use, to the high rate of metabolic disorders has been scarcely investigated.
Use and co-use of alcohol and marijuana often begin during adolescence5. This drug use behavior is worrisome considering the wealth of empirical evidence suggesting that the adolescent brain is susceptible to the influence of exogenous compounds6,7. Notably, alcohol or cannabis use during adolescence can alter the ontogeny of neural systems that regulate cognitive and reward-related behaviors8,9 to provoke aberrant brain functioning later in life10,11. Similar neural alterations can translate into an increased motivation to consume palatable, energy-dense diets during young adulthood12,13. Despite the popularity of alcohol and cannabis co-use14, there is paucity of studies on how such co-use affects energy balance and metabolic outcomes during abstinence.
Epidemiological data have implicated chronic alcohol use with abdominal adiposity and some facets of metabolic syndrome, including leptin and insulin resistance, and type-2 diabetes15. Other researchers conclude that moderate alcohol consumption may either be beneficial to cardio-metabolic health or have null effects16. Meanwhile, few human studies reported the metabolic effects of marijuana use17,18, and there has been no preclinical investigation on this topic. Human laboratory studies have documented that marijuana smoking can acutely increase plasma insulin concentration and alter glucose tolerance19,20 or have no effect on glucose homeostasis21 in adult subjects. A cross-sectional, case-control study reported that chronic marijuana smoking can reduce circulating HDL-cholesterol and increase adipose tissue hypertrophy and insulin resistance without significant detriment to pancreatic β-cell function22. These reports suggest that repeated cannabis use may alter insulin control of glucose homeostasis. Importantly, given that alcohol and cannabis co-administration is common and may be on the rise due to the current social atmosphere of cannabis legalization, how alcohol and ∆9-tetrahydrocannabinol (THC), the main psychoactive constituent of cannabis, interact to affect energy balance and glucose homeostasis deserves investigation.
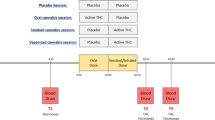
Previously, we demonstrated that male rats that chronically consumed moderate alcohol doses showed early signs of insulin insensitivity23. The lack of systematic investigation of the lasting behavioral and metabolic effects of alcohol and THC co-administration prompted us to undertake this study using a rodent model of adolescent exposure of the two drugs24. Given the negative metabolic outcomes of short-term Western diet consumption in young subjects25,26, we sought to investigate whether high-fat diet (HFD) exposure would exacerbate the impact of prior alcohol and THC use. We hypothesized that, compared to separate administration of alcohol and THC, their co-administration during adolescence would lead to lasting alterations in feeding behavior that will prime the subject for heightened reactivity to metabolic challenges in adulthood. Hence, we predicted that rats previously co-exposed to alcohol and THC would demonstrate hyperphagia and increased adiposity when presented with an HFD compared with the drug naïve rats. We also expected that combined drug co-use and HFD overconsumption would greatly impair glucose tolerance and insulin sensitivity during adulthood. Accordingly, Experiment 1 investigated how repeated alcohol drinking, subcutaneous THC injection, or combinational exposure of the two drugs during adolescence affect metabolic measures e.g., body fat, blood glucose and insulin responses to an oral load of glucose challenge. Consumption of edibles prepared with marijuana extract is popular among medical and recreational marijuana users27. Thus, Experiment 2 investigated how adolescent voluntary consumption of alcohol and THC edibles, alone or in combination, affect feeding and metabolic responses to HFD during early adulthood (See Fig. 1 for experimental timeline).
(A) Timeline of chronic subcutaneous THC injection and voluntary alcohol drinking in Experiment 1 (EtOH, n = 8; CTL, THC, and COM, n = 10/group). The 3 mg/kg/day THC lasted for eight days, while the 5 and 10 mg/kg/day THC each lasted for four days. An oral glucose tolerance test (OGTT) was performed on P54 after an overnight fast. (B) Timeline of chronic oral THC consumption and voluntary alcohol drinking in Experiment 2. The table indicates the schedules and duration for drinking and drug exposure. The shaded portion of the table indicates the last 3 h of the dark cycle when access to the test fluid occurred. From P67, some rats continued consuming regular chow diet while the others received 45% high-fat diet. Glucose and insulin tolerance tests were performed on P71 and P76, respectively [Chow (CTL, n = 14; EtOH, n = 12; THC, n = 10; COM, n = 11) and HF (CTL and COM, n = 11/group; EtOH and THC, n = 12/group)].
Results
Experiment 1: Results
Combined subcutaneous THC injection and alcohol consumption reduced visceral fat composition
Alcohol, food intake, and weight gain during drug treatment and abstinence are presented in our previous published manuscript24. In brief, THC dose-dependently increased food intake during the initial 3 h after subcutaneous injections but did not alter alcohol consumption and blood ethanol concentration (BEC) during the same period. Voluntary alcohol consumption acutely reduced food intake when it was consumed alone (EtOH group) or when THC was on-board in the COM group. Further, THC or alcohol had no effect on daily caloric intake and weight gain during treatment. However, the COM group gained the least amount of weight during the week of abstinence compared with the EtOH and THC groups24. On the eighth day of abstinence (P53), the respective body weights of the CTL (241.7 ± 3.5 g), EtOH (248.4 ± 3.5 g), THC (238.2 ± 6.1 g), and COM (234.0 ± 4.4 g) groups were statistically indistinguishable. Yet, the COM group had lower percent visceral fat on P54 compared with the EtOH group [F(3,34) = 4.41, p < 0.02; post hoc, p < 0.009; Fig. 2A].
Chronic subcutaneous THC injections and voluntary alcohol consumption differently affected visceral fat composition and reduced insulin levels needed to clear glucose in Experiment 1 (CTL, n = 9; EtOH and THC, n = 7; COM, n = 8). (A) THC alone or in combination with alcohol reduced visceral fat composition. THC vs. EtOH: ^p < 0.1; COM vs. EtOH: *p < 0.009. The OGTT was conducted after nine days of abstinence. (B) Alcohol and THC alone or in combination had no effect on glucose clearance; and (C) combined chronic alcohol and THC exposure was associated with reduced plasma insulin levels at 15 min following the oral glucose load. COM vs. EtOH: *p < 0.009 and COM vs. THC: ^p < 0.07. (D) The COM group had a reduced AUC of plasma insulin curve compared with the CTLs (t-test: ^p < 0.05).
Combined subcutaneous THC injection and alcohol consumption reduced plasma insulin concentration following an oral load of glucose
The 16 days of subcutaneous THC and moderate alcohol treatment did not alter fasting blood glucose levels (Fig. 2B). All of the groups of rats comparably cleared blood glucose across time during the OGTT [time effect, F(4,136) = 94.95, p < 0.0001; Fig. 2B]. The fasting plasma insulin concentration of the COM group was mildly lower than that of the THC group [group effect, F(3,27) = 3.00, p < 0.05; post hoc, p < 0.06; Fig. 2C]. Rats in each group had a slightly different profile of plasma insulin concentration during the 2 h following the intragastric 2.0 g/kg glucose load [group, time, and group × time effects: F(3,27) = 2.58, p < 0.08, F(4,108) = 73.90, p < 0.0001, and F(12,108) = 2.01, p < 0.03, respectively]. At the 15-min time point, the plasma insulin level of the COM group was lower than that of both the EtOH (p < 0.009) and THC (p < 0.07) groups. Finally, the COM group demonstrated a trend for an overall reduced insulin response compared with the CTLs [insulin AUC: group effect, F(3,27) = 2.45, p < 0.09; post hoc, p < 0.09; Fig. 2D]. In support of our a priori hypothesis that drug combination will elicit unique effects, independent samples t-test revealed that the area under the insulin curve of the COM group was significantly lower than that of the CTLs [t(15) = 2.19, p < 0.05].
Experiment 2: Results
Male rats readily consumed THC-laced cookies
Most rats consumed the THC-laced cookie within minutes. Two and four rats from the THC and COM groups, respectively, had bits of leftover cookies by the time a second cookie was delivered. The subsequent data of these rats were excluded from analyses.
Consumption of THC edible reduced intakes of saccharin but not alcohol
The CTL and THC rats consumed increasing amounts of saccharin across treatment days [F(3,135) = 78.37, p < 0.0001]. But the THC rats consumed less saccharin compared with the CTLs [F(1,45) = 4.93, p <= 0.04; Fig. 3A]. The EtOH and COM groups consumed similar doses of alcohol across treatment days (Fig. 3B). Despite no group effect on alcohol intake, COM rats appeared to reduce alcohol intake during consumption of higher doses of THC (6 and 10 mg/kg/day). On the days BECs were measured, EtOH (1.36 ± 0.07 g/kg) and COM (1.20 ± 0.08 g/kg) rats consumed similar doses of alcohol within 1 h following 8 mg/kg/day THC consumption. Both groups of rats attained comparable BECs of 25.50 ± 4.08 mg/dl and 25.95 ± 5.67 mg/dl, respectively. After 3 h of alcohol access following 10 mg/kg/day THC consumption, EtOH (1.65 ± 0.10 g/kg) and COM (1.45 ± 0.13 g/kg) rats consumed comparable alcohol doses and attained BECs of 13.57 ± 3.63 mg/dl and 18.35 ± 5.30 mg/dl, respectively. The BECs attained after both 1- and 3-h alcohol drinking significantly correlated with the ingested dose in both the EtOH [r = 0.74, p < 0.0002 and r = 0.64, p < 0.008, respectively] and COM [r = 0.65, p < 0.004 and r = 0.91, p < 0.0001, respectively] groups.
Chronic oral THC consumption reduced saccharin intake and chow intake during the last 3 h of the dark cycle and weight gain, whereas moderate alcohol alone or when combined with oral THC reduced 3-h chow intake (CTL, n = 25; EtOH, n = 24; THC and COM, n = 22/group). (A) THC rats consumed less saccharin compared with the CTL rats. *p < 0.04. (B) EtOH and COM rats consumed similar doses of alcohol during each THC dose. COM rats’ alcohol intake during 6 and 10 mg/kg/day THC consumption was lower than their intake during 3 mg/kg/day THC consumption: ^p < 0.03. (C) Drinking 5% alcohol reduced 3-h chow intake and augmented the somewhat hypophagic effect of oral THC. CTL vs. COM: *p < 0.005; CTL vs. THC and COM: #p < 0.004; CTL vs. EtOH: ^p < 0.06; EtOH vs. COM: @p < 0.03. (D) Oral THC suppressed weight gain in the THC group compared with both the CTL and EtOH groups: *p < 0.02.
Adolescent alcohol and THC co-use facilitated weight gain in response to HFD during early adulthood
Consumption of THC-laced cookies on the previous day blunted chow intake during the last 3 h of the dark cycle on the next day in the THC and COM groups relative to the CTLs [F(3,89) = 8.46, p < 0.0001; post hoc, both p < 0.007; Fig. 3C]. The EtOH group consumed less 3-h chow compared with the CTL group (p < 0.04). The lower 3-h chow intake by THC and COM rats compared with the CTLs was retained after intake was normalized to body weight. There were no group differences in daily caloric intake during the 16-day treatment period, but the THC rats gained less weight than did the EtOH and CTL rats [F(3,89) = 5.39, p < 0.002; post hoc, both p < 0.02; Fig. 3D].
Compared with chow-fed controls, rats that consumed 45% HFD for four days (P67–P70) demonstrated hyperphagia [F(1,38) = 412.58, p < 0.0001] and modest weight gain [F(1,38) = 5.11, p < 0.03]. There were no drug effects on daily chow or HFD intake during the 9-day test period. As expected, the HFD-fed rats consumed approximately 30% more daily calories [F(1,38) = 252.48, p < 0.0001; Fig. 4A] and gained more weight [F(1,38) = 25.23, p < 0.0001; Fig. 4B] compared with the chow-fed rats. Under the HFD condition, the COM group gained more weight than the CTLs did [t(20) = −2.21, p < 0.04].
Chronic oral THC and voluntary alcohol consumption, alone or when combined, had no effect on daily caloric intake and weight gain during abstinence (from P67–P75) [Chow (CTL, n = 14; EtOH, n = 12; THC, n = 10; COM, n = 11) and HFD (CTL and COM, n = 11/group; EtOH and THC, n = 12/group)]. (A) Rats maintained on 45% HFD consumed more average daily calories compared with intake by those maintained on regular chow diet: *p < 0.0001. (B) HFD-fed rats gained more weight compared with the chow-fed rats: *p < 0.0001 by ANOVA. Under the HFD condition, the COM rats gained more weight compared with the CTLs: #p < 0.04 by t-test.
Adolescent THC and alcohol co-use did not affect glucose and insulin tolerance following short-term HFD challenge during early adulthood
The HFD consumers had elevated basal blood glucose (Fig. 5A,B) and insulin concentrations (Fig. 5D,E) relative to the chow consumers following a mild fast [F(1,24) = 42.25 and 28.92, respectively; both p < 0.0001]. During the OGTT, three-way repeated-measures ANOVA uncovered no effect of drug treatment and diet on blood glucose clearance and plasma insulin concentrations across time. The HFD groups demonstrated greater area under curve (AUC) of blood glucose [F(1,24) = 78.52, p < 0.0001; Fig. 5C] and plasma insulin [F(1,24) = 32.78, p < 0.0001; Fig. 5F] concentrations compared with the chow groups. Within each diet condition, there were no effects of prior alcohol and THC exposure on the metabolic parameters. Because there was no effect of prior drug exposure on blood glucose levels during the ITT, we collapsed the groups by diet condition. The HFD-fed groups had higher fasting blood glucose concentrations compared with the chow-fed groups [diet × time effect: F(1,88) = 4.61, p < 0.04; post hoc, p < 0.002; Fig. 6A]. There was, however, no diet effect on blood glucose level at the 75-min time point – indicating that the nine-day HFD consumption did not alter insulin sensitivity in the rats. Furthermore, the HFD-fed rats had higher percent visceral fat [F(1,38) = 84.56, p < 0.0001; Fig. 6B] and trunk plasma leptin concentration [F(1,23) = 10.48, p < 0.004; Fig. 6C], with no effect of prior drug use. Percent visceral fat was positively correlated with plasma leptin in both the chow- [r = 0.39, p < 0.009] and HFD-fed [r = 0.58, p < 0.0007] groups. Under the HFD condition, and mirroring the body weight results, the COM group had slightly higher percent visceral fat compared with the CTLs [t(20) = −2.02, p < 0.06]. Neither drug treatment nor diet condition influenced trunk insulin levels taken 75 min after the insulin injection (Fig. 6D). Finally, a t-test based on our a priori hypothesis that co-use of alcohol and THC would produce distinct effect from the use of either drug alone revealed that, under the HFD condition, the COM group had significantly lower trunk insulin levels than did the EtOH group [t(21) = 3.02, p < 0.007; Fig. 6D].
Effects of oral THC, moderate alcohol, and diet on glucose tolerance (Chow, n = 10–12/group and HFD, n = 7–8/group). Prior drug exposure had no effect on glucose clearance in rats (A) perpetually maintained on chow diet, and those (B) that consumed HFD for 4 days (P67–P70) after the diet switch (see Fig. 1B). (C) Area under curve revealed that 4-day HFD consumption altered glucose tolerance with no significant effect of prior drug exposure: *p < 0.0001. Prior drug exposure had no effect on insulin release in response to the oral glucose loads in both groups of rats that were (D) perpetually maintained on chow diet, and those (E) that consumed HFD for 4 days (P67–P70) after the diet switch. (F) Area under curve revealed that 4-day HFD consumption potentiated insulin release with no significant effect of prior drug exposure: *p < 0.0001.
Effects of oral THC, moderate alcohol, and diet on some metabolic parameters (Chow, n = 10–12/group and HFD, n = 7–12/group). Nine days of HF diet feeding (A) increased fasting blood glucose with no effect on insulin sensitivity during the insulin tolerance test: *p < 0.002, (B) increased visceral fat composition: *p < 0.0001, (C) increased plasma leptin concentration: *p < 0.004, and (D) had no effect on plasma insulin concentration. Visceral fat composition positively correlated with leptin measured in trunk plasma in both the chow- (r = 0.39, p < 0.009) and HFD-fed (r = 0.58, p < 0.0007) groups. Prior alcohol or oral THC consumption did not significantly influence the above measures. However, under the HFD condition, t-test revealed that the COM rats tended to have higher visceral adiposity compared with the CTLs: ^p < 0.06 and lower trunk insulin compared with the EtOH group #p < 0.007.
Discussion
Our data reveal that 16 days of separate and combined alcohol and THC exposure can have distinct effects on subsequent glucose and insulin homeostasis in young male rats. Experiment 1 showed that compared to the CTL, EtOH, and THC groups, the COM rats had the lowest insulin levels to clear glucose during an OGTT that occurred during abstinence. The seemingly increased insulin sensitivity corroborated with low weight gain and the lowest visceral fat in the COM rats among all treatment groups. Experiment 2 investigated glucose and insulin homeostasis under different dietary exposures following longer abstinence from edible THC and alcohol co-use. Although the influences of adolescent drug use on glucose metabolism disappeared after approximately one-month of forced drug abstinence, the COM rats gained the most body weight and visceral fat after a 9-day HFD challenge. The findings of the two experiments suggest that the beneficial effects of moderate alcohol and THC co-administration on glucose and insulin homeostasis manifest soon after 16 days of drug exposure and no benefit is obtained as the length of abstinence increases. Importantly, they highlight that HFD consumption can reverse the beneficial effects of THC and moderate alcohol on energy metabolism and homeostasis.
Under the chow feeding condition, rats that received subcutaneous THC injections (THC or COM group) had reduced adiposity and plasma insulin concentrations (Experiment 1); and those that consumed THC via cookies showed no changes in those parameters (Experiment 2). In addition to the different time points of the adiposity assessment, the differing pharmacokinetic profiles of injected versus oral THC may also contribute to distinct results of the two experiments. The consumption of edibles prepared with marijuana extract is popular among medical and recreational marijuana users27. Consumption of THC via cookie infusion used in Experiment 2 is more reflective of how the drug is consumed today. Past studies show that humans who consumed THC via an edible product can display more than one peak plasma THC concentration; and the duration of the effects of the THC can be longer when it is consumed orally versus when it is inhaled or delivered intravenously28. However, due to first-pass hepatic metabolism, plasma levels of THC are lower when THC or cannabis is consumed orally compared to when smoked or injected24,28,29. Overall, unlike smoked or parenterally administered THC, the bioavailability of orally consumed THC tends to be low and more erratic.
The effects of THC on glucose homeostasis in humans can depend not only on the administered dose, but also on the route of administration19. One study showed that intravenous THC impaired glucose tolerance but smoked THC had no effect. Such observations may be explained by the subjects’ prior experience with marijuana smoking19, by the fact that the bioavailability of THC is slightly greater following intravenous cannabis administration compared with inhalation30, or by unknown variables that await elucidation. Epidemiological reports on the metabolic effects of alcohol or marijuana often statistically control for the use of other drugs or screen for and exclude participants with a history of poly-substance use. Ours is the first preclinical rodent study to 1) investigate the effects of THC administered parenterally and via an edible product on subsequent glucose homeostasis and insulin sensitivity, and 2) examine the long-term effects of adolescent alcohol and oral THC co-exposure on feeding behavior and metabolic outcomes during young adulthood. We demonstrated that moderate daily use of alcohol or THC alone had no significant effects, but co-use of the two can produce additive or even opposite effects on circulating insulin levels and adiposity (Figs 2 and 6).
Alcohol and THC can interact in unique and complex ways to affect physiology. A past study demonstrated that smoking cannabis after drinking alcohol can reduce BECs attained and the psychoactive effects of alcohol in humans31. However, others have observed no effect of smoked THC on BEC32. Intriguingly, other human studies found that drinking before smoking or vaporizing cannabis increases blood levels of THC33,34. In this and our previous study24, subcutaneous or oral THC did not alter BEC in rats. We also observed that moderate alcohol consumption following subcutaneous THC injection resulted in lower plasma levels of the active THC metabolite 11-hydroxy-THC24. Our preliminary finding calls for further investigation of the long-term effects of alcohol and cannabis co-use on glucose homeostasis, including studies to decipher the cellular and pharmacokinetic mechanisms that mediate the outcomes.
In consonance with our previous findings24, subcutaneous and oral THC differently affect saccharin and alcohol intake. Whereas subcutaneous THC had no significant effects, rats that consumed THC-laced cookies subsequently consumed less saccharin and alcohol solution during the last 3 h of the dark cycle. We have previously shown that oral THC can reduce alcohol intake to the extent of complete avoidance24. By contrast, subcutaneous and oral THC had similar effects on food intake and body weight. Except for extremely high doses e.g., 20 mg/kg24, neither route of THC administration altered daily calorie intake. Repeated administration via either route modestly suppressed weight gain during treatment. These findings concur with those of other researchers35,36. There is notable individual variation in the effect of THC on appetite since not all marijuana users overconsume sweet or calorie-rich foods when high37. Epidemiological research has also uncovered that chronic marijuana users can either be of normal weight or have lower body mass index or waist circumference compared with never-users, prompting opinions about the possible weight-reducing effect of cannabis18,38. Furthermore, a very low dose (0.001 mg/kg) of ∆8-THC, which is supposedly a more stable and potent CB1R agonist than ∆9-THC, stimulated food intake but did not promote weight gain in mice39. Notwithstanding, our observed null effect of oral THC on daily caloric intake goes against the acclaimed hyperphagic effects of cannabis or THC13,35,40. Our findings, instead, reinforce the conclusion that depending on its interaction with CB1R on GABAergic or glutamatergic neurons, THC can produce opposite effects on appetite41. The complexities of cannabinoid signaling in the brain feeding circuits, sensory systems, and peripheral tissues involved in energy balance regulation are yet to be untangled, and research efforts in these domains have blossomed in recent years41,42,43.
Contrary to our hypothesis that the impact of alcohol and THC on the adolescent brain will facilitate future overconsumption of caloric dense foods, we observed that separate or combined alcohol and THC exposure did not exacerbate hyperphagia on a palatable 45% HFD during abstinence. This contrary observation can be due to several factors. First, although researchers who have used similar adolescent THC exposure timelines (parenteral administration) observed persistent neural and behavioral abnormalities during abstinence36,44, our 16-day oral drug exposure duration may have been insufficient to elicit sustained brain changes that can engender abnormal feeding behavior during abstinence. Second, any brain abnormalities caused by drug treatments might have been mitigated during the three-week abstinent/washout period. Studies have observed brain-region-specific downregulation and desensitization of CB1R following chronic alcohol or THC exposure that resolved to varying extents during abstinence36,45,46. Hence, a shorter washout period or even an earlier onset and simultaneous consumption of HFD with drug treatment may uncover robust drug/diet interaction. Third, the type of HFD (45%) used and the short duration (nine days) of high-fat diet exposure may have precluded observation of overeating and adverse long-term effects of adolescent drug exposure on energy metabolism47,48. Prolonged exposure to HFD alters the organization and function of neurons and glia in the hypothalamus to promote obesity and metabolic dysfunctions49. An exposure that extends for a longer period may exacerbate the higher weight gain and visceral fat observed in the COM rats to reveal dysregulation of glucose and insulin homeostasis.
An elevated fasting glucose concentration within normal physiological ranges is an independent risk factor for the later onset of type 2 diabetes in otherwise healthy male subjects50. In support of our a priori hypothesis in Experiment 2, pairwise comparison revealed that the COM rats that consumed HFD gained more weight and had higher visceral adiposities compared with the CTLs on the same diet. Although the HFD-consuming rats in our study were not obese per se, it is plausible that the increased visceral adiposity may be associated with alterations to endocannabinoid tone in peripheral tissues like pancreas and visceral fat. Compared with non-obese humans, obese individuals have changes to the peripheral endocannabinoid tone51,52. We found that short-term (4–9 days) HFD feeding resulted in modest signs of glucose intolerance and elevated fasting blood glucose concentration, visceral adiposity, and plasma leptin concentration. It is worth noting that we compared the behavioral and metabolic effects of 45% HFD to that of standard chow diet. Use of control low-fat diet (LFD) is a preferable option for metabolic studies53, but standard chow diet (SD) can be a bona fide substitute when low-fat diet is not available. This point is buttressed by the recent observation that an 18-week consumption of SD or LFD elicited similar phenotypic, behavioral, and metabolic effects in rodents48. More follow-up studies on the effects of moderate alcohol and THC use on energy balance and glucose homeostasis are warranted.
We also observed insignificant effects of drug or diet on insulin sensitivity assessed via the ITT. Besides the fact that we should have measured blood glucose level at least once before the 75-min time point post insulin injection during the ITT, the 1 U/kg insulin dose we administered to the rats may have been too high to reveal subtle drug- or diet-induced changes in insulin sensitivity. Alcohol exposure or cannabinoids acting via CB1/CB2 receptors can stimulate or augment insulin secretion from pancreatic β-cells22,40,54,55. Furthermore, prolonged HFD consumption induces insulin resistance and hyperinsulinemia25. High or low insulin concentration under basal conditions reflects an imbalance in the amount or rate of insulin secretion and degradation by insulin degrading enzymes in the liver and kidneys. Compared with chow-fed rats, we found that those exposed to HFD expressed modest hyperglycemia and hyperinsulinemia following a mild fast and during the OGTT. However, HFD feeding had negligible effects on plasma insulin levels when measured 75 min after exogenous administration of 1 U/kg insulin (Fig. 6A). The differing outcome of insulin levels during the OGTT and following the ITT suggest that 1 U/kg bolus of exogenous insulin may differently affect insulin secretion and/or degradation in chow and HFD rats. Considering that alcohol, cannabinoid, and HFD exposure can compromise hepatic enzyme function to promote hyperinsulinemia and impair insulin signaling56,57, our findings counter the expectation of increased trunk insulin level in the HFD-fed COM group. Instead, our a priori comparisons revealed that the HFD-fed COM group did have lower insulin concentrations compared with the EtOH group (Fig. 6D). Other researchers have posited that cannabinoids can have opposing effects on insulin secretion in ex vivo pancreatic islet preparations58, and the effect of alcohol on insulin secretion is unclear55.
In summary, we aimed to investigate how THC alone or when combined with moderate alcohol during adolescence will affect subsequent glucose and insulin tolerance during young adulthood in male rats. Our data show that combined subcutaneous THC and alcohol drinking can distinctly reduce the amount of insulin needed for the proper clearance of an oral glucose load compared with the effect of either drug alone. We also noticed that following approximately one-month of abstinence from edible THC and alcohol co-use, glucose and insulin tolerance were normalized to that of subjects without drug exposure. Metabolic challenge with an HFD revealed a drug-diet interaction such that rats that co-used THC and alcohol (COM) gained the most weight and visceral fat. These HFD-fed COM rats also appeared to have faster clearance of an exogenous insulin injection than the EtOH rats with the same HFD challenge. Thus, we provide preliminary evidence to show that THC and alcohol co-exposure can distinctly alter the physiology of glucose and insulin homeostasis in a preclinical rodent model. Research on the effects of alcohol and THC co-exposure in females, during other developmental stages, and with other rodent strains will show whether the effects we observed are universal. Such research will also provide a glimpse into how the drugs could impact metabolic health in human populations.
Materials and Methods
Subjects
The subjects comprised of a total of 139 male Long-Evan rats (Envigo, Indianapolis, IN, USA) received on postnatal day 22 (P22). They were semi-pair housed in large polyethylene tubs with transparent Plexiglas cage dividers that enabled measurement of food and fluid intake for each rat. The colony was maintained in a temperature and humidity-controlled vivarium on a 12-h light/dark cycle (lights on at 11:30 AM). During habituation and drug exposure, rats had ad libitum access to a standard rodent chow (3.1 kcal/g; 58% carbohydrate, 24% protein, and 18% fat from soybean oil; 2018 Teklad global rodent diets 2018, Indianapolis, IN, USA). Tap water was provided in glass bottles fitted with stainless steel sippers. Daily animal handling and care occurred at 8:00 AM when body weight, food, and water intakes were recorded. All study protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign, and they conformed to the guidelines stipulated in the Guide for the care and use of laboratory animals by the National Research Council, 2011.
Test fluids (saccharin, alcohol) and ∆9-tetrahydrocannabinol (THC)
Saccharin (0.1%) solution and sweetened 10% or 5% ethanol solution (v/v) were prepared as we previously described24. The appropriate volume of 200 proof alcohol was mixed in 0.1% saccharine solution to make the sweetened alcohol solutions. Test fluids were presented at home cages in plastic bottles fitted with stainless steel sipper tubes that minimize spillage and evaporation. THC for subcutaneous injection or cookie infusion (Goldfish Grahams Fudge Brownie, Pepperidge Farm; Norwalk, CT, USA) was prepared by suspending the contents of 10 mg dronabinol capsules (Actavis Pharm, Inc.; Parsippany, NJ, USA) in a sesame oil vehicle24.
Experiment 1: Procedures
Forty male rats were the subjects of Experiment 1. The experimental procedures that we employed are detailed in Experiment 1a of our past publication24. Briefly, following habituation and drinking training, the rats were divided into four groups (n = 10/group) by matching average body weight and saccharin intake: control (CTL) given saccharin and oil injection, ethanol (EtOH) given 10% ethanol (v/v) and oil injection, THC given saccharin and THC injection, and combination (COM) given 10% ethanol and THC injection. At 8:30 AM from P30–P45, animals received daily subcutaneous THC or oil injections immediately before test fluid access in their home cages. Chow and test fluid intakes were measured at 11:30 AM (3-h intakes) when test fluids were supplanted by tap water. Drug and test fluid exposures spanned 16 days (P30–P45). The 3 mg/kg/day dose lasted for eight days, while 5 and 10 mg/kg/day doses each lasted for four days36. One rat in the EtOH group was discovered dead at 8:00 AM on P42. Another EtOH rat was accidentally injected with 10 mg/kg THC on P43. The subsequent data from this rat were excluded from analysis. Chow and water were freely available for one week after the last drug exposure day. The experimental timeline is summarized in Fig. 1A.
Oral glucose tolerance test (OGTT) and sacrifice
Following an overnight 16-h fast, we performed the OGTT on P54 (Fig. 1A) according to our established protocol23. After fasting blood glucose was measured using an AlphaTRAK glucometer (Abbott Labs) and blood was collected using heparinized microcapillary tubes via a tail clip, a load of 2.0 g/kg 20% glucose solution was administered through oral gavage. Blood glucose levels were measured, and tail blood was collected at 15, 30, 60, and 120 min post glucose gavage. The blood samples were centrifuged at 1,000 × g for 15 min at 4 °C, and the plasma was collected and stored at −80 °C for later insulin measurement using an ELISA kit (ALPCO, Salem, NH, USA) as we have previously done23. Within 1 h after the OGTT, the animals were sacrificed, and their carcasses stored at 4 °C overnight. The epididymal and retroperitoneal fat pads of each rat were dissected and weighed by researchers blinded to the group assignment. Visceral fat composition was calculated with reference to the body weight on the day of sacrifice59.
Experiment 2: Procedures
Drug training and exposure procedures were performed, as we have previously described24. The 99 rats were first habituated to the vivarium for four days. From P26–P29, the animals were trained to consume 0.1% saccharin along with chow during the last 3 h of the dark cycle (8:30–11:30 AM). Following measurement of food and fluid intakes, the first and second oil-laden cookies were respectively provided at 12:30 PM and 9:00 PM. At the end of training, rats were assigned to one of four groups: control (CTL, n = 25) given saccharin and oil-laden cookies, ethanol (EtOH, n = 24) given 5% ethanol (v/v) and oil-laden cookies, THC (n = 24) given saccharin and THC-laden cookies, and combination (COM, n = 26) given 5% ethanol and THC-laden cookies. During the 16-day treatment period, rats first had 3-h access to saccharin or 5% alcohol (v/v) solution followed by a cookie laced with oil or THC (1.5, 3, or 5 mg/kg) presented twice per day at 12:30 PM and 9:00 PM (Fig. 1B). Each cumulative daily dose of 3, 6, 8, and 10 mg/kg THC lasted for four days. To ascertain how oral THC would affect blood ethanol concentration (BEC) in these rats, tail blood was sampled at 1-h during a day of 8 mg/kg/day THC consumption, and at 3-h during a day of 10 mg/kg/day THC consumption. BEC was analyzed in those samples using an ELISA kit (Cell Biolabs, San Diego, CA, USA) as we previously described24.
High-fat diet exposure and oral glucose tolerance test (OGTT)
Following three weeks abstinence, rats in all four groups were subdivided into a chow group that continued consuming regular chow diet (CTL and COM, n = 14/group; EtOH and THC, n = 12/group) or a high-fat diet (HFD) group whose diet was switched to a 45% HFD (4.73 kcal/g; 45% fat from lard and soybean oil, 35% carbohydrate, and 20% protein; D12451, Research Diets, New Brunswick, NJ, USA; CTL, n = 11; EtOH, THC, and COM, n = 12/group). On the fourth day of this diet regimen (P70), rats were overnight food-restricted such that their intake was 70% of their respective intake on P69. OGTT was performed in the middle of the light cycle (between 2:00 and 7:00 PM) on P71 according to our established protocol23 and as described in Experiment 1 above.
Insulin tolerance test (ITT) and tissue harvest
After the OGTT, rats were returned to their home cages to resume ad libitum access to their respective diets for four more days (P71–P74). On P75, they were mildly food-restricted to 90% of their average free-feeding intakes on previous two consecutive days (P72 and P73) following the OGTT. Between 2:00 and 7:00 PM on P76, fasting blood glucose levels were measured before rats were intraperitoneally injected with 1.0 U/kg of insulin (Humulin R, U-100; Lilly USA, LLC, Indianapolis, IN, USA). The 1.0 U/kg insulin dose was chosen based on its use by other researchers to assess systemic insulin sensitivity60. Seventy-five min later, blood glucose was measured before rats were rapidly decapitated. Trunk blood was collected into EDTA-coated tubes, centrifuged and plasma was collected to measure plasma levels of insulin (ALPCO, Salem, NH, USA) and leptin (Cat. #90040; Crystal Chem, Elk Grove Village, IL, USA). Animal carcasses were stored at 4 °C overnight. Epididymal and retroperitoneal fat pads were dissected and weighed by researchers blinded to the group assignment. Percent visceral fat composition was calculated with reference to the body weight on the day of sacrifice59.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) and were analyzed by factorial ANOVA or repeated-measures ANOVA (Statistica 13.3; TIBCO Software Inc.; Palo Alto, CA, USA). Statistically significant main effects and interactions were accompanied with Tukey’s HSD post hoc tests. Comparison between two groups within a diet condition was conducted with independent samples t-test. The linear relationship between ingested alcohol dose and BEC, as well as between plasma leptin and percent visceral fat were analyzed by Pearson’s correlation. P < 0.05 was considered significant.
Data Availability
The raw data of the results presented in this manuscript are available upon request.
References
Bouret, S., Levin, B. E. & Ozanne, S. E. Gene-environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol Rev 95, 47–82, https://doi.org/10.1152/physrev.00007.2014 (2015).
Thompson, B. L., Levitt, P. & Stanwood, G. D. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci 10, 303–312, https://doi.org/10.1038/nrn2598 (2009).
Grant, K. S., Petroff, R., Isoherranen, N., Stella, N. & Burbacher, T. M. Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol Ther 182, 133–151, https://doi.org/10.1016/j.pharmthera.2017.08.014 (2018).
Moody, L., Liang, J., Choi, P. P., Moran, T. H. & Liang, N. C. Wheel running decreases palatable diet preference in Sprague-Dawley rats. Physiol Behav 150, 53–63, https://doi.org/10.1016/j.physbeh.2015.03.019 (2015).
Subbaraman, M. S. & Kerr, W. C. Simultaneous versus concurrent use of alcohol and cannabis in the National Alcohol Survey. Alcohol Clin Exp Res 39, 872–879, https://doi.org/10.1111/acer.12698 (2015).
Ernst, M., Romeo, R. D. & Andersen, S. L. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav 93, 199–211, https://doi.org/10.1016/j.pbb.2008.12.013 (2009).
Nixon, K. & McClain, J. A. Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Curr Opin Psychiatry 23, 227–232, https://doi.org/10.1097/YCO.0b013e32833864fe (2010).
Ellgren, M. et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol 18, 826–834, https://doi.org/10.1016/j.euroneuro.2008.06.009 (2008).
Rubino, T. et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis 73, 60–69, https://doi.org/10.1016/j.nbd.2014.09.015 (2015).
Renard, J., Krebs, M. O., Le Pen, G. & Jay, T. M. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front Neurosci 8, 361, https://doi.org/10.3389/fnins.2014.00361 (2014).
Crews, F. T., Vetreno, R. P., Broadwater, M. A. & Robinson, D. L. Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev 68, 1074–1109, https://doi.org/10.1124/pr.115.012138 (2016).
Figlewicz, D. P. et al. Moderate high fat diet increases sucrose self-administration in young rats. Appetite 61, 19–29, https://doi.org/10.1016/j.appet.2012.09.021 (2013).
Kruger, J. S. et al. Manipulation checking the munchies: validating self-reported dietary behaviors during cannabis intoxication. Human Ethology 34, 10–16, https://doi.org/10.22330/he/34/010-016 (2019).
Midanik, L. T., Tam, T. W. & Weisner, C. Concurrent and simultaneous drug and alcohol use: results of the 2000 National Alcohol Survey. Drug Alcohol Depend 90, 72–80, https://doi.org/10.1016/j.drugalcdep.2007.02.024 (2007).
Fernandez-Sola, J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat Rev Cardiol 12, 576–587, https://doi.org/10.1038/nrcardio.2015.91 (2015).
Baliunas, D. O. et al. Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 32, 2123–2132, https://doi.org/10.2337/dc09-0227 (2009).
Sidney, S. Marijuana Use and Type 2 Diabetes Mellitus: a Review. Curr Diab Rep 16, 117, https://doi.org/10.1007/s11892-016-0795-6 (2016).
Penner, E. A., Buettner, H. & Mittleman, M. A. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med 126, 583–589, https://doi.org/10.1016/j.amjmed.2013.03.002 (2013).
Hollister, L. E. & Reaven, G. M. Delta-9-tetrahydrocannabinol and glucose tolerance. Clin Pharmacol Ther 16, 297–302 (1974).
Podolsky, S., Pattavina, C. G. & Amaral, M. A. Effect of marijuana on the glucose-tolerance test. Annals of the New York Academy of Sciences 191, 54–60 (1971).
Permutt, M. A., Goodwin, D. W., Schwin, R. & Hill, S. Y. The effect of marijuana on carbohydrate metabolism. Am J Psychiatry 133, 220–224, https://doi.org/10.1176/ajp.133.2.220 (1976).
Muniyappa, R. et al. Metabolic effects of chronic cannabis smoking. Diabetes Care 36, 2415–2422, https://doi.org/10.2337/dc12-2303 (2013).
Nelson, N. G., Suhaidi, F. A., Law, W. X. & Liang, N. C. Chronic moderate alcohol drinking alters insulin release without affecting cognitive and emotion-like behaviors in rats. Alcohol 70, 11–22, https://doi.org/10.1016/j.alcohol.2017.12.001 (2017).
Nelson, N. G. et al. Combined (9)-tetrahydrocannabinol and moderate alcohol administration: effects on ingestive behaviors in adolescent male rats. Psychopharmacology (Berl) 236, 671–684, https://doi.org/10.1007/s00213-018-5093-3 (2019).
Brons, C. et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol 587, 2387–2397, https://doi.org/10.1113/jphysiol.2009.169078 (2009).
Kraegen, E. W. et al. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 40, 1397–1403 (1991).
Barrus, D. G. et al. Tasty THC: Promises and Challenges of Cannabis Edibles. Methods Rep RTI Press 2016, https://doi.org/10.3768/rtipress.2016.op.0035.1611 (2016).
Hollister, L. E. et al. Do plasma concentrations of delta 9-tetrahydrocannabinol reflect the degree of intoxication? J Clin Pharmacol 21, 171S–177S (1981).
Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42, 327–360, https://doi.org/10.2165/00003088-200342040-00003 (2003).
Ohlsson, A. et al. Plasma levels of delta 9-tetrahydrocannabinol after intravenous, oral, and smoke administration. NIDA Res Monogr 34, 250–256 (1981).
Lukas, S. E. et al. Marihuana attenuates the rise in plasma ethanol levels in human subjects. Neuropsychopharmacology 7, 77–81 (1992).
Perez-Reyes, M., Hicks, R. E., Bumberry, J., Jeffcoat, A. R. & Cook, C. E. Interaction between marihuana and ethanol: effects on psychomotor performance. Alcohol Clin Exp Res 12, 268–276 (1988).
Hartman, R. L. et al. Controlled vaporized cannabis, with and without alcohol: subjective effects and oral fluid-blood cannabinoid relationships. Drug Test Anal 8, 690–701, https://doi.org/10.1002/dta.1839 (2016).
Lukas, S. E. & Orozco, S. Ethanol increases plasma Delta(9)-tetrahydrocannabinol (THC) levels and subjective effects after marihuana smoking in human volunteers. Drug Alcohol Depend 64, 143–149 (2001).
Williams, C. M., Rogers, P. J. & Kirkham, T. C. Hyperphagia in pre-fed rats following oral delta9-THC. Physiol Behav 65, 343–346 (1998).
Rubino, T. et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 33, 2760–2771, https://doi.org/10.1038/sj.npp.1301664 (2008).
Hollister, L. E. Hunger and appetite after single doses of marihuana, alcohol, and dextroamphetamine. Clin Pharmacol Ther 12, 44–49 (1971).
Alshaarawy, O. & Anthony, J. C. Are cannabis users less likely to gain weight? Results from a national 3-year prospective study. Int J Epidemiol, https://doi.org/10.1093/ije/dyz044 (2019).
Avraham, Y. et al. Very low doses of delta 8-THC increase food consumption and alter neurotransmitter levels following weight loss. Pharmacol Biochem Behav 77, 675–684, https://doi.org/10.1016/j.pbb.2004.01.015 (2004).
Li, C., Jones, P. M. & Persaud, S. J. Role of the endocannabinoid system in food intake, energy homeostasis and regulation of the endocrine pancreas. Pharmacol Ther 129, 307–320, https://doi.org/10.1016/j.pharmthera.2010.10.006 (2011).
Bellocchio, L. et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci 13, 281–283, https://doi.org/10.1038/nn.2494 (2010).
Gatta-Cherifi, B. & Cota, D. New insights on the role of the endocannabinoid system in the regulation of energy balance. Int J Obes (Lond) 40, 210–219, https://doi.org/10.1038/ijo.2015.179 (2016).
Mazier, W., Saucisse, N., Gatta-Cherifi, B. & Cota, D. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol Metab 26, 524–537, https://doi.org/10.1016/j.tem.2015.07.007 (2015).
Renard, J. et al. Adolescent THC Exposure Causes Enduring Prefrontal Cortical Disruption of GABAergic Inhibition and Dysregulation of Sub-Cortical Dopamine Function. Sci Rep 7, 11420, https://doi.org/10.1038/s41598-017-11645-8 (2017).
Hirvonen, J. et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 17, 642–649, https://doi.org/10.1038/mp.2011.82 (2012).
Ceccarini, J. et al. Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J Neurosci 34, 2822–2831, https://doi.org/10.1523/JNEUROSCI.0849-13.2014 (2014).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122, 153–162, https://doi.org/10.1172/JCI59660 (2012).
Almeida-Suhett, C. P., Scott, J. M., Graham, A., Chen, Y. & Deuster, P. A. Control diet in a high-fat diet study in mice: Regular chow and purified low-fat diet have similar effects on phenotypic, metabolic, and behavioral outcomes. Nutr Neurosci 22, 19–28, https://doi.org/10.1080/1028415X.2017.1349359 (2019).
McNay, D. E., Briancon, N., Kokoeva, M. V., Maratos-Flier, E. & Flier, J. S. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest 122, 142–152, https://doi.org/10.1172/JCI43134 (2012).
Park, Y. W. et al. The sequential changes in the fasting plasma glucose levels within normoglycemic range predict type 2 diabetes in healthy, young men. Diabetes Res Clin Pract 73, 329–335, https://doi.org/10.1016/j.diabres.2006.02.006 (2006).
Matias, I. et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 91, 3171–3180, https://doi.org/10.1210/jc.2005-2679 (2006).
Matias, I. & Di Marzo, V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab 18, 27–37, https://doi.org/10.1016/j.tem.2006.11.006 (2007).
Warden, C. H. & Fisler, J. S. Comparisons of diets used in animal models of high-fat feeding. Cell Metab 7, 277, https://doi.org/10.1016/j.cmet.2008.03.014 (2008).
Lee, S. S. et al. Chronic Alcohol Consumption Results in Greater Damage to the Pancreas Than to the Liver in the Rats. Korean J Physiol Pharmacol 19, 309–318, https://doi.org/10.4196/kjpp.2015.19.4.309 (2015).
Steiner, J. L., Crowell, K. T. & Lang, C. H. Impact of Alcohol on Glycemic Control and Insulin Action. Biomolecules 5, 2223–2246, https://doi.org/10.3390/biom5042223 (2015).
Liu, J. et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 142, 1218–1228 e1211, https://doi.org/10.1053/j.gastro.2012.01.032 (2012).
de la Monte, S. M., Longato, L., Tong, M., DeNucci, S. & Wands, J. R. The liver-brain axis of alcohol-mediated neurodegeneration: role of toxic lipids. Int J Environ Res Public Health 6, 2055–2075, https://doi.org/10.3390/ijerph6072055 (2009).
Anderson, R. L., Randall, M. D. & Chan, S. L. The complex effects of cannabinoids on insulin secretion from rat isolated islets of Langerhans. Eur J Pharmacol 706, 56–62, https://doi.org/10.1016/j.ejphar.2013.02.034 (2013).
Nelson, N. G., Suhaidi, F. A., DeAngelis, R. S. & Liang, N. C. Appetite and weight gain suppression effects of alcohol depend on the route and pattern of administration in Long Evans rats. Pharmacol Biochem Behav 150–151, 124–133, https://doi.org/10.1016/j.pbb.2016.10.006 (2016).
Underwood, E. L. & Thompson, L. T. A High-Fat Diet Causes Impairment in Hippocampal Memory and Sex-Dependent Alterations in Peripheral Metabolism. Neural Plast 2016, 7385314, https://doi.org/10.1155/2016/7385314 (2016).
Acknowledgements
Startup funds from the Psychology Department of the University of Illinois and NIH grant R21DA045175-01A1 (both to NCL) supported this study. We thank Sheel Vasavada, Tiffany Yang, Jacquelin Sanchez, and the psychology department animal care staff for their superb technical assistance. We are also grateful to Emily Scott for proof reading and editing the manuscript.
Author information
Authors and Affiliations
Contributions
N.G. Nelson and N.-C. Liang designed and supervised the study; N.G. Nelson, M.J. Weingarten, W.X. Law, D.T. Sangiamo, and N.-C. Liang collected the data; N.G. Nelson and N.-C. Liang analyzed and interpreted the data; N.G. Nelson drafted the manuscript with guidance from N.-C. Liang; all authors read and endorsed the manuscript; N.-C. Liang edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nelson, N.G., Weingarten, M.J., Law, W.X. et al. Joint and separate exposure to alcohol and ∆9-tetrahydrocannabinol produced distinct effects on glucose and insulin homeostasis in male rats. Sci Rep 9, 12025 (2019). https://doi.org/10.1038/s41598-019-48466-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48466-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.