Abstract
In July 2018, certain valsartan-containing drugs were voluntary recalled in Japan owing to contamination with N-nitrosodimethylamine (NDMA), a probable human carcinogen. In this study, an HPLC method was developed for the quantitative detection of NDMA simultaneously eluted with valsartan. Good linearity with a correlation coefficient (R2) > 0.999 was achieved over the concentration range of 0.011–7.4 µg/mL. The limits of detection and quantification were 0.0085 μg/mL and 0.0285 μg/mL, respectively. When the recalled valsartan samples were subjected to this method, the observed NDMA contents were in agreement with the reported values, indicating that our method achieved sufficient linearity, accuracy, and precision to detect NDMA in valsartan drug substances and products. Moreover, six samples (valsartan drug substances and tablet formulations), which had a possibility for NDMA contamination, were analyzed; none of the samples contained NDMA at detectable levels. Our method would be useful for the rapid screening and quantification of NDMA impurity in valsartan drug substances and products.
Similar content being viewed by others
Introduction
Valsartan-containing drugs contain the active pharmaceutical ingredient (API) valsartan. Valsartan [(2S)-3-Methyl-2-(N-{[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}pentanamido)butanoic acid], an angiotensin II receptor antagonist, is mainly used for the treatment of hypertension and congestive heart failure. On 6th July 2018, the Ministry of Health, Labour and Welfare (MHLW) in Japan released that N-nitrosodimethylamine (NDMA) was detected as an impurity in valsartan-containing drugs whose API was supplied by Zhejiang Huahai Pharmaceutical in China. Simultaneously, ASKA Pharmaceutical Co., Ltd. in Japan announced a voluntary product recall of valsartan-containing drugs, because they found that there could be a risk of contamination of NDMA in the API purchased from Zhejiang Huahai Pharmaceutical1. In addition, the MHLW recently notified that NDMA impurity in valsartan drug substances should not exceed 0.599 ppm2.
NDMA is classified as a probable human carcinogen based on results from laboratory animal tests3,4,5 and is listed under WHO/IARC group 2A and EPA group B26,7,8. NDMA contamination was thought to be caused by the following changes in the production process of valsartan API9,10: NDMA was generated during the tetrazole-formation step owing to the presence of dimethylamine as an impurity or a degradant in N,N-dimethylformamide (DMF) solvent and the presence of nitrous acid generated from sodium nitrite under acidic conditions (Fig. 1).
As of September 2018, valsartan-containing products whose APIs were supplied from Zhejiang Huahai Pharmaceutical Co., Ltd., Zhejiang Tianyu Pharmaceutical Co., Ltd., Hetero Labs Ltd., and Zhuhai Rundu Pharmaceutical Co., Ltd. have been recalled in more than 20 countries, including the EU and USA11,12,13,14,15,16. Regulatory bodies, European Medicines Agency (EMA), U.S. Food and Drug Administration (FDA), and the MHLW, cooperatively worked to handle the situation and take necessary measures to mitigate patient risk while estimating the risk of cancer due to NDMA-contaminated valsartan products17,18. Government laboratories, including National Institute of Health Sciences in Japan, parallelly prepared methods to analyze NDMA in valsartan APIs and products in each market.
The API valsartan is listed in the Japanese Pharmacopoeia (JP)19 with methods for testing identity, purity, and assay; it is also listed in the US and European Pharmacopoeias20,21. A purity test mainly focusses on expected impurities from synthesis and/or degradation. The purity test for valsartan focusses on heavy metals and related substances; however, these pharmacopoeias have never mentioned the need for testing NDMA as an impurity.
NDMA is mainly generated in foods and drinks after processing at an elevated temperature22,23,24. It is also detected as a disinfection by-product in ground and drinking water25,26,27. As the toxicity of NDMA is manifested even at μg/kg levels28,29, sensitive and specific methods were developed for the determination of NDMA at trace level. Gas chromatography–mass spectrometry (GC-MS) is the most frequently employed technique for NDMA analyses30,31,32. In addition, several methods using liquid chromatography–mass spectrometry (LC-MS) or LC-MS/MS have been reported in scientific literature33,34,35. However, only few studies have reported NDMA analysis using conventional high-performance liquid chromatography (HPLC)36, especially in drugs. HPLC is the most popular technique for quality control of APIs and products in routine analysis, and it is preferable if NDMA impurity is simultaneously detected with drug substances by a single HPLC analysis. Thus, it is important to develop a fast and simple analytical method for NDMA in drugs by using HPLC.
In view of these situations, we tried to develop an HPLC method for the simultaneous detection of NDMA and valsartan. We analyzed valsartan drug substances and its products, including recalled samples, and confirmed the accuracy and precision of the method. This study provides a simple and accurate method for the quantification of NDMA impurity in valsartan products.
Results and Discussion
HPLC method development
To establish a practical method for the simultaneous detection of NDMA and valsartan, we first assayed each standard solution according to the HPLC condition for the quantitative assay for valsartan API and its tablet formulation defined in the JP19. When each standard solution of valsartan and NDMA was assayed following the modified JP method using isocratic mobile conditions, it was difficult to identify the peak of NDMA as it eluted during the void time (2.5 min) even with a flow rate of 0.80 mL/min, whereas the peak of valsartan was clearly detected at around 11 min (data not shown). Then, we developed a gradient elution program using a water-acetonitrile mobile phase containing 0.1% formic acid to detect NDMA and valsartan simultaneously within 30 min. Under this condition, peaks of NDMA and valsartan were successfully detected at 7.8 and 16.3 min, respectively (Fig. 2). Moreover, we confirmed the simultaneous detection of cilnidipine, another API in valsartan combination products, at 17.1 min under the same condition.
To assess the linearity of the developed method, we prepared a calibration plot using 10 concentration points of NDMA in the range of 0.0111–7.4 μg/mL (0.15–100 μM) and constructed the calibration curve for quantification. The correlation coefficient (R2) of the calibration curve was over 0.999. The limits of detection (LODs) and quantification (LOQs) were 0.0085 μg/mL (at a S/N ratio of 3) and 0.0285 μg/mL (at a S/N ratio of 10), respectively. The standardized limits of NDMA impurity in valsartan drug substances, which was set as 0.559 ppm by the MHLW2, was equivalent to 0.02995 μg/mL when 0.1 g of sample was extracted with 2 mL methanol. This concentration was almost equal to the LOQ. Recently, we reported a GC-MS method for the detection of NDMA in valsartan drug substance and products with much lower LOD (0.001 μg/mL of NDMA corresponded to a S/N of 3)37. However, this method needed the isotopic internal standard (NDMA-d6) and multiple extraction steps as required for other MS-based methods. Generally, an HPLC method is low-cost and more suitable to routine analyses. Thus, the developed method would be useful for the rapid screening of NDMA contamination in valsartan drug substances with sufficient sensitivity.
Quantification of NDMA impurity in the recalled valsartan samples
Following the confirmation of linearity, we evaluated NDMA contents in the recalled valsartan samples using the developed HPLC method. VALSARTAN TABLETS 80 mg [AA] and its substances were provided by ASKA Pharmaceutical Co., Ltd. through the MHLW. NDMA contents were estimated to be 36–74 ppm in the drug substances and 3.1–5.3 μg per tablet, respectively, based on GC-MS analysis according to the press release13. When a sample solution of drug substance (50 mg/mL in methanol) was analyzed using the developed method, the peak at 7.8 min was identified by comparing its retention time and UV spectrum with those of the NDMA reference standard (Fig. 3). NDMA content was calculated to be 54.7 μg/g, which was in agreement with the published value1. A sample solution of the commercial product (150 mg/mL in methanol) provided a similar chromatogram, and its NDMA content was determined to be 17.0 μg/g. The corresponding NDMA content was estimated to be 4.25 μg per tablet (250 mg), which was in agreement with the published data (Table 1)1.
Recovery test
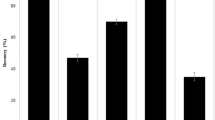
Successively, a recovery test was carried out by analyzing spiked samples in the same way. Forty microliters of 10 mM NDMA (29.6 μg) was added to powdered VALSARTAN TABLETS [AA] (300 mg), and the spiked sample solution fortified at 98.67 μg/g was prepared. Five replicates of the spiked samples and triplicates of blanks (samples not spiked) were analyzed, and the recovery rate was determined to be 96.5% with 0.67% relative standard deviation (RSD). These results indicate that the developed method has sufficient linearity, accuracy, and precision for the quantification of NDMA impurity in contaminated valsartan final products as well as its drug substances.
HPLC assay on APIs and commercial valsartan products
Finally, we investigated NDMA content in APIs and commercially available valsartan-containing products in the same way. Four valsartan APIs and two tablets, including an original drug ATEDIO® Combination Tab. (containing 80 mg valsartan and 10 mg cilnidipine), were supplied by Zhejiang Tianyu Pharmaceutical Co. Ltd. When their sample solutions (50 mg/mL and 150 mg/mL in methanol for API and tablets, respectively) were analyzed, no peak was detected for NDMA in the APIs of VALSARTAN TABLET 80 mg [SAWAI] and VALSARTAN TABLET 80 mg [OHARA]. Any peaks other than those for valsartan and cilnidipine were not detected in the API and tablets of ATEDIO® Combination Tab. Although a small peak was observed at around 8 min on the HPLC chromatogram of VALSARTAN TABLET 80 mg [SANOFI], it had a retention time different from that of NDMA and was not detected in the API. Thus, NDMA contents in all the samples were indicated to be below the LOD (<0.17 μg/g for substances and <0.06 μg/g for tablets) and below the acceptance limit for NDMA (0.599 ppm) (Table 2). The EMA reported that NDMA concentrations in valsartan API from Zhejiang Tianyu Pharmaceutical Co., Ltd. were considerably lower than those from Zhejiang Huahai Pharmaceutical14, and only one batch of valsartan-containing drugs distributed in Germany was recalled13. NDMA was not detectable in the tested samples probably because the manufacturing process of valsartan APIs for Japanese companies could be different from that for other foreign companies. Hence, we successfully developed a practical method for the rapid screening and quantification of NDMA impurity in valsartan-containing products.
Conclusion
A rapid and efficient HPLC method was developed for the quantitative detection of NDMA simultaneously eluted with valsartan. The method was found to have sufficient linearity, accuracy, and precision, and can be applied for the rapid screening and quantification of NDMA impurity in valsartan APIs and commercial products. This HPLC method would be useful for the quality control of APIs and products in routine analysis.
Methods
Reagents and materials
The commercial reagents of valsartan, cilnidipine, and NDMA with high purity (>98.0%, >98.0% and >99%, respectively) were purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan). APIs and its tablet formulations were provided by each company through the MHLW (Table 3).
Other reagents were of analytical grade.
Sample preparation
NDMA (14.8 μL, Mw: 74.08, density: 1.005) was transferred into a 2-mL volumetric flask, dissolved to volume with methanol (NDMA = 7.4 mg/mL, corresponding to 100 mM), and diluted 100-fold to obtain the stock solution at 1 mM. The standard solution of NDMA was prepared by 10-fold dilution of the stock solution for HPLC analysis (100 μM). Valsartan (8.7 mg, Mw: 435.53) and cilnidipine (9.9 mg, Mw: 492.53) reagents were accurately weighed, individually transferred into a 2-mL volumetric flask, and dissolved to volume with methanol to obtain the stock solutions at 10 mM. Each stock solution was diluted 100-fold to a final concentration of 100 μM as standard solutions and filtered through a 0.45-μm Ultrafree-MC centrifugal filter unit (Millipore, Billerica, MA) before HPLC analysis.
The drug substance (100 mg) or powdered tablet (300 mg) was dissolved with 2 mL methanol and centrifuged at 5,000 rpm for 5 min. The supernatant was filtered through a 0.45-μm Ultrafree-MC centrifugal filter unit. Triplicate test samples for each commercial product were prepared from every press-through sheet.
As NDMA is a carcinogenic substance, its handling was carried out in accordance with Safety Data Sheet, and the preparation of samples containing NDMA was performed in a fume hood.
HPLC analysis
HPLC method development and analyses were performed on a Shimadzu UFLC system comprising a binary gradient pump (LC-20AD), an autosampler (SIL-20AC), a column oven (CTO-20A), and a photodiode array detector (SPD-M20A) (Shimadzu, Tokyo, Japan). The first trial was carried out according to a modification of the method cited in the monographs of valsartan and its tablet in JP19. Briefly, 10 μL NDMA standard solution was assayed by isocratic elution with a mixture of water, acetonitrile, and acetic acid (100) (500: 500: 1) on an HPLC system equipped with Unison UK-C18 column (250 × 4.6 mm, 3 μm, Imtakt, Kyoto, Japan) at a flow rate 0.8 mL/min and detected at 235 nm. Although the wavelength of maximum absorption of NDMA standard solution was 228 nm, we set the detection wavelength at 235 nm to achieve detection at lower noise and better baseline stability. In the finalized condition, HPLC analysis was carried out on Inertsil ODS-3 column (150 × 4.6 mm, 5 μm, GL Science, Tokyo, Japan) at 30 °C with a mobile phase comprising water containing 0.1% formic acid (A) and acetonitrile containing 0.1% formic acid (B) and detected at 235 nm. The gradient elution started at 0% B in 10 min and increased linearly to 100% in 5 min at a flow rate of 1.0 mL/min. A 10-μL aliquot of each sample was injected three times, and the reproducibility of the result was confirmed. Each peak obtained from the test samples was identified by comparing its retention time and UV spectrum with those of the reference standard for valsartan, cilnidipine, and NDMA. Peak areas were determined by the automatic integration method. The standard solution of NDMA was diluted for preparing calibration solutions at 0.15, 0.2, 0.3, 0.4, 0.5, 1, 5, 10, 50, 100 μM with methanol. The calibration solutions were analyzed to plot a calibration curve, and its slope, intercept, and coefficient of determination were calculated.
Recovery test
Forty microliters of 10 mM NDMA standard solution (740 μg/mL in methanol) was spiked to 300 mg of powdered VALSARTAN TABLETS [AA] and allowed to stand for 10 min. The spiked sample was dissolved with 1960 μL methanol and centrifuged at 5,000 rpm for 5 min. The supernatant was filtered through a 0.45-μm Ultrafree-MC centrifugal filter unit. The spiked sample was fortified to 98.67 μg/g with NDMA. Five replicates of spiked samples and three blanks (samples not spiked) were prepared and analyzed to determine the percentage of recovery.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Ministry of Health, Labour and Welfare, Japan. Press release: Notice of voluntary collection of pharmaceutical products (Class I) (Valsartan Tablets 20 mg⋅40 mg⋅80 mg⋅160 mg AA, ASKA Pharmaceutical Co., Ltd.) (in Japanese), 6 July 2018, Available online, https://www.mhlw.go.jp/stf/newpage_00086.html (cited 26 September, 2018).
Ministry of Health, Labour and Welfare, Japan. Notification: No. 1109-001, Document 3-1, Notice of the director of the Monitoring and guidance Narcotics Division (in Japanese), Available online, https://www.pmda.go.jp/files/000226684.pdf (cited 9 November, 2018).
Ashley, L. M. & Halver, J. E. Dimethylnitrosamine-induced hepatic cell carcinoma in rainbow trout. JNCI. 41, 531–522 (1968).
Barnes, J. M. & Magee, P. N. Some toxic properties of dimethylnitrosamine. Br. J. Ind. Med. 11, 167–174 (1954).
Jakszyn, P. & González, C. A. Nitrosamine and related food intake and gastric and oesophageal cancer risk: a systematic review of the epidemiological evidence. World J. Gastroenterol. 12, 4296–4303 (2006).
World Health Organization, Guidelines for Drinking-water Quality (2017).
World Health Organization, N-Nitrosodimethylamine In Drinking-water. Background Document for Preparation of WHO Guidelines for Drinking-water Quality, in, Geneva (2008).
International Agency for Research on Cancer, IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans – Some N-Nitroso Compounds, Lyon, (1978).
EMA CHMP List of questions. To be addressed by the API manufacturers for valsartan-containing medicinal products (16 July 2018), Available online, https://www.ema.europa.eu/documents/referral/valsartan-article-31-referral-chmp-list-questions-be-addressed-api-manufacturers-valsartan_en.pdf (cited 3 October, 2018).
Pottegard, A. et al. Use of N-nitrosodimethylamine (NDMA) contaminated valsartan products and risk of cancer: Danish nationwide cohort study. BMJ. 362, k3851, https://doi.org/10.1136/bmj.k3851 (2018).
EMA reviewing medicines containing valsartan from Zhejiang Huahai following detection of an impurity. Some valsartan medicines being recalled across the EU, 05 July 2018, Available online, http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2018/07/WC500251498.pdf (cited 26 September, 2018).
FDA announces voluntary recall of several medicines containing valsartan following detection of an impurity, 13 July 2018, Available online, https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm613532.htm (cited 26 September, 2018).
Ministry of Health, Labour and Welfare, Japan. Notification: Notice of response to the detected cancer-causing substance in Valsartan medicines (in Japanese), 7 September 2018, Available online, https://www.mhlw.go.jp/content/11121000/000360273.pdf, (cited 6 October, 2018).
EMA Update on medicines containing valsartan from Zhejiang Tianyu. Company no longer authorised to manufacture valsartan active substance for EU medicines due to presence of NDMA (20/August/2018), https://www.ema.europa.eu/documents/press-release/update-medicines-containing-valsartan-zhejiang-tianyu_en.pdf, (cited 21 November, 2018).
Valsartan: Slightly elevated NDMA levels were found in a batch of Valsartan HCT Aurobindo 320/25 mg (18/August/2018), https://www.bfarm.de/SharedDocs/Pressemitteilungen/DE/2018/pm8-2018.html.
Company Announcement (8/August/2018). Camber Pharmaceuticals, Inc. Issues Voluntary Nationwide Recall of Valsartan Tablets, USP, 40 mg, 80 mg, 160 mg and 320 mg Due to The Detection of Trace Amounts of N-Nitrosodimethylamine (NDMA) Impurity, Found in an Active Pharmaceutical Ingredient (API), https://www.fda.gov/Safety/Recalls/ucm616405.htm?utm_campaign=FDA%252.
EMA Update on review of recalled valsartan medicines. Preliminary assessment of possible risk to patients (2/August/2018), Available online, https://www.ema.europa.eu/news/update-review-recalled-valsartan-medicines (cited 26 September, 2018).
FDA updates recalled valsartan-containing product information and presents NDMA levels in some foods (20/August/2018), Available online, https://www.fda.gov/Drugs/DrugSafety/ucm613916.htm?utm_campaign=FDA%20updates%20recalled%20valsartancontaining%20product%20information%20to%20incorporate%20recalls%20of%20certain&utm_medium=email&utm_source=Eloqua, (cited 26 September, 2018).
The Ministry of Health, Labour and Welfare, Japan. Monographs for Valsartan and Valsartan Tablets referencing in The Japanese Pharmacopoeia 17th Edition 1248–1250 (2016).
The United States pharmacopeial convention, United States Pharmacopoeia-USP 41 NF36, The United States pharmacopeial convention, Inc., Rockville, (2018).
Council of Europe, European Pharmacopoeia, Council of Europe, Strasbourg (2018).
Rywotycki, R. Meat Nitrosamine Contamination Level Depending on Animal Breeding Factors. Meat Sci. 65, 669–676 (2003).
US Food and Drug Administration, Dimethylnitrosamine in Malt Beverages, (2005).
Yurchenko, S. & Molder, U. The Occurrence of Volatile N-Nitrosamines in Estonian Meat Products. Food Chem. 100, 1713–1721 (2007).
Najm, I. & Trussell, R. NDMA formation in water and wastewater. J. Am. Water Works Assoc. 93, 92–99 (2001).
Mitch, W. et al. N-nitrosodimethylamine (NDMA) as a drinking water contaminant: a review. Environ. Eng. Sci. 20, 389–404 (2003).
Richardson, S. D. & Postigo, C. Drinking water disinfection by-products, in: Emerging Organic Contaminants and Human Health, pp. 93–137 (Springer, 2011).
Issaq, H. J., McConnell, J. H., Weiss, D. E., Williams, D. J. & Saveedra, J. E. High performance liquid chromatography separations of nitrosamines. I. cyclic nitrosamines. J. Liquid Chromatogr. 9, 1783 (1986).
USEPA N-nitrosodimethylamine, CASRN 62-75-9 (12/3/2002). Integrated Risk Information System, http://www.epa.gov/IRIS/subst/0045.html.
Raksit, A. & Johri, S. Determination of N-nitrosodimethylamine in environmental aqueous samples by isotope-dilution GC/MS–SIM. J. AOAC. Int. 84, 1413–1419 (2001).
Feng, D., Liu, L., Zhao, L., Zhou, Q. & Tan, T. Determination of volatile nitrosamines in latex products by HS-SPME–GC–MS. Chromatographia 74, 817–825 (2011).
Qiang, M. et al. Determination of Ten Volatile Nitrosamines in Cosmetics by Gas Chromatography Tandem Mass Spectrometry. Chin. J. Anal. Chem. 39, 1201–1207 (2011).
Zhao, Y. Y., Boyd, J., Hrudey, S. E. & Li, X. F. Characterization of new nitrosamines in drinking water using liquid chromatography tandem mass spectrometry. Environ. Sci. Technol. 40, 7636–7641 (2006).
Herrmann, S. S., Duedahl-Olesen, L. & Granby, K. Simultaneous determination of volatile and non-volatile nitrosamines in processed meat products by liquid chromatography tandem mass spectrometry using atmospheric pressure chemical ionisation and electrospray ionisation. J. Chromatogr. A. 1330, 20–29 (2014).
Ngongang, A. D., Duy, S. V. & Sauvé, S. Analysis of nine N-nitrosamines using liquid chromatography-accurate mass high resolution-mass spectrometry on a Q-Exactive instrument. Anal. Methods 7, 5748–5759 (2015).
Cha, W., Fox, P. & Nalinakumari, B. High-performance liquid chromatography with fluorescence detection for aqueous analysis of nanogram-level N-nitrosodimethylamine. Anal. Chim. Acta. 566, 109–116 (2006).
Tsutsumi, T. et al. Analysis of an impurity, N-nitrosodimethylamine, in valsartan drug substances and associated products using GC-MS. Biol. Pharm. Bull. 42, 547–551 (2019).
Acknowledgements
This work was supported by a Health Labour Sciences Research Grant provided by the Ministry of Health, Labour and Welfare of Japan. This study was supported in part by grants from AMED under Grant Number JP19mk0101129j0101 (to Y.D.).
Author information
Authors and Affiliations
Contributions
Y.D., H.A., T.H., K.I., Y.G. and H.O. conceived and supervised the study. N.U. and Y.D. designed and conducted the experiments. S.M., G.T., R.A., T.T. and Y.A. performed the experiments. S.M., G.T., N.U. and Y.D. analyzed the results and wrote the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Masada, S., Tsuji, G., Arai, R. et al. Rapid and efficient high-performance liquid chromatography analysis of N-nitrosodimethylamine impurity in valsartan drug substance and its products. Sci Rep 9, 11852 (2019). https://doi.org/10.1038/s41598-019-48344-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48344-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.