Abstract
Both earthworms and plants may affect the soil nematode community. However, the effects of earthworms and plant species interactions on soil nematode community are poorly understood. We explored how an epigeic earthworm Eisenia fetida affects the soil nematode community in systems with three representative plants (wheat, cotton and cabbage) which were grown in pots with or without added earthworms under greenhouse conditions. Earthworm presence decreased the abundance of total nematode and all four nematode trophic groups, except for the fungivore and predator/omnivore nematodes in wheat systems, but increased the genus richness of nematode in all treatments. Due to plant identity and different root exudates, plants had significant effects on soil nematode abundance. Compared with the no plant and without earthworm treatment, wheat and cabbage had the higher stimulation of the abundance of total nematode, bacterivores and fungivores, and cotton had the higher stimulation of the abundance of fungivores and predators-omnivores; whereas earthworm presence mostly weakened the stimulation effects of plant species on soil nematode abundance which indicated earthworms had the enhanced effects in the presence of plants. The interaction affected soil nematode abundance (total nematodes, bacterivore, fungivore and omnivore-predators) and community diversity indices (diversity index H′, evenness index J′, community maturity index ∑MI, Simpson dominance index λ and nematode channel ratio NCR). Principal component analysis showed that plant species affected soil nematode community composition. Redundancy analysis indicated plant species and biomass accounted for 41.60% and 34.13% of the variation in soil nematode community structure, respectively; while earthworms explained only 6.13%. Overall, current study suggest that earthworm could inhibit nematode abundance; whereas, plants have exerted greater influences on nematode community structure than earthworm presence due to their species-specific effects on different trophic groups of nematodes.
Similar content being viewed by others
Introduction
Soil nematodes are an important part of soil biota and occupy positions at the primary, secondary and tertiary consumer levels in soil food webs1,2,3. As the most abundant and diverse type of soil invertebrate comprising all major trophic groups, soil nematodes may represent the complexity of soil food webs4. Nematodes can serve as model soil invertebrates that provide a holistic measure of the biotic and functional statuses of soils5. Living in soil microecosystems, nematode communities are sensitive to environmental changes and are therefore considered useful bioindicators for soil health assessments6,7.
Earthworms represent the largest component of the soil animal biomass and are commonly known as ‘ecosystem engineers’8. Previous studies have shown that endogeic and anecic earthworms have negative, positive or neutral effects on soil nematodes in different ecosystems9,10,11,12,13. In addition, reports have indicated that the epigeic earthworms have effects on soil nematode populations. Studies have found that epigeic earthworm suppressed bacterivorous nematode numbers (more than 50%) in fresh manures and sludge wastes14 and 30% in pig manure15. However, the epigeic earthworms could also increase the number of plant-parasitic nematodes in maize with earthworms by 27% compared with maize without earthworms16. In addition, in peat meadow soil, compared to the control (without earthworms), the introduction of epigeic earthworms (Lumbricus rubellus) decreased the total number of nematodes after 30 days, while 120 days after earthworm addition, the total nematode numbers showed no significant difference17. These results indicated that the effects of epigeic earthworms depended on the soil or substrate systems, nematode trophic groups or even the duration of earthworm presence. Most studies have focused on substrate conditions such as those in manure, sludge or peat meadow soil; whereas, few studies18,19,20 have focused on the effects of earthworms on soil nematode community under planting crops.
In addition, there have been considerable studies in how plants affect the abundance and diversity of soil nematodes21,22,23. Studies have demonstrated that different plant species could affect the numbers of plant-feeding nematodes, the nematode taxonomic diversity and the ratio of fungal to bacterial plus fungal feeders24 although they did not affect the omnivore-predators feeding groups25,26,27. Reasons may be due to the quantity and quality of organic matter produced by different plant species28, as well as the different harmful substances exuded by certain plant species for plant-feeding nematodes29 and the different amounts of bacteria populations or plant species modify nematode community in bacterivorous nematodes30. Wheat, cotton and cabbage are the main agricultural crops in northern China. Different plant species may produce specific root exudates which may result in a stimulation or inhibition on specific trophic group of nematodes.
Although the effect of earthworms on plant growth is well recognized, the combined effects of plants and earthworms on soil nematodes have not been well investigated. Study indicated plants could modify the effects of earthworm on the soil microbial community and its activity31. Whether the effect of earthworms on soil nematode community are more significant than that of plant species has not been clarified. The effects of earthworms on soil nematodes may be reduced or enhanced by plants. The combined effects of earthworms and plant species can be expected to affect soil nematode communities in different ways. A laboratory research can be an effective way to research these effects and elucidate novel aspects of interactions among plants, earthworms and nematodes. In the current study, we hypothesized: (1) that earthworm presence may decrease nematode abundance under planting crops, and such effect may be enhanced in a system where earthworms stimulated plant growth; (2) that either plant species or earthworms may have a more significant effect on the soil nematode community composition; (3) that the interactive effects of earthworms and plant species would influence soil nematode abundance and community structure.
Materials and Methods
Microcosms
A sandy mineral soil (5–20 cm below the surface) from ordinary farmland (continuous cropping winter wheat in October, every year) collected at Henan University was used in the experiment. The soil was sieved (2 mm mesh). Maize straw (C/N = 52) was sundried and milled to pass through a 2 mm diameter mesh before use. 40 kg soil (dry weight) and 2400 g maize powder (earthworm food) were mixed thoroughly, and divided into 80 pots (diameter 12 cm and height 15 cm) (500 g soil + 30 g maize powder/pot). Other soil fauna, including macrofauna was manually removed. The starting soil contained approximately 0.08 g/kg total nitrogen, 0.38 g/kg organic carbon, and 1.58 g/kg total carbon (C/N ratio 21.07) and had a pH of 8.08.
Eisenia fetida is an easily cultured, and has a broad distribution in China. The gut contents of 80 earthworms were emptied on moistened tissue paper for 24 h at room temperature before the experiment, and these earthworms (fresh weight 0.32 ± 0.02 g/earthworm) were added into 40 pots (2 earthworms/pot). Another 40 pots, with 10 no plant pots and 30 pots with plants, were earthworm free. The earthworm numbers corresponded to a density of 216 individuals/m2, which was observed in winter wheat fields32. To prevent the earthworms from escaping, 80 gauzes (height 50 cm, diameter 15 cm) which would affect the growth of plants were wrapped around the pots.
Seeds of winter wheat cultivar Zhoumai 18 (Plant Genetic Resources and Genetic Engineering Laboratory, Henan University), cotton cultivar Zhongmian 79 (State Key Laboratory of Cotton Biology, Henan University), and cabbage cultivar Zhonggan 11 (Vegetable and Flower Research Institute, Chinese Academy of Agricultural Sciences) were surface sterilized with sodium hypochlorite (1%), sown on wet paper in Petri dishes and placed in a climate chamber (14 h/10 h, light/dark, 25 °C). Germinated plant seeds (one cotyledon in wheat; two cotyledons in cotton and cabbage) were transplanted into every pot. Earthworms were added after the plants had grown for one week. All 80 pots were placed in five blocks on greenhouse benches, and each block contained 2 replicates (pots) of the 8 treatments. The blocks were redistributed randomly in the greenhouse every week. The experiment began on December 3rd in 2015. No fertilizers were applied during the experiment, and other weed seedlings germinating from seeds in the soil were removed. The soil was maintained at 70% soil field capacity (checked via regular weighing of the pots).
Experimental design
The experiment was conducted in a greenhouse in Henan University, China, and included two factors: plant species and earthworm treatments. Earthworms were added or not under the no plant treatment and three different agricultural plant species treatments, resulting in 8 treatments. Each treatment was replicated ten times, resulting in 80 microcosms. Control microcosms consisted of no plant and each species of plant without earthworms. No plant, wheat, cotton, and cabbage systems were grown in greenhouses for 15 weeks under the following controlled conditions: relative humidity = 60–85%, temperature = 23–25 °C, light intensity = 600 μmol/m2/s and photo period = 14 h.
Harvesting plants, soil sampling and analysis
After 15 weeks, all plants were cut at ground level and separated into two parts: shoot biomass and root biomass. The shoot dry weight was determined after drying for 72 h at 70 °C. We only analyzed samples in which the number of earthworms found was the same as the number of earthworms introduced. In order to keep the same effective replicates in all the treatments, 3 replicates were eliminated for the no plant, wheat, cotton, and cabbage treatments. After cleaning, the roots were dried at 60 °C for 72 h and weighed. The fresh soil samples were stored in a refrigerator at 4 °C for subsequent soil analysis and nematode extraction.
The analyzed soil properties included the soil pH (H2O), total carbon (TC), total nitrogen (TN), organic carbon (SOC), and the ratio of TC to TN (C/N). Soil pH was measured in a 1:2.5 soil-distilled H2O suspension using a glass electrode (Sartorius PB-10). TC, TN and SOC were analyzed using an element analyzer (Vario MACRO cube, Elementar Inc., Germany).
Soil nematode extraction and identification
Nematodes were extracted from 50 g fresh soil from each pot by using the modified Baermann method33. Another 50 g fresh soil was used to determine the soil water content. Soil water content was measured gravimetrically using soil sample from each pot dried at 105 °C for 24 h. The extracted nematodes were preserved in TAF fixation (40% formaldehyde 7 ml, triethanolamine 2 ml, and distilled water 91 ml)34. Nematode abundance was measured as individuals per 100 g dry soil, and after counting the total number of nematodes, 100 nematode individuals from each sample were identified to the genus level according to Bongers35 using an optical microscope (Motic, BA210, Motic Corporation). For samples in which there were fewer than 100 nematodes, all specimens were identified. The soil nematodes were assigned to four trophic groups: bacterivores (Ba), fungivores (Fu), plant parasites (Pp), and omnivores-predators (Om) with their corresponding colonizer-persister (cp) groups36,37.
Soil nematode community analysis
The following nematode community indices were calculated: (1) dominant genera (Relative abundance > 10%, dominant genera; 1% < relative abundance < 10%, common genera; relative abundance < 1%, rare genera); (2) species richness (SR); (3) the Shannon-Wiener diversity index (H′); (4) the modified maturity index (ΣMI); (5) evenness (J′); (6) Simpson’s index for dominance (λ); and (7) the nematode channel ratio (NCR)7,38.
Margalef richness index, \({\rm{SR}}=\frac{S-1}{lnN}\)
Shannon-Wiener diversity index, \(H^{\prime} =-\mathop{\sum }\limits_{i=1}^{S}\,Pi\times lnPi\)
The modified maturity index, \(\sum {\rm{MI}}={\sum v(i)}^{cp1-5}\times f{(i)}^{cp1-5}\)
Evenness index, \(\,J^{\prime} =\frac{H^{\prime} }{lnS}\)
Simpson dominance index, \(\,{\rm{\lambda }}=\sum P{i}^{2}\)
Nematode channel ratio, \({\rm{NCR}}=\frac{{\rm{B}}}{{\rm{B}}+{\rm{F}}}.\)
In the above equations, ‘S’ is the total number of nematode genera in the community, ‘N’ is the total number of nematodes in the community, ‘Pi’ is the proportion of the individuals of “ith” group in the community, ‘\(v(i)\)’ is the c-p value of “ith” taxon, ‘\(f(i)\)’ is the frequency of “ith” taxon, and ‘B’ and ‘F’ are the numbers of bacterivores and fungivores in the total soil nematode population. SR, H′ and J′ indices are calculated and used as an indication of the diversity of the soil nematodes. Lower and higher values of \(\sum {\rm{MI}}\) indicate disturbed and stable nematode communities, respectively. The higher λ reflects the more uneven distributions of different genera in soil nematode community and the lower diversity of the soil nematodes. The NCR is a powerful index to assess the decomposition pathway of soil matter and might indicate the contribution of bacteria and fungi to the rate of mineralisation.
Statistical analyses
Results are shown as the means ± SE. Data normality was checked to ensure that the data distribution met the underlying assumptions for further statistical analysis. If necessary, nematode abundance and generic richness were ln(x + 1) transformed. The percentage of trophic groups of soil nematode was arcsine-transformed before two-way analysis. Paired t tests were used to compare the difference (P < 0.05) of plant biomasses between with and without earthworm addition treatments under each plant species. Two-way analysis of variance was performed to test the effects of earthworms and plant species on soil characteristics, nematode total genera richness, nematode abundance, relative nematode abundance and ecological indices. Significant differences in the main effects were further analyzed by paired comparison with the Tukey HSD test. All statistical tests were conducted using the SPSS version 19.0 statistical software (SPSS Inc., Chicago, IL, USA).
Principal component analysis (PCA) in CANOCO version 4.5 was used to measure the soil nematode community composition according to the relative abundances of nematodes39 in the absence or presence of earthworms under the no plant treatment and three different plant species treatments. A total of 56 PCAs were analyzed. Each PCA represented a microcosm. Redundancy analysis (RDA) in CANOCO version 4.540 was performed to explore the nematode community composition in relation to environmental factors (plant biomass, plant species, with or without added earthworms, soil properties). Interpretation proportions of environmental factors were calculated by using manual selection referring to a reference23. Monte Carlo permutation tests were conducted using 499 random permutations in order to determine the statistical significance of the first and all ordinations axes.
Results
Earthworm and crop biomasses
At the end of the experiment, 56 earthworms (0.34 ± 0.01 g/per earthworm) were obtained. Plant biomasses were shown in Table 1. Paired t tests indicated earthworm presence significantly increased the shoot biomass of wheat and cotton.
Soil nematode community composition
Forty nematode genera were identified during the study (Table 2). The two most abundant feeding groups were bacterial feeders and plant parasite nematodes. In the absence of earthworms, the dominant genera Cephalobus and Rhabditis were identified in no plant and wheat; Cephalobus and Tylenchus which has been classified as “plant associated”41 were identified in cotton; and Cephalobus, Acrobeles and Rhabditis were identified in cabbage. However, in the presence of earthworms, the dominant genera Cephalobus was found in no plant and cotton; Aphelenchoides and Aphelenchus were in wheat; and Cephalobus and Tylenchus were in cabbage (Table 2).
Nematode abundance and the relative abundance of trophic groups
Earthworm addition significantly suppressed the abundance of total soil nematodes in three different plant species (Fig. 1a), when compared with no addition of earthworm, which showed the significant interaction between plant species and earthworms (Table 3). Compared to without earthworm treatments, earthworm additions significantly increased the total genus richness in all treatments except for in wheat. However, no significant interaction between earthworms and plant species was observed in total genus richness (Fig. 1b and Table 3).
Earthworm addition reduced the abundance of bacterivores in wheat and cabbage (Fig. 2a, Table 3). Furthermore, significant decrease in abundance of fungivores was also observed in cotton (39.99%) and cabbage (69.39%) (Fig. 2b and Table 3) when compared with no addition of earthworm. However, there was no significant difference with plant parasites among no plant and three different plant species (Table 3, Fig. 2c). Finally, we found the abundance of omnivores-predators was remarkably decreased by earthworm addition in cotton (Table 3, Fig. 2d).
Compared with the no plant without added earthworm treatments, wheat and cabbage had the higher abundance of total nematode (Fig. 1a), bacterivores (Fig. 2a) and fungivores (Fig. 2b) and cotton had the higher abundance of fungivores (Fig. 2b) and omnivore-predators (Fig. 2d). However, the abundance of the total nematodes, bacterivores, fungivores and omnivore-predators had no significant difference compared with in the no plant with added earthworm treatments under the three plant species treatments except for in the wheat treatments.
The maximum and minimum relative bacterivore abundances were found in the wheat without and with added earthworm treatments, respectively. Earthworms decreased the relative abundance of fungivores except for in wheat but increased the relative abundance of plant parasites in wheat, cotton and cabbage. Except for in cotton, the relative abundance of omnivore-predator was enhanced by earthworm activity (Table 4).
Nematode community diversity and ecological indices
Two-way ANOVA showed that earthworm addition and plant species significantly affected the nematode community indices H′, SR, ΣMI and NCR (P < 0.01) but not J′. The values of λ were significantly influenced by earthworm activity (P < 0.01). The interactive effects earthworms and plant species was identified for the values of H′, J′, λ, ΣMI, and NCR (Table 5).
Relationships among nematode abundances, plant species and soil environmental parameters
PCA (Fig. 3) showed that the nematode community compositions in no plant, wheat, cotton and cabbage were distinguished by the fist canonical axis, which explained 25.20% of the total variation. The distances between samples under the 3 plant species treatments were longer than that between with and without added earthworm treatments, which indicated plant species had more effects on soil nematode community composition than earthworm addition. The second axis explained 13.40% of the total variation.
Based on the different community composition of soil nematodes under no plant and 3 different plant species, RDA (Fig. 4) was performed to analyze the relationship between nematodes and environmental properties. Results showed that eigenvalues were 0.187 (F = 3.069, P = 0.002) and 0.092 for axis 1 and axis 2, respectively, and the first two axes explained 27.90% of species-environment variation. The interpretation amount of plant species, root biomass, shoot biomass and earthworm addition accounted for 41.60%, 21.33%, 12.80% and 6.13% of the explanation variations of all environmental factors in soil nematode compositions, respectively. The soil TN explained 4.80% of the variation in soil nematode community (Fig. 4).
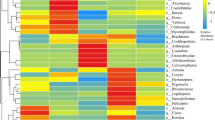
Redundancy analysis (RDA) diagram of soil nematode genera, soil properties, and treatment variables (plant species, with or without added earthworms, shoot biomass, root biomass). Ceph, Cephalobus; Eucp, Eucephalobus; Acro, Acrobeloides; Chil, Chiloplacus; Cerv, Cervidellus; Acrob, Acrobeles; Rhab, Rhabditis; Prot, Protorhabditis; Alai, Alaimus; Plac, Placodira; Caen, Caenorhabditis; Plec, Plectus; Pris, Prismatolaimus; Meso, Mesorhabditis; Rhabd, Rhabditophanes; Aphe, Aphelenchoides; Tyle, Tylencholaimus; Aphel, Aphelenchus; Cepha, Cephalenchus; Tylen, Tylenchus; Heli, Helicotylenchus; Roty, Rotylenchus; Psil, Psilenchus; Noth, Nothotylenchus; Xiph, Xiphinema; Prat, pratylenchus; Eudo, Eudorylaimus; Thor, Thorneella; Disc, Discolaimus; Dory, Doryllium; Mesod, Mesodorylaimus; Apor, Aporcelaimus; Ench, Enchodelusthorne; Doryl, Dorylaimus; Labr, Labronema. TC: total carbon; TN: total nitrogen; C/N, total carbon/total nitrogen; SOC: soil organic carbon.
Soil physical and chemical properties
Two-way ANOVA showed that the presence of earthworms increased the soil TC, and SOC values. Significant differences in the values of TC, TN, and C/N were observed among the different plant species (Table 6). No significant interaction effect on soil properties was found between earthworm addition and plant species.
Discussion
Influence of earthworm presence
This study demonstrated epigeic earthworm addition suppressed the soil nematode abundance, which is consistent with previous reports9,10,14,17,42 and demonstrated partly our first hypothesis “earthworm presence may decrease nematode abundance under planting crops”. Direct grazing by earthworms causes a decline in nematode numbers, as indicated by the presence of living and dead nematodes43 and nematode cuticles44 in the digestive systems of earthworms. The second reason may be that the increasing content of soil total carbon and organic carbon by earthworm addition had the negative effects on the abundance of soil nematode. Another reason was that earthworms and nematodes competed for food resources, which resulted in the decreasing nematodes. Except for in wheat, the total genera richness was increased by earthworm activity, indicating a shift of soil food web to a relatively complex community.
Earthworm addition suppressed the bacterivore abundance in wheat and cabbage but not in cotton, which may be related to the important role of plant species in the bacterial composition of the rhizosphere45. As soil passes through the earthworm gut, the suppressing effects have been shown to be selective towards soil bacteria46,47; In fact, decreases in the bacterial biomass have been found in three types of animal manure after transit through the earthworm gut48. We found that the fungivore abundance was lower in cotton and cabbage in the presence compared with the absence earthworms. In this study, the mean abundances of two fungivores genera (Aphelenchoides and Aphelenchus) were decreased in cotton and cabbage by earthworm activity. However, this change was not observed in the fungivore abundances in wheat (Fig. 2b), which may be due to a high number of fungal pathogens found in continuous cropping wheat49. Microorganisms, especially fungi, might be the main constituents of the epigeic earthworm diet50. A decrease in the abundance of fungivores in cotton and cabbage was attributed to the transit of soil through the earthworm gut. We speculated that the increase of the fungi number in the wheat treatment counteracted the quantity of earthworms ingesting the fungi, which led to the lack of change in the abundance of fungivores. However, the results of this study were not consistent with reports that showed an increasing abundance of fungal populations in the presence of earthworms12,15. The abundance of plant parasites was not affected by earthworm addition which was consistent with the study51. The omnivore-predator abundance in cotton with earthworms was lower than that without earthworms. Specifically, the mean abundances of Dorylaimus, Eudorylaimus and Labronema were depressed in cotton (from 17.43, 30.57 and 20.23 individuals/100 g dry soil to 8.00, 3.43 and 8.33 individuals/100 g dry soil in the absence and presence of earthworms, respectively), which suggested that these genera were possibly directly ingested by earthworms.
Except for cotton, the relative abundance of bacterial feeders decreased in all treatments when the earthworms were present. Such a pattern could be ascribed to the decreasing percent of the two dominant genera Cephalobus and Rhabditis in no plant and wheat; and the decreasing percent of the genera Acrobeles and Rhabditis in cabbage. The lower relative abundance of plant parasites in the presence of earthworms was ascribed to the decreasing relative abundance of the genera Cephalenchus and Pratylenchus in no plant; the increasing relative abundance the genera Cephalenchus, Helicotylenchus and Nothotylenchus in the presence of earthworms resulted in the higher relative of abundance of plant parasites under the three different plant species. In addition, earthworms increased the relative abundance of the genera Tylenchus and Pratylenchus in wheat, and enhanced the relative abundance of the genera Cephalenchus and Xiphinema in cotton, and increased the relative abundance of the genera Tylenchus and Rotylenchus in cabbage.
The higher H′ and SR in the presence of earthworms indicated a high number of soil nematode genera and a more stable soil nematode community structure, respectively. The lower λ values also showed the diversity of soil nematode community structure. The increasing values of ΣMI suggested that the nematode community structure was better and the soil food web complexity was increased under the earthworm treatment. The increase in the NCR value under earthworm addition suggested that earthworms increased the contribution of the bacterial decomposition channels in the soil food web (Table 5). However, the lower NCR value in wheat was ascribed to the higher fungivore abundance in the presence of earthworms (mean 127.89 individuals/100 g dry soil) when compared to that without earthworms (112.67 individuals/100 g dry soil).
Effects of different agricultural plant species
In the absence of earthworms, the bacterivore abundance showed varying responses to the three different plant species compared with no plant, which may related to different plant species supporting specific bacterial communities due to variations in the spectra of root exudates52,53,54,55. The fungivorous abundance appeared to be influenced by different plant species. In the soil, fungal-feeding nematodes can feed on saprophytic, pathogenic and mycorrhizal fungi. Unfortunately, we do not have any data on the number of mycorrhizal hyphae. Differences in the omnivore-predators abundance may be explained by differences in food sources. The different H′, SR, λ, ∑MI and NCR values were ascribed to the different soil nematode community compositions (Fig. 3) under different agricultural plant species. Reasons may be related to the effects of different root exudates from plant species on the soil nematode population. Furthermore, RDA revealed plant species could explain the higher percent of total variance of soil nematode community than earthworm addition which indicated the plant species effects on the soil nematode community composition were more significant than earthworm presence and answered our second hypothesis.
Interactive effects of plant species and earthworms on soil nematodes
Compared with the no plant with added earthworm treatments, the abundance of total nematode, bacterivores, fungivores and omnivore-predators under 3 agricultural plant species were weakened (Figs 1 and 2); whereas the abundance of fungivores, and omnivore-predators in wheat was not decreased and verified partly our first hypothesis “such effect may be enhanced in a system where earthworms stimulated plant growth”. These indicated in the wheat system treatments the mechanism of earthworm effects on the fungivores and omnivore-predators was not different from that in the cotton and cabbage treatments. The co-presence of both earthworms and plant species in soils could significantly affect the abundance of total soil nematode, bacterivores, fungivores and omnivore-predators. This is consistent with our third hypothesis “the interaction effects between earthworms and plant species would affect the soil nematode abundance”. We speculated that the system where earthworms stimulated plant growth may enhance the interaction effects. Earthworms significantly increased the shoot biomasses of wheat and cotton, the biomass of cabbage and the root biomass of wheat and cotton (Table 1). The enhancing plant biomasses in the presence of earthworms might have the effects on the abundance of total nematodes, bacterivores, and fungivores resulting in the decreasing abundance of soil nematode. In addition, significant different in the diversity and ecological indices H′, J′, λ, ∑MI, and NCR under the earthworm addition and different plant species treatments which was consistent with our third hypothesis were likely to related to the enhancing effects of earthworms in the presence of plants. Obviously, more studies are required to determine the complex interplay between earthworms and plants. The results obtained in the present study proposed the necessity that we should broaden the ecological context of soil biota by considering the interactive effects of plants and earthworms on the soil nematode community.
Conclusions
In conclusion, earthworm presence weakened the crop species effects on soil nematode abundance in the wheat, cotton and cabbage treatments. Plant species were crucial for the distribution of soil nematode communities. The interaction effects of earthworms and plant species changed the trophic structure of the soil nematode community, and earthworms mostly decreased the soil nematode abundance but significantly increased the values of the soil nematode community structure indices. The effects of plant species on the soil nematode community composition were more significant than the effects of earthworm addition.
Overall, the present study provides insights into the interaction effects of earthworms and plants on soil nematode communities. Future studies should determine whether the effects of earthworms on soil nematode community structure are modified through plants. Ultimately, this knowledge will help us to better understand the interplay among plants, earthworms, soil microorganisms, and soil nematodes.
References
Moore, J. C. & de Ruiter, P. C. Temporal and spatial heterogeneity of trophic interactions within below-ground food webs. Agr. Ecosyst. Environ. 34, 371–397 (1991).
Neher, D. A. Role of Nematodes in Soil Health and Their Use as Indicators. J. Nematol. 33, 161–168 (2001).
Yeates, G. W., Shepherd, T. G. & Francis, G. S. Contrasting response to cropping of populations of earthworms and predacious nematodes in four soils. Soil. Til. Res. 48, 225–264 (1998).
Wall, D. H. & Moore, J. C. Interactions Underground: Soil biodiversity, mutualism, and ecosystem processes. BioScience 49, 109–117 (1999).
Ritz, K. & Trudgill, D. L. Utility of nematode community analysis as an integrated measure of the functional state of soils: perspectives and challenges. Plant Soil 212, 1–11 (1999).
Neher, D. A. & Olson, R. K. Nematode communities in soils of four farm cropping management systems. Pedobiologia 43, 430–438 (1999).
Yeates, G. W. & Bongers, T. Nematode diversity in agroecosystems. Agr. Ecosyst. Environ. 74, 113–135 (1999).
Blouin, M. et al. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Biol. 64, 161–182 (2013).
Senapati, B. K. Biotic interactions between soil nematodes and earthworms. Soil Biol. Biochem. 24, 1441–1444 (1992).
Räty, M. & Huhta, V. Earthworms and pH affect communities of nematodes and enchytraeids in forest soil. Biol.Fert. Soils 38, 52–58 (2003).
Tao, J. et al. Earthworms change the abundance and community structure of nematodes and protozoa in a maize residue amended rice–wheat rotation agro-ecosystem. Soil Biol. Biochem. 41, 898–904 (2009).
Villenave, C., Rabary, B., Kichenin, E., Djigal, D. & Blanchart, E. Earthworms and Plant Residues Modify Nematodes in Tropical Cropping Soils (Madagascar): A Mesocosm Experiment. Appl. Environ. Soil Sci. 2010, 323640 (2010).
Ilieva-Makulec, K. & Makulec, G. Does the activity of the earthworm Aporrectodea caliginosa modify the plant diversity effect on soil nematodes? Eur. J. Soil Biol. 43, S157–S164 (2007).
Dominguez, J., Parmelee, R. W. & Edwards, C. A. Interactions between Eisenia andrei (Oligochaeta) and nematode populations during vermicomposting. Pedobiologia 47, 53–60 (2003).
Aira, M., Sampedro, L., Monroy, F. & Domínguez, J. Detritivorous earthworms directly modify the structure, thus altering the functioning of a microdecomposer food web. Soil Biol. Biochem. 40, 2511–2516 (2008).
Brown, G. et al. Effects of earthworms on plant production in the tropics. In Earthworm Management in Tropical Agroecosystems (eds Lavelle, P., Brussard, L. & Hendrix, P.) (CAB International, Wallingford (U.K.), 87–148 (1999).
Ilieva-Makulec, K. & Makulec, G. Effect of the earthworm Lumbricus rubellus on the nematode community in a peat meadow soil. Eur. J. Soil Biol. 38, 59–62 (2002).
Loranger-Merciris, G., Cabidoche, Y. M., Deloné, B., Quénéhervé, P. & Ozier-Lafontaine, H. How earthworm activities affect banana plant response to nematodes parasitism. Appl. Soil Ecol. 52, 1–8 (2012).
Lafont, A. et al. Effects of the earthworm Pontoscolex corethrurus on banana plants infected or not with the plant-parasitic nematode Radopholus similis. Pedobiologia 51, 311–318 (2007).
Demetrio, W., Dionísio, J. & Maceda, A. Earthworms and root-knot nematodes: effect on soil biological activity and tomato growth. Semina: Ciências Agrárias 38, 1437–1446 (2017).
Viketoft, M. & Sohlenius, B. Soil nematode populations in a grassland plant diversity experiment run for seven years. Appl. Soil Ecol. 48, 174–184 (2011).
Viketoft, M. et al. Temporal dynamics of soil nematode communities in a grassland plant diversity experiment. Soil Biol. Biochem. 43, 1063–1070 (2011).
Lu, Z.-b et al. Effects of crop species richness on the community of soil nematodes in an experimental agro-ecosystem. Eur. J. Soil Biol. 73, 26–33 (2016).
Viketoft, M., Palmborg, C., Sohlenius, B., Huss-Danell, K. & Bengtsson, J. Plant species effects on soil nematode communities in experimental grasslands. Appl. Soil Ecol. 30, 90–103 (2005).
De Deyn, G. B., Raaijmakers, C. E., van Ruijven, J., Berendse, F. & van der Putten, W. H. Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web. Oikos 106, 576–586 (2004).
Franco-Navarro, F. & Godinez-Vidal, D. Soil nematodes associated with different land uses in the Los Tuxtlas Biosphere Reserve, Veracruz, Mexico. Revista Mexicana de Biodiversidad 88, 136–145 (2017).
Wardle, D. A., Yeates, G. W., Williamson, W. & Bonner, K. I. The response of a three trophic level soil food web to the identity and diversity of plant species and functional groups. Oikos 102, 45–56 (2003).
Wardle, D. Communities and ecosystems: Linking the aboveground and belowground components (MPB-34). Princeton University Press, Princeton, NJ. 34 (2002).
Wurst, S. et al. Plant defence against nematodes is not mediated by changes in the soil microbial community. Funct. ecol. 23, 488–495 (2009).
Griffiths, B. S., Welschen, R., van Arendonk, J. J. C. M. & Lambers, H. The effect of nitrate-nitrogen supply on bacteria and bacterial-feeding fauna in the rhizosphere of different grass species. Oecologia 91, 253–259 (1992).
Lv, M. et al. Plants modify the effects of earthworms on the soil microbial community and its activity in a subtropical ecosystem. Soil Biol. Biochem. 103, 446–451 (2016).
Gu, Y., Zhang, L., Ding, S. & Qin, S. The soil macrofaunal community structure under a long-term fertilization in wheat field. Acta Ecologica Sinica 31, 4900–4906 (2011).
Ruess, L. Studies On the Nematode Fauna of an Acid Forest Soil: Spatial Distribution and Extraction. Nematologica 41, 229–239 (1995).
Minagawa, N. & Mizukubo, T. A Simplified Procedure of Transferring Nematodes to Glycerol for Permanent Mounts. Jpn J Nematol 24, 75 (1994).
Bongers, T. De Nematoden van Nederland, Koninklijke Nederlandse Natuurhistorische Vereniging. (1988).
Bongers, T. & Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 10, 239–251 (1998).
Bongers, T. The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia 83, 14–19 (1990).
Yeates, G. W. Nematodes as soil indicators: functional and biodiversity aspects. Biol. Fert. Soils 37, 199–210 (2003).
Dong, C. H. et al. Effects of long-term organic manure and inorganic fertilizer combined application on rice yield and soil organic carbon content in reddish paddy fields. J. Plant Nutr. Fert. 20, 336–345 (2014).
Braak, C. J. F. T. & Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (version 4.5). (www.canoco.com, Ithaca NY, USA, 2002).
Yeates, G. W., Wardle, D. A. & Watson, R. N. Relationships between nematodes, soil microbial biomass and weed-management strategies in maize and asparagus cropping systems. Soil Biol. Biochem. 25, 869–876 (1993).
Tao, J. et al. Earthworms Reduce the Abundance of Nematodes and Enchytraeids in a Soil Mesocosm Experiment Despite Abundant Food Resources. Soil Sci. Soc. Am. J. 75, 1774–1778 (2011).
Dash, M., Senapati, B. & Mishra, C. Nematode Feeding by Tropical Earthworms. Oikos 34, 322–325 (1980).
Monroy, F., Aira, M. & Dominguez, J. Changes in density of nematodes, protozoa and total coliforms after transit through the gut of four epigeic earthworms (Oligochaeta). Appl Soil. Ecol. 39, 127–132 (2008).
Berg, G. et al. The rhizosphere effect on bacteria antagonistic towards the pathogenic fungus Verticillium differs depending on plant species and site. FEMS Microbiol. Ecol. 56, 250–261 (2006).
Byzov, B. A., Khomyakov, N. V., Kharin, S. A. & Kurakov, A. V. Fate of soil bacteria and fungi in the gut of earthworms. Eur. J. Soil Biol. 43, S149–S156 (2007).
Koubová, A., Chroňáková, A., Pižl, V., Sánchez-Monedero, M. A. & Elhottová, D. The effects of earthworms Eisenia spp. on microbial community are habitat dependent. Eur. J. Soil Biol. 68, 42–55 (2015).
Gomez-Brandon, M., Aira, M., Lores, M. & Dominguez, J. Epigeic Earthworms Exert a Bottleneck Effect on Microbial Communities through Gut Associated Processes. Plos One 6, e24786 (2011).
Lebreton, L. et al. Changes in population structure of the soilborne fungus Gaeumannomyces graminis var. tritici during continuous wheat cropping. Environ. Microbiol. 6, 1174–1185 (2004).
Bonkowski, M., Griffiths, B. S. & Ritz, K. Food preferences of earthworms for soil fungi. Pedobiologia 44, 666–676 (2000).
Shao, Y. et al. Nitrogen deposition cancels out exotic earthworm effects on plant-feeding nematode communities. J. Animal Ecol. 86, 708–717 (2017).
Grayston, S. J., Wang, S., Campbell, C. D. & Edwards, A. C. Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol. Biochem. 30, 369–378 (1998).
Inderjit & Weston, L. A. In Root Ecology (eds Hans de Kroon & Eric J. W. Visser) 235–255 (Springer Berlin Heidelberg, 2003).
Johnson, D. et al. Plant community composition affects the biomass, activity and diversity of microorganisms in limestone grassland soil. Eur. J. Soil Biol. 54, 671–677 (2003).
Marschner, P., Crowley, D. & Yang, C. H. Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 261, 199–208 (2004).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31070394) and the Natural Science Project of Henan province (162300410009).
Author information
Authors and Affiliations
Contributions
X.N. and Y.G. contributed to the design of the study. X.N. and P.Z. performed the experiments. X.N. analyzed the data. X.N., Y.G. and W.Z. wrote or critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Niu, X., Zhai, P., Zhang, W. et al. Effects of Earthworms and Agricultural Plant Species on the Soil Nematode Community in a Microcosm Experiment. Sci Rep 9, 11660 (2019). https://doi.org/10.1038/s41598-019-48230-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48230-0
This article is cited by
-
Long-Term Plant Community Removal Alters Soil Nematode Communities Mainly Through the Trophic Cascading Effects of Fungal Channel
Journal of Soil Science and Plant Nutrition (2023)
-
Effect of fly ash and vermicompost amendment on rhizospheric earthworm and nematode count and change in soil carbon pool of rice nursery
Environmental Science and Pollution Research (2022)
-
Effects of plant species diversity on nematode community composition and diversity in a long-term biodiversity experiment
Oecologia (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.