Abstract
The objective of this in vitro study was to investigate the effect of nanostructured silver vanadate decorated with silver nanoparticles (AgVO3) on antimicrobial activity, hardness, roughness, and adhesion of a soft denture liner. The antimicrobial efficacy of the Trusoft (Boswoth) liner incorporated with different concentrations of AgVO3 against Enterococcus faecalis, Pseudomonas aeruginosa, Candida albicans, and Staphyloccocus aureus (n = 5) was evaluated by the agar diffusion method. Roughness, hardness, and adhesion properties were also evaluated. The data were analyzed by analysis of variance (ANOVA) and Tukey’s multiple comparison test with significance at the p < 0.05 level. At concentrations of 1 and 2.5%, AgVO3 incorporation was effective only against E. faecalis, and at 5 and 10%, against E. faecalis, P. aeruginosa, and C. albicans. None of the concentrations was effective against S. aureus. A decrease in hardness was found for the 1, 2.5, and 10% AgVO3 concentrations (p < 0.001) and at 5%, hardness was not affected. None of the concentrations affected the roughness of the material. A significant increase in tensile values was observed between the liner and heat-curing acrylic resin for 2.5% (p < 0.001) and 10% (p = 0.042) concentrations. AgVO3 incorporation to a soft denture liner promoted antimicrobial activity against E. faecalis, P. aeruginosa, and C. albicans without affecting roughness, maintaining the hardness properties recommended for soft and extra soft liners, and improving the adhesion between the liner and the acrylic resin used for dentures.
Similar content being viewed by others
Introduction
Soft denture liners based on poly-methyl methacrylate or silicone elastomers are used for repairs, rebuilding the surface of the prosthesis in contact with the oral mucosa1,2,3, and reducing forces to supporting tissues4,5,6,7. These materials are recommended for patients with extremely resorbed residual ridges3,4,8, and for implant osseointegration1,9 as they promote an even distribution of the supporting loads. However, because of their structural form, composition, and properties, soft liners have a porous surface1,10 and thus function as microbial reservoirs10. Liner contamination may cause serious local problems such as implant loss1,11, peri-implant infections11,12, delay in implant osseointegration, pain13, and discomfort, in addition to systemic diseases such as bacterial endocarditis, pneumonia, chronic obstructive pulmonary disease, and oropharyngeal, esophageal, and respiratory infections14,15,16,17 interfering with treatment success, general health, and quality of life. Thus, the addition of antimicrobial properties to denture liners would have many advantages.
The antimicrobial property of denture liners is usually achieved with the incorporation of antimicrobial agents, which often causes changes in the physical-chemical and mechanical properties of the materials, hindering their use. The incorporation of ketoconazole, chlorhexidine, nystatin, and miconazole have shown to cause significant increase in the material’s hardness (although itraconazole did not affect hardness)18. The use of silver nanoparticles caused a decrease in adhesion to the prosthesis, modified the type of adhesive failure, and decreased the hardness of the material with increasing concentrations of antimicrobial agent19. Inhibition of C. albicans and reduction of surface roughness was observed with the incorporation of essential oils20. The addition of silver nanoparticles to a tissue conditioner in concentrations varying between 0.1 and 3.0% was effective against S. aureus, S. mutans (at 0.1%), and C. albicans (at 0.5%) after a period of 24 and 72 h16.
To add antimicrobial properties to denture liners while maintaining good physical and chemical properties, this study tested the incorporation of nanostructured silver vanadate, since its use was shown to be efficient in acrylic resin21,22.
Nanostructured silver vanadate decorated with silver nanoparticles (AgVO3) is formed by a precipitation reaction between silver nitrate and ammonia vanadate and was described by Holtz et al.23,24. This substance showed promising antibacterial activity against strains of gram-positive bacteria, including methicillin-resistant S. aureus.
Therefore, the objective of this study was to incorporate AgVO3 at 1, 2.5, 5, and 10% to a resin denture liner and evaluate the antimicrobial capacity, adhesion properties, hardness, and roughness. The null hypothesis was that there is no significant difference in the properties of the liner and antimicrobial effectiveness as a function of AgVO3 concentration.
Material and Method
Synthesis of nanostructured silver vanadate
The nanostructured silver vanadate is synthesized through a precipitation reaction between silver nitrate (AgNO3, Merck 99.8%) and ammonium vanadate (NH4VO3, Merck 99%), as described previously21,22,23,24. After the solubilization of 0.9736 g of NH4VO3 and 1.3569 g of AgNO3 in 200 mL of distilled water, the AgNO3 solution was added dropwise to the NH4VO3 solution under constant stirring at 65 °C. The obtained precipitate was washed with distilled water and absolute alcohol, filtered, and dried under vacuum for 10 hours23,24.
Materials characterization
Analysis of transmission electron microscopy (TEM)
The presence and morphology of silver nanoparticles (AgNPs) on the surface of the crystals formed was verified by transmission electron microscopy (TEM) using the JEOL microscope JEM-100CX II (Fig. 1).
Analysis of Fourier Transform Infrared (FTIR) spectroscopy
The chemical composition of AgVO3 was evaluated by Fourier Transform Infrared (FTIR) spectroscopy; that was performed using ATR technique with a diamond crystal detector coupled to the FTIR Spectrometer Nicolet 380 (Thermo Fisher Scientific Inc.; Waltham, MA, USA). For the measurement, the AgVO3 were positioned in contact with the diamond crystal of the ATR setup. The infrared absorbance spectrum of the samples was obtained over the range of 4000–400 cm−1 at 1 cm−1 resolution using 32 scans and was recorded using the OMNIC Spectra Software (Fig. 2).
For the peak identification we employ the second derivative to the absorption spectrum to identify precisely the band position.
Analysis of X-ray diffraction (XRD)
The structural changes were evaluated by x-ray diffraction (XRD) patterns. The patterns were collected using a Bruker AXS D2 diffractometer equipped with a Cu tube, using Cu Kα radiation (λ = 1.54 Å); in the range of 5–50°, with steps of 0.05°, with 0,5 s for each step (Fig. 3).
Preparation of the resin liner and specimens containing AgVO3
The powder of Trusoft (Boswoth) denture liner was weighed to obtain the amount of nanomaterial to be added. Fractions from 1 to 10% of the liner weight were subtracted to incorporate that amount of the nanomaterial, as described in Table 1.
For the antimicrobial, hardness and roughness analyses, the AgVO3 + liner combinations were mixed for 30 to 40 seconds on an unpolished glass slab. The mix was placed in a metal mold (Ø 8 mm × 3 mm) and pressed between two glass plates to create sample disks. The entire procedure was done according to the manufacturer’s recommendations.
For the adhesion analysis, a mold with 10 mm diameter and 20 mm height was used to obtain specimens in wax that were invested with plaster in metal flasks, creating a matrix for packing with heat or self-curing acrylic resin (Clássico Produtos Odontológicos, SP, Brazil). One hundred disks of each acrylic resin were obtained, which were polished under water with 320-grit sandpaper. Two disks of each resin type were positioned in a spacer and joined with the liner, generating 50 specimens for each resin (Ø10 mm × 43 mm, n = 10 for each percentage of nanomaterial).
Analysis of antimicrobial activity
The microorganisms Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 27853), Candida albicans (ATCC 90028), and Enterococcus faecalis (ATCC 29212) were obtained from thawed strains and cultured at 37 °C for 24 hours in a microbiological oven. Cultures were centrifuged at 6,000 g for 5 minutes, the supernatant was discarded, and the pellet was washed twice with 10 mL of PBS (Phosphate Buffered Saline, containing NaCl, KCl, Na2HPO4, KH2PO4). The inoculum suspensions were made in BHI medium and the concentration confirmed by optical density using a spectrophotometer (BEL Photonics, model 1105, Piracicaba, SP, Brazil), with absorbance reading of 0.120 for P. aeruginosa, 0.150 for E. faecalis, 0.8 for S. aureus at 625 nm wavelength, which corresponds to 108 cells/mL. For C. albicans, cells were counted in the Newbauer chamber due to the variable morphology of the genus. After standardization of the suspensions, the inocula were diluted to 106 CFU/mL.
TSA (tryptic soy agar) culture medium was used for P. aeruginosa and E. faecalis, Saboraud Dextrose Agar was used for C. albicans, and BHI (Brain Heart Infusion) was used for S. aureus.
To evaluate the 4 microorganisms, the agar diffusion method was performed with 5 specimens for each percentage of nanomaterial. Denture liner disks (Ø 8 mm × 3 mm) were exposed to UV light in a laminar flow hood for 30 min on each side for disinfection and then placed in petri dishes containing the culture media. The culture media for diffusion were heated to 50 °C for addition of the microorganisms. After 2-h rest, dishes were stored for 24 hours in an oven at 37 °C.
Hardness and roughness tests
The surface hardness analysis was performed using the microdurometer Microtest SD 300 (Moema, SP, Brazil) with five random equidistant measurements in each specimen using a Shore A indenter. The roughness was analyzed using the Mitutoyo analogue roughness tester (Mitutoyo Suzano, São Paulo, Brazil) with three measurements in each specimen: one in the center and two 1-mm from the central measurement.
Tensile test
The tensile test was performed according to ISO 20795-1: 2008 in a universal testing machine (EMIC, São José dos Pinhais, Paraná, Brazil) with a speed of 5 mm/min. Data were collected using Tesc® software v.3.01 (EMIC).
The failure types of the test groups were observed and classified as adhesive failure - total separation at the interface between the liner and the acrylic resin; cohesive failure - separation within the liner; and mixed failure – a mix of adhesive and cohesive failures.
Tensile bond strength was calculated using the equations below:
where σ = stress (MPa), F = maximum recorded strength at failure (N), and A = original cross sectional area (mm2).
Data analysis
After verifying the normal distribution of the data (Kolmogorov-Smirnov test), ANOVA with Tukey’s Multiple Comparison test (α = 0.05) was applied for comparisons and the Two Proportions Equality test to analyze the distribution of the relative frequencies (percentages). The software used was SPSS v 20.0.
Results
FTIR, SEM and X-ray diffraction (XRD) analysis
The AgVO3 morphology was examined by TEM, and it shows that the synthesized sample is mostly rod-like particles (Fig. 1). The AgVO3 are composed of nanowires with length in the order of micrometers. The wires are coated with semispherical metallic silver particles (Ag).
Figure 4 shows the fourier transform infrared (FTIR) spectrum of AgVO3 nanorods acquired in the range of 400–4000 cm−1 which is very similar to that reported for AgVO322,23.
It was observed absorption bands at 908 cm−1, 892 cm−1, 860 cm−1 (broad band), 827 cm−1, 817 cm−1 and 809 cm−1. We didn’t find the same bands in the literature, but for the same Ag/V molar ratio of the two used in this work, it was observed bands at 964, 915, 895, and 849 cm−1 23. These absorption bands area was assigned to stretching vibration mode of the V = O bound.
In Fig. 3 it is observed the x-ray diffraction pattern of the silver vanadate. The x-ray diffraction pattern is a mean value of silver vanadate nanowires who are decorated with Ag nanoparticles. The data depicts that the sample is crystalline, but we didn’t identify in this work its crystalline structure, remembering that we have certainly at least two-phase as suggested by the literature23, metallic Silver (Ag°) and silver vanadate (AgVO3).
Antimicrobial activity
Antimicrobial activity was tested by the Kirby Bauer agar diffusion method. The incorporation of 1 and 2.5% AgVO3 to the denture liner did not result in antimicrobial activity for P. aeruginosa and C. albicans, but it was effective at 5 and 10%; for P. aeruginosa, the 10% showed higher antimicrobial capacity. For C. albicans, both 5 and 10% concentrations showed similar antimicrobial effect. All preparations showed antimicrobial activity for E. faecalis in a concentration-dependent manner (with 10% having the highest effect). None of the concentrations was effective against S. aureus (Fig. 4).
Mechanical properties
The incorporation of 1, 2.5, and 10% AgVO3 promoted a decrease in Shore A values (p < 0.001), while the 5% dose had no effect. The incorporation of 1% AgVO3 produced specimens with a lower Shore A hardness value (average of 43.77), which is within the ISO standard of 40 units (DIN 53505 and ASTM D2240 / 75) for soft denture liners (Fig. 5).
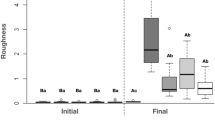
The addition of the nanomaterial at all concentrations did not significantly affect the roughness of the denture liner. (Table 2).
For the heat-curing acrylic resin, a significant increase in tensile values was found for 2.5% (p < 0.001) and 10% AgVO3 (p = 0.042), showing that the nanomaterial caused an increase in the adhesive property of the liner (Table 3). The 2.5% concentration promoted the highest adhesion with a mean of 0.664 MPa. For the self-curing acrylic resin, no significant effect was found in strenght (N/cm and Kgf) and tension (MPa) in any of the groups evaluated (Table 4).
Regarding the type of failure for the heat-curing resin, a significant difference was found in the 2.5% group, with 50% of mixed failures and the 10% group with 60% of adhesive failures. For the self-curing resin, a difference was found only in the 10% group, with 80% of adhesive failures (p = 0.007) (Table 5). When comparing the two resin types, a significant difference was found only for the 2.5% group (p = 0.010), with 50% of failures within the heat-curing resin.
Discussion
The AgVO3 formed had acicular morphology and length in the order of micrometers, and they are decorated with AgNPs, similar to the pattern observed in the images obtained by transmission electron microscopy (TEM) by de Castro et al.22 and Holtz et al.24. The characterization showed a vanadate wire with a heterogeneous distribution and few AgNPs decorating the surface of the nanowires, and both vanadate and Ag present antibacterial effect, which characterizes the antibacterial potential of the material.
The infrared absorption bands show very broad bands, and different works from the literature diverge from the nominal values of the bands position observed in this work. These differences can be assigned for different Ag/V molar ratio and also to the synthesis.
AgVO3 is a promising alternative to provide antimicrobial capacity to various materials23 such as water-based paints24 and, in dentistry, acrylic resin21,22.
In relation to the efficacy of silver vanadate antimicrobial properties it was seen that the antimicrobial mechanism of AgVO3 is attributed to the contact between the hybrid nanomaterial and bacteria22 and occurs when Ag + is absorbed and released from the Ag nanoparticles through a direct dissociation between the nanoparticle and the bacterial cell wall, causing toxic effects23,24. Besides that, the AgVO3 maintains a large surface area in contact with microorganisms, promoting changes in bacterial membranes morphology and loss of DNA’s ability to replicate, which can cause cell death23. The surface of the bacterium has a negative charge at biological pH due to the dissociation of a large number of functional groups, including carboxylic and other chemical groups on the membrane. According to Li et al.25 the semiconductor antibacterial mechanism is attributed to the oxidative stress caused by the production of OH*, O2 and and O2H *, that is, when reactive species with complex groupings such as AgO3VO are formed a cluster in the reaction where O3 decomposes into hydroxyl radicals and protons. At the same time, an electron transferred to the molecule of O interacts with the proton, forming an O2H* radical. Thus, bacterial death occurs through collaboration between surfaces26.
In this study, nanostructured silver vanadate was incorporated into a resin denture liner and the following properties were evaluated: antimicrobial effectiveness, roughness, hardness, and adhesion. The null hypothesis that the addition of AgVO3 does not significantly affect the properties of the liner and antimicrobial effectiveness in a concentration-dependent manner was partially accepted.
Soft resins used for denture lining can serve as reservoirs for microorganisms1 even when hygienized10 due to their porous and irregular surface. Thus, research has been carried out for finding antimicrobial possibilities1,6,20,26,27,28 to simultaneously treat infected mucosa and decrease denture fungal infections29. However, antimicrobial agents generally affect the physicochemical and mechanical properties of the material6,18. Denture liners contamination favors numerous diseases of the respiratory tract that cause serious health problems14,17. Even with the proven effectiveness of silver nanoparticles (AgNPs)16,30,31, silver nanoparticles aggregation reduces the surface area of the nanoparticles and the emission of silver ions, reducing the antimicrobial effect of the particles27,31,32,33. Holtz et al.24 found that, in addition to a possible antibacterial action, silver vanadate acts as a carrier for the silver nanoparticles, allowing a continuous release of silver ions, and avoiding possible accumulations of AgNPs, and consequently maintaining a large surface area.
In this study, the resin liner incorporated with 1 and 2.5% AgVO3 showed antimicrobial activity for E. faecalis, and 5 and 10% for E. faecalis, P. aeruginosa, and C. albicans. None of the concentrations was effective against S. aureus (Fig. 4).
The cell wall of Gram-negative bacteria comprises a fine peptidoglycan sacculus which is covered by an outer membrane. In contrast, Gram-positive bacteria have the much thicker cell wall, with multiple layers of peptidoglycan, and the presence of additional glycolipers in addition to other protective surface structures34,35. These additional glycopolymers are essential in maintaining the bacterial architecture, replication and other major cell functions, are highly diversified and generally species or strain-specific. Most Gram-positive bacteria have two types of glypolymers, one peptidoglycan-attached and one lipid-attached, although some bacterial species have three or four different glypolymers35.
Our research evidenced the presence of a different antibacterial effect for two of the gram positive bacteria tested, with effect for E. faecalis and no effect for S. aureus. This difference may be related to the presence of different additional glypolymers in the cell wall of both bacteria. The effect of AgVO3 on P. aeruginosa is probably due to the thin cell wall, characteristic of gram negative bacteria.
According to Holtz et al.23 the contact of AgNPs change the bacterial membrane morphology, and when bound to DNA, they affect bacterial metabolic processes, in particular cell division, causing cell death. As for C. albicans, AgNPs function by breaking down the membrane potential and forming pores, causing leakage of ions and other materials inducing apoptosis and causing ultrastructural changes36,37.
In one of our previous studies22 where cell metabolism analysis was performed using the XTT method, a decline in the metabolism of C. albicans was observed with the incorporation of 5 and 10% b-AgVO3 and in the present study, 5 and 10% promoted antimicrobial effect.
Several studies1,16,20,27,28 using antimicrobial agents such as silver zeolite1, silver nanoparticles16, Plant-Derived Component6,20,27,28, drugs38, and photocatalysts14 found activity against C. albicans, gram-positive, and gram-negative bacteria. However, some disadvantages were encountered such as short duration of antimicrobial capacity14, and alterations in the material’s roughness14, hardness18, fluidity28, adhesion6,19,39 and gellyfication properties6.
Comparisons of the mechanical properties of modified denture liners among studies are difficult because of the various methods used to evaluate the properties7,18,39,40. Trusoft has been tested for different properties such as porosity7, hardness18,39, absorption39, solubility39, and traction39. Although the composition of Trusoft is not specified by the manufacturer, its liquid component contains ethyl alcohol and low concentration of plasticizers, which guarantees a clinically acceptable smoothness for up to 6 months.
Soft denture liners should exhibit low hardness levels18,39, however, during use, they are susceptible to changes related to the leaching of plasticizers40. Mancuso et al.39 found that thermocycling exerted a significant effect on the hardness of Trusoft due to the hydrophilic character of the material, which can lead to hardening through ethanol loss, water absorption, and loss of the plasticizer7,41. Urban et al.18 observed a hardness increase of Trusoft when antimicrobial drugs were added, but the change was insufficient to interfere with clinical use. In the present study, the incorporation of 1% AgVO3 produced specimens with hardness values in compliance with ISO requirements for soft liners (ISO 10139-2: 2009)40 of approximately 40 units (DIN 53505 and ASTM D2240/75).
The bond strength of AgVO3-modified Trusoft to the heat-curing resin was higher for the 2.5 and 10% concentration, compared to the control group. In the attempt to elucidate this difference in the tensile strengh values, the tensile test was repeated for the 5% group, however, the same results were found. We suggest in a future study the accomplishment of the microstructural characterization proposing to analyze the structure-properties correlation in the attempt to find the answer for this difference in tensile strength means values for groups 2.5 and 10%.
Similar results were reported with the addition of 40 ppm silver nanoparticles (1.33 ± 0.22 MPa) compared to control (1.18 ± 0.17 MPa)19. In contrast, different results have also been found, where the control group (0.91 ± 0.52 N) presented greater tensile strength compared to the modified group (0.16 ± 0.05 N)6.
A previous study has reported a decrease of adhesive strength and changes in the type of failure with the increase of silver nanoparticles concentration13. In this study, the heat-curing resin had 50% of mixed failures in the 2.5% AgVO3 group and 60% of adhesive failures in the 10% group. Takahashi et al.38 found cohesive and mixed failures and a lower tensile strength for Trusoft without additives, compared to our results. The authors believed the findings were related to the high elasticity of the liner rather than bonding deficiencies. They also associated the failure of the liner to the low cohesive resistance of the material, which in turn provides information on the strength of the liner, rather than an accurate measure of the bond strength between liner and denture material38.
In the present study, the incorporation of different concentrations of AgVO3 did not affect roughness, which is an important parameter, since the incorporation of antimicrobials to denture liners may increase the recommended roughness parameter of 0.2 μm42,43.
The properties evaluated in this study were selected due to their clinical influence. An appropriate hardness allows the material to provide cushioning for the supporting mucosa. Adequate adhesion to the prosthesis and low levels of roughness are important to avoid the adhesion and proliferation of microorganisms.
Conclusions
The incorporation of 5% AgVO3 to a soft denture liner was efficient in the control of P. aeruginosa, E. faecalis, and C. albicans, and 2.5 and 10% improved the adhesion properties between the liner and the denture base material. There was no effect on roughness and the 1% concentration maintained the recommended hardness properties of a soft material (ISO 10139-2: 2009). In conclusion, the AgVO3-incorporated denture liner is an efficient, practical, effective and accessible alternative for denture users with an oral infection.
References
Saravanan, M. et al. Viscoelastic properties and antimicrobial effects of soft liners with silver zeolite in complete dental prosthesis wearers: an in vivo study. International Journal of Prosthodontics 28, 265–269 (2015).
Dimiou, A. M. et al. Influence of thickness increase of intraoral autopolymerizing hard denture base liners on the temperature rise during the polymerization process. The Journal of prosthetic dentistry 111, 512–520 (2014).
Kasuga, Y. et al. Basic evaluation on physical properties of experimental fluorinated soft lining materials. Dental materials journal 30, 45–51 (2011).
Bail, M. et al. Surface roughness of acrylic and silicone-based soft liners: in vivo study in a rat model. Journal of Prosthodontics 23, 146–15 (2014).
Zarb G. A., Carlsson G. E., Bolender C. L. Boucher’s prosthodontic treatment for edentulous patients. 11th ed. (St. Louis, Mosby, p. 144–149, 2013).
Srivatstava, A. et al. Evaluation of the properties of a tissue conditioner containing origanum oil as an antifungal additive. The Journal of prosthetic dentistry 110, 313–319 (2013).
Lima, J. B. G. et al. Analysis of stress on mucosa and basal bone underlying complete dentures with different reliner material thicknesses: a three‐dimensional finite element study. Journal of oral rehabilitation 40, 767–773 (2013).
Moffa, E. B. et al. Colour stability of relined dentures after chemical disinfection. A randomised clinical trial. Journal of dentistry 39, e65–e71 (2011).
Bacchi, A. et al. Influence of different mucosal resiliency and denture reline on stress distribution in peri‐implant bone tissue during osseointegration. A threedimensional finite element analysis. Gerodontology 29, e833–e837 (2012).
Valentini, F. et al. Surface Roughness Changes in Denture Liners in Denture Stomatitis Patients. International Journal of Prosthodontics 30, 561–564 (2017).
Santos, M. B. F. D. et al. Influence of different soft liners on stress distribution in peri-implant bone tissue during healing period. A 3D finite element analysis. Journal of Oral Implantology 39, 575–581 (2013).
Radi, I. A. E. & Elmahrouky, N. Effect of two different soft liners and thicknesses mediating stress transfer for immediately loaded 2-implant supported mandibular overdentures: A finite element analysis study. The Journal of prosthetic dentistry 116, 356–361 (2016).
Chladek, G. et al. Long-term soft denture lining materials. Materials 7, 5816–5842 (2014).
Uchimaru, M. et al. Antimicrobial and antifungal effects of tissue conditioners containing a photocatalyst. Dental materials jornal 30, 691–699 (2011).
Mayer, F. L. et al. Candida albicans pathogenicity mechanisms. Virulence 4, 119–128 (2013).
Nam, K. Y. In vitro antimicrobial effect of the tissue conditioner containing silver nanoparticles. The journal of advanced prosthodontics 3, 20–24 (2011).
Przybyłowska, D. et al. Potential respiratory pathogens colonisation of the denture plaque of patients with chronic obstructive pulmonary disease. Gerodontology 33, 322–327 (2016).
Urban, V. M. et al. Effect of the addition of antimicrobial agents on Shore A hardness and roughness of soft lining materials. Journal of Prosthodontics 24, 207–214 (2015).
Chladek, G. et al. Sorption, solubility, bond strength and hardness of denture soft lining incorporated with silver nanoparticles. International journal of molecular sciences 14, 563–574 (2012).
Muttagi, S. & Subramanya, J. K. Effect of incorporating seed oils on the antifungal property, surface roughness, wettability, weight change, and glucose sorption of a soft liner. The Journal of prosthetic dentistry 117, 178–185 (2017).
De Castro, D. T. et al. In vitro study of the antibacterial properties and impact strength of dental acrylic resins modified with a nanomaterial. The Journal of prosthetic dentistry 115, 238–246(a) (2016).
De Castro, D. T. et al. Evaluation of antibiofilm and mechanical properties of new nanocomposites based on acrylic resins and silver vanadate nanoparticles. Archives of oral biology 67, 46–53(b) (2016).
Holtz, R. D. et al. Development of nanostructured silver vanadates decorated with silver nanoparticles as a novel antibacterial agent. Nanotechnology 21, 1–8 (2010).
Holtz, R. D. et al. Nanostructured silver vanadate as a promising antibacterial additive to water-based paints. Nanomedicine:Nanotechnology, Biology, and Medicine 8, 935–940 (2012).
Li, Y. et al. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered[page9image34307264]Metal-Oxide Nanoparticles. ACS Nano 6, 5164–5173 (2012).
De Oliveira, R. C. et al. Mechanism of antibacterial activity via morphology change of α-AgVO3: theoretical and experimental insights. ACS applied materials & interfaces 9, 11472–11481 (2017).
Guzman, M. et al. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria.Nanomedicine: Nanotechnology. Biology and Medicine 8, 37–45 (2012).
Sharma, S. & Hegde, V. Comparative evaluation of antifungal activity of melaleuca oil and fluconazole when incorporated in tissue conditioner: an in vitro study. Journal of Prosthodontics 23, 367–373 (2014).
Naito, Y. et al. Antifungal and Mechanical Properties of Tissue Conditioner Containing Plant‐Derived Component: An In Vitro Study. Journal of Prosthodontics 27, 665–669 (2018).
Hotta, J. et al. In vivo biocompatibility of an interim denture resilient liner containing antifungal drugs. The Journal of Prosthetic Dentistry 1–8 (2018).
Durán, N. et al. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine: Nanotechnology. Biology and Medicine 12, 789–799 (2016).
Franci, G. et al. Silver nanoparticles as potential antibacterial agents. Molecules 20, 8856–8874 (2015).
Radzig, M. A. et al. Antibacterial effects of silver nanoparticles on gram-negative bacteria: influence on the growth and biofilms formation, mechanisms of action. Colloids and Surfaces B: Biointerfaces 102, 300–306 (2013).
Weidenmaier, C. & Peschel, A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nature Reviews Microbiology 6, 276–287 (2008).
Romero-Urbina, D. G. et al. Ultrastructural changes in methicillin-resistant Staphylococcus aureus induced by positively charged silver nanoparticles. Beilstein journal of nanotechnology 6, 2396–2405 (2015).
Kim, K. et al. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals 22, 235–242 (2009).
Lara, H. H. et al. Effect of silver nanoparticles on Candida albicans biofilms: an ultrastructural study. Journal of nanobiotechnology 13, 1–12 (2015).
Takahashi, J. M. F. K. et al. Effect of accelerated aging on permanent deformation and tensile bond strength of autopolymerizing soft denture liners. Journal of Prosthodontics: Implant, Esthetic and Reconstructive Dentistry 20, 200–204 (2011).
Neppelenbroek, K. H. et al. Effect of incorporation of antifungal agents on the ultimate tensile strength of temporary soft denture liners. Journal of Prosthodontics 27, 177–181 (2018).
Mancuso, D. N. et al. Effect of thermocycling on hardness, absorption, solubility and colour change of soft liners. Gerodontology 29, e215–e219 (2012).
Cha, H. S. et al. Changes in stress relaxation property and softness of soft denture lining materials after cyclic loading. Dental materials 27, 291–297 (2011).
ISO International Organization for Standardization. EN ISO 10139-2:2009 Dentistry—Soft Lining Materials for Removable Dentures-Part 2: Materials for Long-Term Use, (2009).
Bollen, C. M. et al. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dental materials 13, 258–269 (1997).
Acknowledgements
This work was supported by São Paulo Research Foundation (FAPESP) grant # 2017/25923-5, 2012/02460-6 and 12/09124-1.
Author information
Authors and Affiliations
Contributions
K.S. carried out the research methodology and paper drafting. O.V. carried out laboratory methodology. B.L. was responsible for materials characterization. A.O. synthesized the nanostructured silver vanadate. R.A. contributed to the study design, acquisition of the materials, interpretation of data, paper revision and supervised the research.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kreve, S., Oliveira, V.C., Bachmann, L. et al. Influence of AgVO3 incorporation on antimicrobial properties, hardness, roughness and adhesion of a soft denture liner. Sci Rep 9, 11889 (2019). https://doi.org/10.1038/s41598-019-48228-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48228-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.