Abstract
As a result of the increased urban and agricultural development in coastal environments, estuaries are among the most modified and threatened aquatic ecosystems. This study used stable isotopes to examine the effects of human impacts by contrasting the food web structures of two Iberian estuaries exposed to different degrees of human pressure. More complex feeding pathways were found in the more altered estuary (Guadalquivir). Greater spread among species along the carbon axis suggests that the primary consumers exploit organic matter with various origins, whereas different nitrogen signals of the secondary consumers suggest that they feed on different suites of prey. In contrast, the similar isotopic signals of secondary consumers in the relatively little influenced estuary (Guadiana) suggests similarity in diet composition and feeding on the same organic matter sources. Understanding trophic interactions in estuaries is vital for defining proper management and conservation, and the preliminary data provided here are one step in this direction.
Similar content being viewed by others
Introduction
Estuaries are some of the most biologically productive ecosystems in the world1,2,3. They play an important role in the continental shelf environment, acting as nursery habitats and providing other habitats with invaluable ecosystem services4,5,6. However, with more than 60% of Earth’s population living in coastal areas, estuarine ecosystems have been extensively altered by human activities7. Rapid urban and agricultural development is the major factor contributing to wetland loss and the deterioration of water quality in these coastal areas8,9. Nutrient load inputs to estuaries are directly related to intensive agriculture and large populations10 and have the potential to alter nutrient dynamics, in turn modifying the functioning and structure of estuarine ecosystems11.
Elevated loads of nutrient input of anthropogenic origin into aquatic ecosystems may affect different ecological processes such as basal resource production, nutrient dynamics and energy transfer12. These impacts can alter a system’s trophic structure12,13 defined as the distribution of organisms in terms of biomass among producers and consumers12. For example, nutrient loading of ecosystems may shift primary production to a single basal source, which is exploited by fewer intermediate consumers, thereby converting a structured and compartmented ecosystem into one with a less stable food web14,15,16. In contrast, these impacts can favour autotrophs and increase the nutritional quality of basal resources12. Analysing community trophic structure is one way to assess the nature and magnitude of human impacts17. Additionally, trophic niches, which describe the overall trophic role of species within an ecosystem, including all the trophic interactions, and it is often realised as the dietary resource base of consumers12,18,19, respond quickly to modification of basal resources and biotic interactions12,20,21. These niches therefore provide insights into the functional effects of nutrient loading in aquatic ecosystems.
Stable isotope analysis (SIA) is one of the primary tools used to examine the structure and dynamics of food webs22 and may represent a unifying methodology with which to compare anthropogenic pressures among different coastal ecosystems23. SIA provide time- and space-integrated information on the trophic interactions of species18 in disturbed, undisturbed or restored ecosystems24. An example of SIA applicability is the assessment of the effects of invasive species on the trophic structure of native communities. It can be useful to analyse both direct predatory behaviour and indirect impacts on local food webs and to predict potential spread by comparing trophic niche metrics with those of the native species23,25. Moreover, stable isotopes is used to track the source of nutrients in a food web, to characterize the trophic niche of species (isotopic niche18,21). In this context, analysis of δ15N and δ13C stable isotope ratios is frequently used in estuarine systems to assess nutrient pollution, organic matter origin and trophic interactions3,8,26,27. δ13C is typically used to determine the origin of carbon sources28. δ15N values allow the study of trophic levels of consumers28, and the enriched nitrogen isotopic composition of biota can be an indicator of anthropogenic wastewater27,29. However, nutrient contamination studies that include multiple taxa and different trophic levels in the food webs are not common30.
Another approach based on SIA used to quantitatively characterize the community trophic niche aspects of food webs includes the “Layman metrics18”. As Layman et al.18 proposed, these metrics provide an integrated estimate of multiple anthropogenic-related impacts on food webs, including parameters such as trophic diversity or food web stability and trophic resilience22,23,25. While these metrics have been applied in marine ecosystems, their use in estuarine systems remains limited. As Mitchell et al. suggested31, more studies are needed to assess the implications of this approach as a monitoring and management tool.
In this study, we used SIA to contrast the food web structure of two Iberian estuaries exposed to different degrees of urban and agricultural perturbations. First, we hypothesized that the higher nitrogen isotope values of focal species would reflect greater anthropogenic pressures within the estuary. Second, we hypothesized that human impacts in the more impacted estuary would result in a more homogeneous basal resource pool. Third, we predicted a more complex food web in the less impacted estuary.
Study system
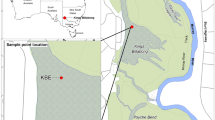
The Guadiana and Guadalquivir Rivers are the largest rivers in the southern Iberian Peninsula. The estuaries of these two rivers are located in the Mediterranean climate region, and both flow to the Gulf of Cadiz on the Atlantic coast (Fig. 1). Both estuaries have hydrological regimes regulated by dams. The flow is low in summer, with episodic freshwater runoff in winter32,33. The estuaries are mesotidal and vertically well mixed with a longitudinal salinity gradient. Despite the similarities of these estuaries, they have not been subjected to the same level of disturbances over recent years.
Sampling locations in the oligohaline zone of the Guadiana estuary (left) and Guadalquivir estuary (right). The salinity gradient of the estuaries is provided in grey scale from the euhaline zone (black) to the oligohaline zone (hatched area). The maximum turbidity zone is within the oligohaline zone.
The Guadalquivir estuary is an example of a highly impacted estuarine environment. It crosses extensive rural areas and has been exposed to increasing human activity34. Such activity includes desiccation of tidal marshes, isolation of the estuary course from the original tidal marshes, a reduction in freshwater inputs, and eutrophication from urban and agricultural wastes due to continual dredging work35,36. All these impacts have caused the Guadalquivir estuary to be characterized by high turbidity levels and increased nutrient loadings37,38.
In contrast, the Guadiana estuary is characterized by relatively lower anthropogenic pressures39,40. Although it is also influenced by agriculture, agroindustrial activities, and dams41,42, this estuary has been catalogued as one of the least polluted European estuaries42,43. Furthermore, it is considered one of the best preserved and most vulnerable estuaries of the Iberian Peninsula41. Comparative studies of both rivers have found concentrations of N one order of magnitude higher in the Guadalquivir estuary than in the Guadiana estuary associated with the influence of agricultural runoff in the waters44, as well as, twice as high pollution in modern sediments of the Guadalquivir estuary45. Although there are differences in human pressures between the estuaries, their biological communities contain a very similar set of species46. For this reason, the Guadiana estuary has been used as a reference area in other biological studies39. To understand human impacts on each estuary, analysis carried out in summer 2017 showed that in the Guadalquivir estuary, ammonia ranged from 0.11 to 0.13 mg/L (0.12 ± 0.012 mg/L mean ± SD), and nitrate ranged from 1.33 to 3.73 mg/L (2.68 ± 1.23 mg/L mean ± SD), while in the Guadiana estuary, the levels were under the detection limits (0.05 mg/L for ammonia and 0.15 mg/L for nitrate). Turbidity in the Guadalquivir estuary is significantly higher than that in the Guadiana estuary (316 ± 94 NTU (Nephelometric Turbidity Unit) in the Guadalquivir estuary, while turbidity in the Guadiana estuary was 80 ± 31.21 NTU).
Results and Discussion
Our results suggest that the higher human pressures found in the Guadalquivir estuary lead to more complex feeding pathways, as shown by a greater trophic niche width (−23.05 to −29.32 and 6.75 to 21.34 max and min values of δ13C and δ15N respectively in the Guadalquivir estuary while in the Guadiana they were −25.81 to −27.20 for δ13C and 5.83 to 17.28 for δ15N) and by the greater variability in organism position within isotope niche space (Figs 2 and 3). A greater distribution among species along the carbon axis suggests that primary consumers exploit organic matter of various origins, whereas the different nitrogen signals of secondary consumers suggest that they feed on different prey items (Fig. 2). There was greater intraspecific variability in isotope signals in this estuary, suggesting more variation in diet composition. In contrast, the similar isotope signals of secondary consumers in the Guadiana estuary suggest similarity in their diet composition, implying that they feed on the same organic matter sources.
δ13C and δ15N (mean ± standard deviation) of the primary producers, invertebrates and fishes collected in the Guadalquivir (left) and Guadiana (right) estuaries in summer 2017. Horizontal bars below the x-axis represent the δ13C ranges of primary producers extracted from the literature (Supplementary Table S1). POM: particulate organic matter, SOM: sedimentary organic matter, MPB: microphytobenthos (upper limit of MPB range, −14‰, is out of the axis limit). Figures of the different organisms are provided for a better understanding of the species. Colours indicated trophic position: Secondary consumers: red. Primary and/or secondary consumers: yellow. Primary consumers: blue. Producers: green.
Trophic niche width according to the convex hull area (dotted lines) and standard ellipse areas corrected for a small sample size (SEAc) for the Guadalquivir (black lines) and Guadiana estuaries (blue lines). Triangles represent individuals of all the species measured in the Guadalquivir estuary, and circles represent species found in the Guadiana estuary.
The trophic structure of both estuarine communities, measured with the standard ellipse area (SEAc, where c indicates that the SEA was corrected for a small sample size) and the total area (TA), were distinct (Guadalquivir TA = 41.03 vs Guadiana TA = 5.74) (Fig. 3). The total overlap between the ellipses was the size of the Guadiana ellipse; it was much smaller and located inside the Guadalquivir SEAc (Guadiana SEAc = 1.66 vs Guadalquivir SEAc = 16.89, Fig. 3). Furthermore, the probability that the Bayesian standard ellipse (SEAB) value of Guadalquivir was larger than the SEAB value of Guadiana was 100% (Supplementary Fig. S1). The community metrics18 also showed large differences between estuaries (Fig. 4). All indices were smaller in the Guadiana estuary than in the Guadalquivir estuary. Therefore, the smaller SEA and mean distance to the centroid (CD) in the Guadiana estuary suggest a more compact food web and lower trophic diversity than in the Guadalquivir47. Trophic redundancy (low mean nearest neighbour distance (M-NND)) and its standard deviation (SD-NND) were also higher in the Guadiana estuary.
Bayesian results for the estuarine community-wide metrics that provide information on trophic diversity: carbon range (CR), nitrogen range (NR), mean distance to the centroid (CD) and trophic redundancy measured as the mean nearest neighbour distance (M-NND) and its standard deviation (SD-NND). Black dots are the modes, and boxes indicate the 50%, 75% and 95% credible intervals. The numbers above the red crosses represent the values of the crosses, which are the true population values.
Individuals within populations can exhibit variation in their trophic niche20. Variance in stable isotope values among individuals within populations can be used as a proxy of diet variation. This individual specialization is determined by biotic interactions such as predation and competition and by resource diversity20,48. Experimental and comparative studies suggested that while intraspecific competition increases individual specialization, interspecific competition reduces it48. In contrast, an increase in ecological opportunity, defined as the diversity of available resources, favours individual specialization48. Although other factors can influence the intraspecific variability of SIA, such as the size of individuals49,50, the higher diversity of resources in the Guadalquivir estuary seems to explain the higher intraspecific variability. Moreover, species that feed on more than one trophic level can also increase the intraspecific variability49, which is consistent with the higher trophic diversity found in the Guadalquivir estuary.
Organisms in the Guadalquivir food web tended to have higher δ15N values than those in the Guadiana food web, as reflected by the higher SEA position on the nitrogen axis of the Guadalquivir estuary. The high nitrogen range (NR) value in the Guadalquivir estuary suggests more trophic levels and more energy transfer to higher trophic levels51, and could be explained by the higher nutrient load. The similarity of the δ15N values of sedimentary organic matter (SOM) and vegetal matter in the two estuaries, in contrast to our hypothesis, suggests that this baseline variation does not drive the differences. However, the quick turnover rate of nitrogen in the primary producers could hide differences between estuaries in those resources since these values could be a snapshot of the temporal variation in the two systems52. Organisms at higher trophic levels integrate the stable isotope values of primary producers over time in their tissues, which helps to capture potential variation in basal carbon isotope signatures52. For this reason, the use of fish larvae and juveniles in planktonic communities is often a better long term indicator of water nitrogen content27,53.
Individual species, however, exhibited small differences between estuaries (Table 1). The variation was greatest in the large size class of the anchovy (Engraulis encrasicolus), the mysid (Neomysis integer) and the shrimp (Palaemon sp.). Differences in trophic position between estuaries of the mysid species Neomysis integer could be explained by the addition of an intermediate consumer to its diet or a change in its degree of trophic omnivory in the Guadalquivir estuary54. N. integer has been described as an opportunistic omnivore species that utilizes mesozooplankton and detritus as food sources and is able to feed on juveniles of the other mysid species such as Mesopodopsis slabberi55. In contrast, M. slabberi, which showed no difference between estuaries, feeds mostly on primary producers56. In the case of the anchovy E. encrasicolus, only the largest size class showed enriched δ15N in the Guadalquivir estuary. This result could be explained by the addition of an intermediate consumer such as the juveniles of the invasive isopod Synidotea laticauda which were only found in the Guadalquivir estuary (Fig. 2) or a change in trophic omnivory. In the Guadalquivir estuary, E. encrasicolus larvae change their diet from copepods to mysids as they grow57, which could explain the δ15N differences. Additionally, these differences could be explained in part by E. encrasicolus entering these two estuaries in early life history stages from the same spawning area58 and leaving in a later developmental period57,59. Consequently, the larger individuals of this species would feed longer on enriched sources in the Guadalquivir and would show larger differences with respect to the Guadiana than would the smaller individuals.
In the Guadiana estuary, the δ13C values of all organisms were similar, as indicated by the low carbon range (CR) and high redundancy values. These similar values could indicate the use of freshwater or brackish water particulate organic matter (POM), rather than marine inputs, as a carbon source; the latter would have more enriched carbon values. The similar isotope signatures between consumers suggest a more confined suite of prey resources than in the Guadalquivir estuary; these prey resources potentially include copepods and mysids (Mesopodopsis slabberi). Potential high trophic redundancy in this estuary showed by the Layman’s metrics could indicate a higher capability of species to play similar trophic roles and could support resistance to disturbances without the loss of connectivity in the food web60. However, pairing this information with stomach content analysis is necessary to truly assess trophic redundancy60. Moreover, the small number of links between primary consumers and secondary consumers may lead to an increase in fragility61. Niche width collapse and homogenization in the energy flow pathway have been described in fragmented systems, resulting in a less stable food web structure15. Low SEA values and low trophic diversity in estuarine fish food webs have been related to the low availability of aquatic producers as a consequence of the high level of suspended solids, which would limit primary production, which is not the case for Guadiana47. The simplification of the complex food web is also related to an increase in the vulnerability to environmental changes that can affect productivity and secondary extinctions62.
Conversely, in the Guadalquivir estuary, consumers showed greater differences in their carbon stable isotope signals, a greater niche width, more trophic diversity and lower redundancy values. These differences could indicate that the organic matter sources are different63. Thus, the Guadalquivir estuary would have a relatively more reticulated food web with multiple trophic pathways towards upper-level consumers.
Since the high turbidity present in the Guadalquivir estuary limits primary production, the allochthonous organic matter inputs in the oligohaline zone may be an important basal source. This detritus contribution can compensate limited phytoplankton production in highly turbid estuaries64,65. In the maximum turbidity zone of estuaries (MTZ), primary production was primarily bacterial, fed by detrital terrestrial and estuarine organic matter64,66. In the MTZ sediment, where organic matter aggregates by flocculation65, particles act as substrates for microorganisms that serve as prey for protozoa and other microorganisms67; the detrital energy is thus transferred to copepods and can also act as a food source for mysids65. The high abundance of copepods and mysids64,66 is supported by energy from detrital sources, which differs from nutritionally poor systems, in which food webs are based on algae in lower-turbidity areas66. This is also the case for the Guadalquivir estuary, in which the MTZ is located within the oligohaline zone55. In this estuary, the high biomass of copepods and mysids who feed on these detrital sources would support fish larvae and other crustaceans68,69. This finding agrees with those of other studies that have found that the turbidity maximum zone is a significant nursery area that positively influences fish growth and condition66,70. In addition, a positive relationship between the number of organic matter basal sources and fish production71 has been suggested.
These results could also explain the differences between estuaries in the mysid species; when detritus is present, M. slabberi shows a detritivorous/herbivorous tendency, and N. integer exhibits omnivorous behaviour with a carnivorous feeding tendency64. These authors described a 2- or 3-stage route from bacteria and vegetal matter to copepods. This route agrees with the high isotopic signatures of nitrogen of some species in the Guadalquivir estuary. In contrast, M. slabberi probably directly feed on detritus or phytoplankton, which would explain the lack of differences between estuaries in terms of the trophic enrichment factors.
The greater trophic niche width of the planktonic community, as well as the higher trophic diversity, could thus be explained by the different organic matter sources that are present in the Guadalquivir estuary28. Furthermore, another possible organic matter source would be linked to microphytobenthos in Guadalquivir mudflats. This particular organic matter source has been reported in other estuaries as one of the principal basal sources for the pelagic food web72. In contrast, the lower turbidity in the Guadiana estuary would permit higher phytoplankton primary production in the water column, which would be the base for copepods and M. slabberi, in turn sustaining all the secondary consumers. The smaller mudflats would also limit the contribution of microphytobenthos to the food web, but the overlapping values of carbon isotopes in the basal resources make it difficult to identify the main resources. Therefore, these conclusions are a first overview of organic matter origins for this two estuarine food webs.
The species found in each estuary were a good representation of the native planktonic macrofaunal communities. The same species were found in both estuaries except of the invasive isopod S. laticauda mentioned before (found in the Guadalquivir estuary) and the invasive clam Corbicula fluminea (found in the Guadiana estuary)73,74,75. Stable isotope studies assessing the effects of invasive species have been more frequently used in terrestrial and freshwater systems76. However, recent studies have applied stable isotopes to assess the effects of invasive species on marine ecosystems23. For example, the trophic niche of the benthic food web was wider in sites invaded by the macroalgae Caulerpa cylindracea than in non-invaded sites due to an increase in the diversity of basal resource pools25. In contrast, another study found a compacted food web structure in Caulerpa prolifera meadows77. There is a large degree of overlap in the utilization of basal sources, which is related to intra- and interspecific competition77 and is characteristic of degraded systems15. In this sense, our results showed that the invasive species S. laticauda may have an effect on the food web in the Guadalquivir estuary. First, this organism has isotopic signatures similar to those found in small anchovies (E. encrasicolus), suggesting that they are potential competitors. This potential risk caused by an invasive species has been also suggested78. Second, small individuals of this isopod could act as prey and could explain higher nitrogen signatures found in secondary consumers in the Guadalquivir. Additionally, this species showed the most enriched carbon signatures (Fig. 2), suggesting that it feeds on microphytobenthos and/or more marine basal sources than other species, which would explain the higher trophic diversity of the Guadalquivir estuary. In contrast, the impacts of C. fluminea, the invasive species found in the Guadiana estuary, do not appear to be readily visible in this study, and further investigation into the impacts of this species on planktonic food webs is certainly warranted.
Although our results show that the isotopic signal of nitrogen in the planktonic community generally seems to reflect the higher anthropogenic pressure present in the Guadalquivir estuary (Table 1), other factors could contribute to these differences. In contrast to our hypothesis and previous related research16, even though the Guadalquivir estuary has higher human pressure, the food web is more complex, with more feeding pathways, a greater niche width, more trophic diversity and lower trophic redundancy than the Guadiana estuary. The different organic matter sources present in the Guadalquivir estuary and the detrital processes in the MTZ could explain these distinctions. These results could be related to the higher nitrogen loads in the Guadalquivir estuary, which would have a positive effect on food web consumers by improving the nutritional quality and palatability of basal resources79. This finding also agrees with Warry et al.12 that found higher trophic diversity and less redundancy in fish food webs in estuaries with high nitrogen loads and suggested that the same pattern may be found in systems where the nitrogen loads are high and there is not a single dominant organic source.
Conclusions
This study concludes that the Bayesian approach to the “Layman metrics18” is a useful tool with which to detect ecological differences in food webs between estuaries under different human pressures, as has been demonstrated in other studies47. Furthermore, stable isotope analysis revealed differences in the trophic interactions of species that were present in both estuaries, which is important information which complements traditional species surveys24 by providing key, additional ecological information about differences between these estuaries. Because this study is a comparison made during the summer season, the results obtained must be considered carefully, keeping in mind that the conclusions obtained are applicable to the oligohaline zone. Nevertheless, the important trophic differences observed between these two systems allow us to extract some conclusions and also to point out some characteristics that would be worth being further investigated. Thus, more extensive research on the spatial and temporal variability of the origin of basal resources, as well as the bottom-up interactions of estuarine food webs and their relationships with other environmental factors, is needed to better understand the food web dynamics in both systems. Understanding the trophic interactions present in estuaries with a strong human presence is crucially important for defining proper management and conservation strategies80. Furthermore, the management of factors that influence an estuary, such as freshwater discharges and urban and agricultural wastes, can regulate inputs of basal resources and modulate a phytoplankton or detrital dominated food web.
One important environmental implication of this research is that, even under altered conditions, the community of the Guadalquivir estuary seems to have reached a comparatively complex structure, which ensures a high productivity and some important ecosystem services such as the nursery function59,81. A word of caution should be included here for the future environmental management of this estuary since, any change, even with the objective to improve the environmental quality, should be done slowly and closely monitored. For instance, the high and permanent turbidity in this estuary is a present concern and its reduction is a commonly claimed objective82,83. However, the possibility that introduced sediment could be partly associated with the main sources of carbon for the community (allochthonous organic matter), makes it advisable to proceed with any potential restoration measure with caution, since any abrupt change in the present equilibrium would probably affect the nursery function and the fisheries production in the nearby marine areas. Assessing trophic structure and its relationships with other factors is crucial for understanding the consequences of increasing human pressure on estuaries.
Methods
Sampling
To eliminate any seasonal bias, sampling was carried out in summer 2017. Sampling was performed in the oligohaline zone of the estuaries to avoid marine influences on species isotopic niche breadth and assemblage architecture12. Several samples of the planktonic community were collected along the oligohaline zone to characterize the possible variation within this area. Samples were collected with a zooplankton net with a 1-m mouth diameter and 1000-µm mesh size. Twelve oblique tows were performed from the surface to the bottom during flood tide in the main channel at a constant speed of 2 knots. Parallel, copepods were collected with a bongo net with a 200-µm mesh size following the same process. Samples of soft bottom community were collected with a van Veen grab (0.05 m2) although only the clam C. fluminea, found in the Guadiana estuary, had enough biomass for isotopic characterization. All organisms were sorted by species, and juvenile anchovies (Engraulis encrasicolus) were sorted into three size classes: large, juveniles of 34.2 to 43 mm; medium, postlarvae of 27.8 to 31.5 mm; and small, postlarvae of 18.5 to 25.1 mm. The organisms were transferred to the laboratory in refrigerated containers and kept alive for 24 h to allow stomach evacuation to avoid any possible interference with the isotope signatures of their prey. Permission to gather the samples was obtained from the local authority “Consejeria de Medio Ambiente de Andalucía”. There are no ethical concerns associated with our study based on Directive 2010/63/UE and order ECC/566/2015. Primary producers were sampled by first sieving the sample collected with the bongo net through a sieving column and then selecting the vegetal matter under the stereoscopic microscope. Three sediment organic matter samples were collected with the van Veen grab from the uppermost 2 cm of the sediment. Other possible primary producers were extracted from the literature (see supplementary information).
Isotope analyses
We rinsed animal and plant samples with distilled water. Muscle tissue samples of fish, clams and shrimp abdomens were used for isotopic analysis. Multiple organisms (>50) were pooled when the individuals had low biomass values (Supplementary Table S2). Samples were dried at 60 °C and ground to a powder. Sediment samples were acidified with 0.1 M HCl to remove carbonates and then oven dried. Organismal tissues were not acidified to avoid alterations in isotopic values84. Subsamples of powdered materials were weighed to the nearest 0.3 μg and placed into tin capsules for δ13C and δ15N analysis. Isotope analyses were carried out at the Laboratorio de Isótopos Estables of the Estación Biológica de Doñana (LIE-EBD, Spain; www.ebd.csic.es/lie/index.html). All samples were combusted at 1020 °C using a continuous flow isotope-ratio mass spectrometry system with a Flash HT Plus elemental analyser coupled to a Delta-V Advantage isotope ratio mass spectrometer via a CONFLO IV interface (Thermo Fisher Scientific, Bremen, Germany).
To investigate changes in trophic diversity within both estuaries, analysis of community niche space was performed using a novel Bayesian approach with the metrics proposed by Layman et al.18 for quantitative comparison of food webs85. This method returns a posterior distribution of estimates of the original metrics, which include the δ13C range (CR), δ15N range (NR), mean distance to the centroid (CD), mean nearest neighbour distance (M-NND) and SD of the M-NND (SD-NND). The Bayesian inference technique provides measures of uncertainty for these metrics reported as sampling error for the estimates of the means. Thus, the technique permits robust statistical comparisons to be made between communities independently of the number of groups within the communities47,85. Briefly, the CR is indicative of niche diversification at the base of food webs. The NR is a representation of the vertical structure of a food web, and larger ranges suggest more trophic levels and a greater degree of trophic diversity. The CD provides a measure of the average degree of trophic diversity within a food web. M-NND represents trophic redundancy, and food webs with species with similar trophic ecologies will show smaller values. Finally, SD-NND is a measure of the evenness of the food web, and large values suggest more diversification of trophic niches (see18 for more details).
The total convex hull area (TA) and the standard ellipse area (SEAc) were also calculated (c indicates that the SEA was corrected for a small sample size). The two metrics were estimated as quantitative proxies of the isotopic niche width, although the SEA is less sensitive to outliers and sample size than the TA85. Differences in the SEAc between the communities were evaluated via Bayesian interference (SEAB) according to85. All measures were calculated using the SIBER package in R.
Differences in the isotopic values between estuaries were investigated using two-way permutational multivariate analysis of variance (PERMANOVA)86. Each δ13C and δ15N isotope variable was analysed based on two fixed factors: estuaries (Guadalquivir (GDQ)/Guadiana (GDN)) and species. Posterior pairwise tests were used to test for differences between species in the estuaries. The Monte Carlo P-value was used instead when small unique values in the permutation distribution were available (<100)87. The tests were based on Euclidean distance matrices of the untransformed data using 9999 permutations. Statistical analyses were conducted using Primer v.6 and PERMANOVA (Primer-E Ltd., Plymouth, UK).
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Wetzel, M.A, Wahrendorf, D. S. & von der Ohe, P. C. Sediment pollution in the Elbe estuary and its potential toxicity at different trophic levels. Sci. Total Environ. 449, 199–207 (2013).
Robb, C. K. Assessing the impact of human activities on British Columbia’s estuaries. PLoS One 9, e99578 (2014).
França, S. et al. Assessing food web dynamics and relative importance of organic matter sources for fish species in two Portuguese estuaries: A stable isotope approach. Mar. Environ. Res. 72, 204–215 (2011).
Dauvin, J. et al. The evolution of habitat areas and carrying capacity for Western Cotentin Estuaries (North-Western France) 77–89 (2014).
Kostecki, C. et al. Dynamics of an estuarine nursery ground: the spatio-temporal relationship between the river flow and the food web of the juvenile common sole (Solea solea, L.) as revealed by stable isotopes analysis. J. Sea Res. 64, 54–60 (2010).
Costanza, R. et al. The value of the world’s ecosystem services and natural capital. Nature 387, 253–260 (1997).
Ray, G. C. The coastal realm’s environmental debt. Aquat. Conserv. Mar. Freshw. Ecosyst. 16, 1–4 (2006).
Morris, C., Lee, S. Y. & Van De Merwe, J. δ15N of estuarine fishes as a quantitative indicator of urbanization. Ecol. Indic. 56, 41–49 (2015).
Lee, K. M., Lee, S. Y. & Connolly, R. M. Combining process indices from network analysis with structural population measures to indicate response of estuarine trophodynamics to pulse organic enrichment. Ecol. Indic. 18, 652–658 (2012).
González-Ortegón, E. & Drake, P. Effects of freshwater inputs on the lower trophic levels of a temperate estuary: Physical, physiological or trophic forcing? Aquat. Sci. 74, 455–469 (2012).
Mazumder, D., Saintilan, N., Alderson, B. & Hollins, S. Inputs of anthropogenic nitrogen influence isotopic composition and trophic structure in SE Australian estuaries. Mar. Pollut. Bull. 100, 217–223 (2015).
Warry, F. Y. et al. Nitrogen loads influence trophic organization of estuarine fish assemblages. Funct. Ecol. 30, 1723–1733 (2016).
Olsen, Y. S., Fox, S. E., Teichberg, M., Otter, M. & Valiela, I. δ15N and δ13C reveal differences in carbon flow through estuarine benthic food webs in response to the relative availability of macroalgae and eelgrass. Mar. Ecol. Prog. Ser. 421, 83–96 (2011).
Rooney, N., McCann, K., Gellner, G. & Moore, J. C. Structural asymmetry and the stability of diverse food webs. Nature 442, 265–269 (2006).
Layman, C. A., Quattrochi, J. P., Peyer, C. M. & Allgeier, J. E. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol. Lett. 10, 937–944 (2007).
Rooney, N., McCann, K. S. & Moore, J. C. A landscape theory for food web architecture. Ecol. Lett. 11, 867–881 (2008).
Hussey, N. E. et al. Rescaling the trophic structure of marine food webs. Ecol. Lett. 17, 239–50 (2014).
Layman, C. A. et al. Can Stable Isotope Ratios Provide for Community-Wide Measures of Trophic Structure? Published by: Ecological Society of America can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88, 42–48 (2007).
Leibold, M. The Niche Concept Revisited: Mechanistic Models and Community Context. Ecology 76, 1371–1382 (1995).
Evangelista, C., Boiche, A., Lecerf, A. & Cucherousset, J. Ecological opportunities and intraspecific competition alter trophic niche specialization in an opportunistic stream predator. J. Anim. Ecol. 83, 1025–1034 (2014).
Bearhop, S., Adams, C. E., Waldron, S., Fuller, R. A. & Macleod, H. Determining trophic niche width: A novel approach using stable isotope analysis. J. Anim. Ecol. 73, 1007–1012 (2004).
Layman, C. A. et al. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 87, 545–562 (2012).
Mancinelli, G. & Vizzini, S. Assessing anthropogenic pressures on coastal marine ecosystems using stable CNS isotopes: State of the art, knowledge gaps, and community-scale perspectives. Estuar. Coast. Shelf Sci. 156, 195–204 (2015).
Nigro, K. M. et al. Stable isotope analysis as an early monitoring tool for community-scale effects of rat eradication. Restor. Ecol. 25, 1015–1025 (2017).
Alomar, C. et al. Caulerpa cylindracea Sonder invasion modifies trophic niche in infralittoral rocky benthic community. Mar. Environ. Res. 120, 86–92 (2016).
Baker, R., Fry, B., Rozas, L. & Minello, T. Hydrodynamic regulation of salt marsh contributions to aquatic food webs. Mar. Ecol. Prog. Ser. 490, 37–52 (2013).
Baeta, A. et al. Use of stable isotope ratios of fish larvae as indicators to assess diets and patterns of anthropogenic nitrogen pollution in estuarine ecosystems. Ecol. Indic. 83, 112–121 (2017).
Post, D. M. Using stable isotopes to estimate trophic position: models, methos, and assumptions. Ecology 83, 703–718 (2002).
Watson, E. B., Szura, K., Powell, E., Maher, N. & Wigand, C. Cultural Eutrophication Is Reflected in the Stable Isotopic Composition of the Eastern Mudsnail. J. Environ. Qual. 47, 177 (2018).
Connolly, R. M., Gorman, D., Hindell, J. S., Kildea, T. N. & Schlacher, T. A. High congruence of isotope sewage signals in multiple marine taxa. Mar. Pollut. Bull. 71, 152–158 (2013).
Mitchell, S. B., Jennerjahn, T. C., Vizzini, S. & Zhang, W. Changes to processes in estuaries and coastal waters due to intense multiple pressures - An introduction and synthesis. Estuar. Coast. Shelf Sci. 156, 1–6 (2015).
Wolanski, E., Chicharo, L., Chicharo, M. A. & Morais, P. An ecohydrology model of the Guadiana Estuary (South Portugal). Estuar. Coast. Shelf Sci. 70, 132–143 (2006).
González-Ortegón, E. et al. Freshwater scarcity effects on the aquatic macrofauna of a European Mediterranean-climate estuary. Sci. Total Environ. 503–504, 9 (2014).
Ruiz, J., Macías, D., Losada, Ma, Díez-Minguito, M. & Prieto, L. A simple biogeochemical model for estuaries with high sediment loads: Application to the Guadalquivir River (SW Iberia). Ecol. Modell. 265, 194–206 (2013).
Taglialatela, S., Ruiz, J., Prieto, L. & Navarro, G. Seasonal forcing of image-analysed mesozooplankton community composition along the salinity gradient of the Guadalquivir estuary. Estuar. Coast. Shelf Sci. 149, 244–254 (2014).
Llope, M. The ecosystem approach in the Gulf of Cadiz. A perspective from the southernmost European Atlantic regional sea. ICES J. Mar. Sci. 74, 382–390 (2017).
Prieto, L. et al. Oceanographic and meteorological forcing of the pelagic ecosystem on the Gulf of Cadiz shelf (SW Iberian Peninsula). Cont. Shelf Res. 29, 2122–2137 (2009).
Díez-Minguito, M. Tidal Wave Reflection From the Closure Dam in the Guadalquivir Estuary (Sw Spain). Coast. Eng. Proc. 1, 1–8 (2012).
Sánchez-Moyano, J. E. et al. BENFES, a new biotic index for assessing ecological status of soft-bottom communities. Towards a lower taxonomic complexity, greater reliability and less effort. Mar. Environ. Res. 132, 41–50 (2017).
Morais, P. Review on the major ecosystem impacts caused by damming and watershed development in an Iberian basin (SW- Europe): Focus on the Guadiana estuary Review on the major ecosystem impacts caused by damming and watershed development in an Iberian basin (SW-. Ann. Limnol. - Int. J. Limnol. 44, 105–117 (2008).
Barbosa, A. B., Domingues, R. B. & Galvão, H. M. Environmental Forcing of Phytoplankton in a Mediterranean Estuary (Guadiana Estuary, South-western Iberia): A Decadal Study of Anthropogenic and Climatic Influences. Estuaries and Coasts 33, 324–341 (2009).
Sánchez-Moyano, J. E. & García-Asencio, I. Crustacean assemblages along the Guadiana River estuary (south-western Iberian Peninsula). J. Mar. Biol. Assoc. United Kingdom 91, 127–138 (2011).
Vasconcelos, R. P. et al. Assessing anthropogenic pressures on estuarine fish nurseries along the Portuguese coast: a multi-metric index and conceptual approach. Sci. Total Environ. 374, 199–215 (2007).
González-ortegón, E., Laiz, I., Sánchez-quiles, D., Cobelo-garcia, A. & Tovar-sánchez, A. Trace metal characterization and fluxes from the Guadiana, Tinto-Odiel and Guadalquivir estuaries to the Gulf of Cadiz. Sci. Total Environ. 650, 2454–2466 (2019).
Hanebuth, T. J. J. et al. Hazard potential of widespread but hidden historic offshore heavy metal (Pb, Zn) contamination (Gulf of Cadiz, Spain). Sci. Total Environ. 637–638, 561–576 (2018).
Miró, J. M. et al. Assessing the fish nursery function in the main estuaries of the gulf of cadiz during the summer season. (2018).
Abrantes, K. G., Barnett, A. & Bouillon, S. Stable isotope-based community metrics as a tool to identify patterns in food web structure in east African estuaries. Funct. Ecol. 28, 270–282 (2014).
Araújo, M. S., Bolnick, D. I. & Layman, C. A. The ecological causes of individual specialisation. Ecol. Lett. 14, 948–958 (2011).
Pasquaud, S. et al. A preliminary investigation of the fish food web in the Gironde estuary, France, using dietary and stable isotope analyses. Estuar. Coast. Shelf Sci. 78, 267–279 (2008).
Chouvelon, T. et al. Trophic ecology of European sardine Sardina pilchardus and European anchovy Engraulis encrasicolus in the Bay of Biscay (north-east Atlantic) inferred from δ13C and δ15N values of fish and identified mesozooplanktonic organisms. J. Sea Res. 85, 277–291 (2014).
Cooper, R. N. & Wissel, B. Loss of trophic complexity in saline prairie lakes as indicated by stable-isotope based community-metrics. Aquat. Biosyst. 8, 6 (2012).
Van De Merwe, J. P., Lee, S. Y., Connolly, R. M., Pitt, K. A. & Steven, A. D. L. Assessing temporal and spatial trends in estuarine nutrient dynamics using a multi-species stable isotope approach. Ecol. Indic. 67, 338–345 (2016).
Hoffman, J. C. et al. Using δ15N in Fish Larvae as an Indicator of Watershed Sources of Anthropogenic Nitrogen: Response at Multiple Spatial Scales. Estuaries and Coasts 1–15 (2012).
Post, D. M. & Takimoto, G. Proximate structural mechanisms for variation in food-chain length. Oikos 116, 775–782 (2007).
Vilas, C., Drake, P. & Fockedey, N. Feeding preferences of estuarine mysids Neomysis integer and Rhopalophthalmus tartessicus in a temperate estuary (Guadalquivir Estuary, SW Spain). Estuar. Coast. Shelf Sci. 77, 345–356 (2008).
Modéran, J., David, V., Bouvais, P., Richard, P. & Fichet, D. Organic matter exploitation in a highly turbid environment: Planktonic food web in the Charente estuary, France. Estuar. Coast. Shelf Sci. 98, 126–137 (2012).
Baldó, F. & Drake, P. A multivariate approach to the feeding habits of small fishes in the Guadalquivir Estuary. J. Fish Biol. 61, 21–32 (2002).
Baldó, F. et al. Spatial and temporal distribution of the early life stages of three commercial fish species in the northeastern shelf of the Gulf of Cádiz. Deep. Res. Part II Top. Stud. Oceanogr. 53, 1391–1401 (2006).
Drake, P. et al. Spatio-temporal distribution of early life stages of the European anchovy Engraulis encrasicolus L. within a European temperate estuary with regulated freshwater inflow: Effects of environmental variables. J. Fish Biol. 70, 1689–1709 (2007).
Matich, P., Kiszka, J. J., Gastrich, K. R. & Heithaus, M. R. Trophic redundancy among fishes in an East African nearshore seagrass community inferred from stable-isotope analysis. J. Fish Biol. 91, 490–509 (2017).
Vinagre, C., Salgado, J., Cabral, H. N. & Costa, M. J. Food Web Structure and Habitat Connectivity in Fish Estuarine Nurseries—Impact of River Flow. Estuaries and Coasts 34, 663–674 (2010).
Careddu, G., Calizza, E., Costantini, M. L. & Rossi, L. Isotopic determination of the trophic ecology of a ubiquitous key species – The crab Liocarcinus depurator (Brachyura: Portunidae). Estuar. Coast. Shelf Sci. 191, 106–114 (2017).
Dias, E., Morais, P., Faria, A. M., Antunes, C. & Hoffman, J. C. Benthic food webs support the production of sympatric flatfish larvae in estuarine nursery habitat. Fish. Oceanogr. 26, 507–512 (2017).
David, V., Sautour, B., Galois, R. & Chardy, P. The paradox high zooplankton biomass-low vegetal particulate organic matter in high turbidity zones: What way for energy transfer? J. Exp. Mar. Bio. Ecol. 333, 202–218 (2006).
Kudryavtsev, I. V. & Subbotovskaya, A. I. Application of Six-Color Flow Cytometric Analysis for Immune Profile Monitoring. Med. Immunol. 17, 19 (2015).
Islam, M. S. & Tanaka, M. Spatial variability in nursery functions along a temperate estuarine gradient: role of detrital versus algal trophic pathways. Can. J. Fish. Aquat. Sci. 63, 1848–1864 (2006).
Artolozaga, I., Valcárcel, M., Ayo, B., Latatu, A. & Iriberri, J. Grazing rates of bacterivorous protists inhabiting diverse marine planktonic microenvironments. Limnol. Oceanogr. 47, 142–150 (2002).
De Carvalho-Souza, G. F. et al. Natural and anthropogenic effects on the early life stages of European anchovy in one of its essential fish habitats, the Guadalquivir estuary. Mar. Ecol. Prog. Ser, (2018).
Vilas, C., Drake, P. & Pascual, E. Inter- and intra-specific differences in euryhalinity determine the spatial distribution of mysids in a temperate European estuary. J. Exp. Mar. Bio. Ecol. 369, 165–176 (2009).
Escalas, A. et al. Spatial variations in dietary organic matter sources modulate the size and condition of fish juveniles in temperate lagoon nursery sites. Estuar. Coast. Shelf Sci. 152, 78–90 (2015).
Hoffman, J. C., Kelly, J. R., Peterson, G. S. & Cotter, A. M. Landscape-Scale Food Webs of Fish Nursery Habitat Along a River-Coast Mixing Zone. Estuaries and Coasts 38, 1335–1349 (2015).
David, V. et al. Estuarine habitats structure zooplankton communities: Implications for the pelagic trophic pathways. Estuar. Coast. Shelf Sci. 179, 99–111 (2016).
Cuesta, J. A., Serrano, L., Bravo, M. R. & Toja, J. Four New Crustaceans in the Guadalquivir River Es- Tuary (Sw Spain), Including an Introduced Species (1996).
Ruiz-delgado, C., Dbo, S. L., González-ortegón, E. & Herrera, I. Distribution and salinity tolerance of the invasive isopod Synidotea laticauda in the Guadalquivir estuary (SW Spain): Field and laboratory observations Distribution and salinity tolerance of the invasive isopod Synidotea laticauda in the Guadalquivir e. Conf. ECSA 56, Bremen (2016).
Pérez-Quintero, J. C. Revision of the distribution of corbicula fluminea (Müller, 1744) in the Iberian Peninsula. Aquat. Invasions 3, 355–358 (2008).
Mancinelli, G. & Vizzini, S. Assessing anthropogenic pressures on coastal marine ecosystems using stable CNS isotopes: State of the art, knowledge gaps, and community-scale perspectives. Estuar. Coast. Shelf Sci. 156, 195–204 (2014).
Deudero, S., Box, A., Vázquez-Luis, M. & Arroyo, N. L. Benthic community responses to macroalgae invasions in seagrass beds: Diversity, isotopic niche and food web structure at community level. Estuar. Coast. Shelf Sci. 142, 12–22 (2014).
Carrozzo, L. et al. Seasonal abundance and trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in a Mediterranean coastal habitat. Rend. Lincei 25, 201–208 (2014).
Cebrian, J. et al. Producer nutritional quality controls ecosystem trophic structure. PLoS One 4, 1–5 (2009).
Vermeiren, P., Abrantes, K. & Sheaves, M. Generalist and Specialist Feeding Crabs Maintain Discrete Trophic Niches Within and Among Estuarine Locations. Estuaries and Coasts 38, 2070–2082 (2015).
Fernández-Delgado, C. et al. Effects of the river discharge management on the nursery function of the Guadalquivir river estuary (SW Spain). Hydrobiologia 587, 125–136 (2007).
González-Ortegón, E. et al. The impact of extreme turbidity events on the nursery function of a temperate European estuary with regulated freshwater inflow. Estuarine, Coastal and Shelf Science 87, 311–324 (2010).
Ruiz, J. et al. The Guadalquivir Estuary: A Hot Spot for Environmental and Human Conflicts. 8, (2015).
Schlacher, T. A. & Connolly, R. M. Effects of acid treatment on carbon and nitrogen stable isotope ratios in ecological samples: A review and synthesis. Methods Ecol. Evol. 5, 541–550 (2014).
Jackson, A. L., Inger, R., Parnell, A. C. & Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER - Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602 (2011).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance 32–46 (2001).
Anderson, M., Gorley, R. N. & Clarke, K. PERMANOVA+ for primer: Guide to software and statistical methods. (Plymouth: Primer-E, 2008).
Acknowledgements
We thank Autoridad Portuaria de Sevilla (APS) and Acuario de Sevilla for financial and logistical support. This work was partially supported by the V Plan Propio Universidad de Sevilla via two pre-doctoral grants (I. Donázar-Aramendía, J.M. Miró). We thank all the members of the LBM who participate in field surveys, in samples processing and in the revision of the manuscript. We thank M. Florido for the drawings provided for the figure. Finally, we thank Dr. Craig Layman for his invaluable help with stable isotope interpretation.
Author information
Authors and Affiliations
Contributions
The authors contributed equally in designing the study. I.D., J.E.S., I.G and J.M. performed the research. I.D., C.M. and J.C.G. analysed the data. I.D. and J.E.S. wrote the first manuscript and all authors contributed substantially to revisions.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Donázar-Aramendía, I., Sánchez-Moyano, J.E., García-Asencio, I. et al. Human pressures on two estuaries of the Iberian Peninsula are reflected in food web structure. Sci Rep 9, 11495 (2019). https://doi.org/10.1038/s41598-019-47793-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47793-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.