Abstract
Chronic pain, including chronic low back and leg pain are prominent causes of disability worldwide. While patient management aims to reduce pain and improve daily function, prescription of opioids remains widespread despite significant adverse effects. This study pooled data from two large prospective trials on 10 kHz spinal cord stimulation (10 kHz SCS) in subjects with chronic low back pain and/or leg pain and performed post hoc analysis on changes in opioid dosage 12 months post 10 kHz SCS treatment. Patient-reported back and leg pain using the visual analog scale (VAS) and opioid dose (milligrams morphine equivalent/day, MME/day) were compared at 12 months post-10 kHz SCS therapy to baseline. Results showed that in the combined dataset, 39.3% of subjects were taking >90 MME dose of opioids at baseline compared to 23.0% at 12 months post-10 kHz SCS therapy (p = 0.007). The average dose of opioids in >90 MME group was significantly reduced by 46% following 10 kHz SCS therapy (p < 0.001), which was paralleled by significant pain relief (P < 0.001). In conclusion, current analysis demonstrates the benefits of 10 kHz SCS therapy and offers an evidence-based, non-pharmaceutical alternative to opioid therapy and/or an adjunctive therapy to facilitate opioid dose reduction whilst delivering significant pain relief. Healthcare providers involved in management of chronic non-cancer pain can include reduction or elimination of opioid use as part of treatment plan when contemplating 10 kHz SCS.
Similar content being viewed by others
Introduction
Chronic pain, defined as pain that remains beyond normal healing time, is a debilitating group of conditions and a prominent cause of disability worldwide1. While acute pain is generally nociceptive pain associated with somatosensory stimuli, chronic pain is thought to involve a shift from peripheral damage to more prominent central sensitization and central nervous system mechanisms2. In general, the worldwide prevalence of chronic pain in developed nations is around 20% and can be grouped into seven etiologies: primary pain that is not explained by another pain condition, cancer pain, neuropathic pain, posttraumatic and postsurgical pain, musculoskeletal pain, visceral pain and headache and orofacial pain3,4,5,6,7. Chronic low back pain (CLBP) is one of the most prevalent types of chronic pain; it is the leading global cause of disability, one of the common reasons for visits to physician’s office, correlates with work absence and is therefore associated with significant economic costs8,9,10,11,12,13.
Management of CLBP, like other chronic pain conditions, aims to reduce pain and improve daily function. If non-pharmacological options such as bed rest, superficial heat, cryotherapy, exercise and physiotherapy are unsuccessful, pharmacological therapies are a second-line option8,12,14. Of these, opioids are amongst the most commonly prescribed drugs for low back pain15, yet their use remains controversial16. Limited head-to-head evidence suggests opioids to be more effective than naproxen or placebo for relieving CLBP17,18, but the average effect is little more than a 10-mm reduction on a 100-mm visual analog scale (VAS), a decrease that is not considered to be clinically meaningful19,20. In addition, opioids have well-described short and long-term side effects such as constipation, nausea, vomiting, sedation, dizziness, respiratory depression, hormonal imbalance, changes in immune response and physical dependence; opioid overdose deaths are mainly due to acute respiratory depression caused by opioids21,22,23. Moreover, opioid use for chronic pain conditions like CLBP can cause tolerance, hyperalgesia, misuse, abuse and diversion24,25,26,27. Therefore, international guidelines agree that opioids need to be cautiously prescribed and should be discontinued if the benefits do not outweigh risks28.
In 2016, the Centers for Disease Control and Prevention (CDC) published guidelines on using opioids for management of chronic pain and capped the maximum opioid dose at 90 milligrams morphine equivalent (MME)/day as the risk of abuse and misuse rises with higher doses29. Even at doses ≥50 MME/day, the risk of death from opioid overdose is doubled30. The challenge for patients and prescribers is for patients taking ≥90 MME/day, the CDC recommends other approaches to pain management and a taper and discontinue approach for the opioid29. However, no definite alternatives are recommended.
Therefore, for improved quality of life, patients may desire and benefit from a reduction in opioid usage just as much as they seek relief from chronic pain through additional treatment options that enable them to achieve both goals. A minimally invasive alternative to opioid therapy is spinal cord stimulation (SCS), a type of neuromodulation typically used to treat chronic intractable neuropathic pain conditions like failed back surgery syndrome (FBSS)31. With SCS, the painful region is stimulated using electrical pulses and the transmission of abnormal pain signals to the brain is altered31. Stimulation is generally provided through electrode arrays placed into the epidural space percutaneously, or through a surgical paddle lead delivered via a laminotomy. First generation SCS devices generally deliver pulse frequencies between 40 to 60 Hz with the intent of replacing the pain sensation with a stimulation-produced sensation known as paresthesia31,32. However, it is challenging to produce reliable and tolerable paresthesis in the axial back region33. For this reason, it’s use has been largely limited to leg pain. Moreover, some patients report paresthesia as an unpleasant sensation34 and the perceived intensity of paresthesia can increase with body movement35. Another challenge of previous devices and new iterations of these devices is providing durable pain relief for back and leg pain. Multiple studies demonstrated that approximately 50% of the patients achieve ≥50% pain relief with traditional SCS and even in the patients who respond initially, therapy effectiveness was found to diminish with time36,37,38,39,40,41,42,43,44.
More recently, SCS has evolved into a variety of different modalities. One of these is 10 kHz SCS therapy that applies a 10,000 Hz waveform and provides paresthesia-free pain relief31,45,46,47. Its use has been approved for patients with chronic refractory pain of the trunk and/or limbs and is particularly suitable for CLBP.31,45,48,49. Two studies described previously (SENZA-EU and SENZA-RCT) showed 10 kHz SCS therapy relieves low back pain and leg pain for up to 24 months post-implant50,51. Compared to traditional SCS, 10 kHz SCS demonstrated superior pain relief as well as long-term improvement in quality of life in subjects with leg pain and/or CLBP51,52.
Despite their increasing use, to date there has been limited investigation on the role of SCS in facilitating a reduction or elimination of opioids in chronic pain management53. Prospective studies have shown that traditional SCS can reduce reliance on medication for management of pain. In a randomized controlled trial, North et al. reported that 87% (20/23) of FBSS patients in the SCS group were either stably using opioids or decreased their opioid use and only 13% (3/23) patients needed to increase their use of opioids whereas 58% (15/26) patients had a stable or decreased opioid use and 42% (11/26) patients required increased opioid use in reoperation group37. The results were further supported by systematic reviews on safety and efficacy of SCS for chronic pain including CLBP and FBSS, which reported stable or reduced medication usage in 65% and 53% patients treated with SCS respectively41,54. However, the definition of medication included opioid as well as non-opioid drugs in both studies and so far, to our knowledge, no chronic pain SCS study has assessed reduction in opioid dose as the primary endpoint and none have explored the ≥90 MME high-risk category.
This post hoc analysis of the SENZA-EU and SENZA-RCT studies examines the effect of 10 kHz SCS therapy on opioid analgesic pain management in the ≥90 MME high-risk category for subjects with leg pain and/or low back pain. This analysis will determine if 10 kHz SCS therapy may facilitate successful reduction of opioids in subjects taking the ≥90 MME doses for management of chronic pain in routine clinical practice.
Results
Patient demographics
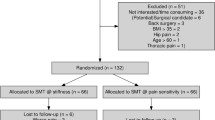
In SENZA-RCT, 89 subjects were successfully included through 12 months and in SENZA-EU, 68 subjects were included through 12 months. Out of the subjects with pain relief data, 83 subjects in SENZA-RCT and 54 subjects in SENZA-EU (137 subjects in the combined population) had opioid usage data at baseline or 12-month assessment. They were included in initial analyses of demographics and clinical characteristics.
As listed in Tables 1, 60.2% subjects were female in SENZA-RCT, 53.7% in SENZA-EU and 57.7% in combined dataset. The average age of the combined population was 51.8 ± 11.7 years (mean ± standard deviation [SD]) and the mean duration since diagnosis was 11.3 ± 9.5 years. Majority of subjects reported having previous back surgery (85.4%) and were diagnosed with FBSS (81.8%). Due to minor differences in inclusion criteria between SENZA-EU and SENZA-RCT studies, such as requirement of back pain with VAS score ≥5 cm regardless of leg pain in the former study and requirement of both back and leg pain VAS scores ≥5 cm in the latter study, the baseline average back pain VAS score (mean ± standard error of the mean [SEM])was 1 cm higher than SENZA-EU study (8.4 ± 0.2 cm compared with 7.5 ± 0.1 cm), while the average leg pain score was 2 cm lower (5.1 ± 0.4 cm compared with 7.1 ± 0.2 cm). In the combined dataset, 39.5% of subjects were taking >90 MME dose of opioids prior to 10 kHz SCS treatment; 48.0% in SENZA-RCT and 27.8% in SENZA-EU.
Mean opioid reduction
Further analyses on mean opioid reduction, reduction in pain intensity scores and classification of subjects based on opioid dose were carried out in subjects with information on opioid dose. At baseline, dose information was available in 75 subjects in SENZA-RCT, 54 subjects in SENZA-EU and 129 subjects in the combined dataset (Fig. 1). At 12-month follow-up, dose information was available in 68 subjects in SENZA-RCT, 54 subjects in SENZA-EU and 122 subjects in the combined data set (Fig. 1).
As illustrated in Fig. 2, though the primary endpoint of the studies was pain scores and opioid reduction was not emphasized in the study, decreases in opioid dosage were noted in the subjects following 10 kHz SCS treatment. In both studies, there was a reduction in the mean opioid dose 12-months following 10 kHz SCS therapy compared with baseline (Fig. 2A). In the combined dataset, there was a 41.0% reduction (p < 0.001) in the mean opioid dose at 12 months from 104.2 ± 9.0 MME (N = 129) to 61.4 ± 6.9 MME (N = 122). Separately, in the SENZA-EU study, the mean opioid dose at 12 months post-10 kHz SCS therapy significantly reduced from 92.3 ± 15.6 MME (N = 54) to 28.1 ± 6.0 MME (N = 54, p < 0.001). Whereas in the SENZA-RCT study, the mean opioid dose significantly decreased from 112.7 ± 10.5 MME (N = 75) to 87.9 ± 10.3 MME (N = 68) at 12 months post-10 kHz SCS therapy (p < 0.001).
Pain response
When the studies were compared for pain reduction following 10 kHz SCS therapy, there were significant reductions in pain relief (VAS score) across both back and leg pain (Fig. 2B, p < 0.001). Overall in the combined dataset, there was a 67.9% reduction in back pain from 7.8 ± 0.1 cm (N = 129) to 2.5 ± 0.2 cm (N = 122) and 68.2% reduction in leg pain from 6.3 ± 0.2 cm (N = 129) to 2.0 ± 0.2 cm (N = 122) reported by subjects that were opioid users at 12 months post-10 kHz SCS therapy compared with baseline.
Proportion of subjects and mean MME
Changes in medication and opioid doses (MME) are shown in Table 2 and Fig. 3. In the combined dataset at 12 months post-10 kHz SCS therapy, 27.0% (N = 33/122) of subjects were taking no opioids, 23.0% (N = 28/122) of subjects were on >90 MME compared with only 1.6% (N = 2/129) and 39.5% (N = 51/129) at baseline, respectively (Fig. 3E, p < 0.0001). Across all doses in both SENZA-EU and SENZA-RCT, there was a significant shift towards lower MMEs following 10 kHz SCS treatment (Fig. 3C, p = 3.9 × 10−7 and Fig. 3A, p = 0.046 respectively).
Change in opioid dose categories in SENZA-RCT (top), SENZA-EU (middle) and the combined dataset (bottom). Distribution of subjects by morphine milligram equivalent (MME) doses of opioids and distribution of high-risk (>90 MME) versus lower-risk (≤90 MME) subjects at baseline and 12 months following 10 kHz SCS therapy. BL, baseline; mo, months. ***p < 0.001, *p < 0.05 chi-square test; ###p < 0.001, Fischer’s exact test.
Proportion of subjects ≤90 and >90 MME
Figure 3B,D,F show the proportion of subjects in doses ≤90 MME and >90 MME categories. Overall there was a significant reduction in the percentage of subjects on high-risk MME doses >90 in the combined dataset (Fig. 3F, p = 0.007).
Changes in opioid dose in subjects in high-risk category (>90 MME)
Subjects taking >90 MME of opioids at baseline were then studied further for changes in opioid dose following 10 kHz SCS therapy. At baseline, 36 subjects in SENZA-RCT, 15 subjects in SENZA-EU and 51 subjects in the combined dataset were taking >90 MME of opioids for management of pain (Fig. 1). Across both studies, within the high-risk category (>90 MME) there was a trend towards a reduced opioid dose in most individual subjects 12 months post-10 kHz SCS therapy (Fig. 4A). Twelve out of 15 subjects in SENZA-EU and 11 out of 36 subjects in SENZA-RCT were able to cease or reduce their opioid dose to ≤90 MME at 12 months. Despite more subjects remaining on lower MMEs of opioids compared to baseline, significant pain reduction was reported across both studies following 10 kHz SCS therapy. As shown in Fig. 4B, following 10 kHz SCS therapy, subjects initially on >90 MME dose of opioids at baseline reported a 72.4% reduction in back pain from 8.7 ± 0.3 cm (N = 15) to 2.4 ± 0.6 cm (N = 15) and 72.0% reduction in leg pain from 5.0 ± 0.8 cm (N = 15) to 1.4 ± 0.5 cm (N = 15) in SENZA-EU and a 68.9% reduction in back pain from 7.4 ± 0.2 cm (N = 36) to 2.3 ± 0.3 cm (N = 36) and 73.6% reduction in leg pain from 7.2 ± 0.3 cm (N = 36) to 1.9 ± 0.3 cm (N = 36) in SENZA-RCT.
Change in opioid dose by subject in patients with a baseline MME >90 (A) and change in patient-reported back and leg pain (B) in SENZA-RCT (left) and SENZA-EU (right) at baseline and 12 months following 10 kHz SCS therapy. Bars show the mean ± SEM. BL, baseline; mo, months. ***p < 0.001, paired t-test.
Mean opioid reduction in high-risk category (>90 MME)
For subjects on high doses of opioids prior to treatment (>90 MME), in the combined dataset, 12 months post-10 kHz SCS therapy resulted in a 45.9% reduction in the mean opioid dose at follow-up from 196.8 ± 14.2 to 106.5 ± 14.1 (N = 51 for both baseline and follow-up, Fig. 5A, p < 0.0001). In these subjects, significant reductions in both back and leg pain were recorded, with subjects reporting on average a 70.5% reduction in back pain and 73.8% reduction in leg pain at 12 months (N = 51 for both baseline and follow-up, Fig. 5B, both p < 0.001).
Discussion
In North America, the opioid crisis is still at its peak55. Mortality rates associated with prescription opioid-related addiction and overdose have surpassed motor-vehicle accidents and patients with Human Immunodeficiency Virus, affecting people from all socio-economic classes55. Clear evidence is now available that demonstrates the rate of overdose of prescription opioids is directly proportional to the prescribed dose56. Despite efforts from regulatory bodies and clinical guidelines, opioids continue to be prescribed for patients with chronic pain conditions. In 2016, the CDC released its guideline on prescribing opioids for chronic pain and recommended avoiding or carefully justifying doses of above 90 MME29. Patients who do not achieve adequate pain relief and function at this dose are advised to seek alternative pain management options29. This recommendation received widespread criticism for its lack of guidance on suitable alternative options, leaving prescribers and patients with a dilemma regarding how to manage pain57. Patients are feeling left without options, and their quality of life is suffering as a consequence58.
Despite clear guidance on maximum doses of opioids and the rationale for high-dose opioids becoming weaker, patients continue to rely on doses > 90 MME for pain management. A recent retrospective analysis of 1,066 primary health care records of patients prescribed opioids for chronic non-cancer pain, 9.7% were receiving ≥90 MME59. In 2012, the CDC reported that 10% of patients on prescription opioids were receiving a high-dose (>100 MME) from one doctor, and of those who overdosed, 40% were also prescribed their pain relief from a single doctor29. Surveys have reported that 70% of long-term opioid users suffer from a chronic disease or disability which limits their daily function29,60. Assuming 10% of these patients may be on high-doses to maintain an adequate level of function and pain relief, feasible alternative options are required to reduce the risk of overdose. Clearly, patients suffering from CLBP and leg pain need evidence-based, safe and effective alternatives to long term opioid therapy.
In the current study, patients had CLBP and leg pain for an average of 11.3 ±0.8 years. They experienced significant baseline pain, despite being managed by prescription opioid therapy. Over a third (39.3%) of subjects were taking > 90 MME of opioids at baseline. 10 kHz SCS therapy was successful in reducing the mean opioid dose and patient reported pain at 12 months. Figure 2 shows following 10 kHz SCS therapy, the mean dose was reduced below 90 MME to 61.4 MME in the combined dataset. Despite a substantial reduction in the mean opioid dose, 10 kHz SCS therapy significantly improved patient-reported back and leg pain at the 12-month follow-up.
Not only was 10 kHz SCS therapy able to reduce the overall dose of opioids used by subjects with CLBP and leg pain, it reduced the proportion of subjects requiring high-risk doses >90 MME per day at the same time. Figure 3 shows in both studies fewer subjects remained on >90 MME per day, and more subjects were able to withdraw their opioid altogether following 10 kHz SCS therapy. In the combined dataset, nearly 80% of the subjects were now on dosing range associated with a reduced risk doses of opioids, with over 20% shifting from >90 MME to <90 MME at follow-up. The dose reduction was observed across individual subjects, as shown in Fig. 4, with most of them demonstrating a reduction in dose at 12 months. A reduction in pain with a reduction in opioid dose is a clinically important finding, demonstrating 10 kHz SCS therapy is not only an alternative to opioid therapy, regardless of high- or low-dose, it is able to provide more effective pain relief than baseline opioid therapy.
Interestingly, compared to SENZA-RCT, higher reductions in opioid dose and higher proportion of subjects taking lower doses of opioids at 12 months were observed in the SENZA-EU study. There may be multiple factors contributing to these observed differences. While SENZA-EU was a prospective, single-arm study designed to assess the efficacy of 10 kHz SCS system, SENZA-RCT was a randomized controlled trial designed to compare 10 kHz SCS with traditional SCS in order to support regulatory approval. Thus, the SENZA-RCT was highly focused on pain management rather than opioid reduction, whereas more emphasis was placed on weaning SENZA-EU subjects off of opioids. In SENZA-RCT, subjects were allowed to continue on stable doses of opioids but were considered as treatment failures regardless of pain scores (VAS) if they increased opioid dose. Related, there were also differences in pain management practice between study centers in SENZA-EU and SENZA-RCT. Investigators from SENZA-EU study actively encouraged the subjects to titrate and taper their opioid dose, whereas investigators from SENZA-RCT did not actively encourage opioid reduction. Lastly, chronic pain patients who are on long term opioids but still have high pain scores underscores the observation that opioids seem to have little benefit in improving chronic pain, especially as measured by VAS, yet are difficult to discontinue. Over the past decade, especially in the US, patients remained on opioids despite their dubious clinical benefit. This may have translated into clinical management changes that were not part of the goals of the RCT or EU studies.
In the past few years, several studies have documented lack of clinically relevant efficacy with the use of opioids for chronic non-cancer pain and highlighted the harmful side effects of using opioids61,62,63,64,65,66,67. The CDC guidelines published in 2016, recommended caution when increasing the opioid doses above 50 MME and avoiding opioid doses above 90 MME29. To prescribe safe doses of opioids and to wean the chronic pain patients off opioids, pain management specialists need durable alternatives for management of chronic pain. The current findings indicate that 10 kHz SCS can be considered as an alternative to opioids. A case-controlled retrospective study by researchers from Boston, USA showed that opioid usage was reduced or eliminated in 71% (n = 15/21) of patients treated with 10 kHz SCS for back and/or leg pain68. Another retrospective study by pain management specialist from Perth, Australia showed that percentage of patients not taking opioids increased nearly 2-fold in patients treated with 10 kHz SCS therapy and 70% of patients either eliminated or reduced their opioid dose at last follow-up. More importantly, average opioid dose in patients taking high dose opioids at baseline was reduced by 47% at last follow-up69. Current study further confirms the findings from the retrospective studies and shows that 10 kHz SCS can provide long-lasting pain relief and allow reduction or elimination of opioids even in patients who were prescribed high-risk doses. Pain physicians need to take note of the findings from the studies and encourage patients treated with 10 kHz SCS to discontinue opioids. They need to include reduction or elimination of opioid use as part of treatment plan when contemplating 10 kHz SCS. Establishing opioid reduction as part of the treatment goal even prior to the trial could help in setting up expectations and result in meaningful improvement in quality of life.
The main limitation of this study is that it involved post hoc analysis of the dataset and there was no prospectively defined statistical analysis plan (SAP) for predetermined outcomes. Although the combined dataset in the study included 129 subjects with opioid dose information, there were differences between the two studies and the number of subjects taking > 90 MME was relatively small. Additional prospective studies and real-world studies may be needed to validate the findings of this analysis. Nevertheless, current analysis shows that despite the differences between the two studies and the patient cohorts, 10 kHz SCS therapy resulted in significant reduction in opioid dosage while maintaining pain relief when the data was analysed individually and when combined.
Based on the results of this study, 10 kHz SCS therapy has the potential to assist physicians and patients in the wake of the opioid crisis, offering a non-pharmacological alternative to the suite of pain management tools for patients on high-risk doses of opioids (>90 MME).
Methods
SENZA-EU
As described previously, SENZA-EU was a prospective, multicentre, open-label study in 83 subjects conducted at two European centers including AZ Nikolaas Pain Centre, St Niklaas Belgium and Guy’s and St Thomas’ Pain and Neuromodulation Centre, London, United Kingdom50. The study protocol and informed consent forms were approved by each study site’s ethic committee (Commissie voor Medische Ethiek AZ Nikolaas, Belgium and NRES Northern & Yorkshire REC, UK respectively). Local clinical research and data protection regulations, good clinical practice guidelines (ISO 14155), and the Declaration of Helsinki were followed during the study. Selection bias in the study was minimized by the involvement of independent medical monitors, which assured high level diligence for the recruitment of subjects. Inclusion and exclusion criteria for enrollment of patients has been previously reported50. Briefly, patients who signed informed consent forms, with chronic back pain (defined as lumbo-sacral pain) with or without leg pain with pain intensity of ≥5.0 cm (average score over the last 30 days) on the 0 to 10 cm, visual analog scale (VAS), who were ≥18 years of age and who were candidates for SCS were included in the study. Patients were evaluated at baseline and then received a trial 10 kHz SCS (Senza® SCS system Nevro Corp., Redwood City, CA, USA) therapy for 14–30 days50. Patients who had at least 50% reduction in pain intensity received a permanent IPG implant and followed for 24 months with assessments at 1, 3, 6, 12, and 24 months50. Subjects were enrolled in the study between August 2009 and February 2011. The follow-up for the last implanted patient was completed in April 2013. The study design allowed adjustment of stimulation parameters and clinical judgement-based adjustment to pain medication dosage throughout the follow-up period50.
SENZA-RCT
SENZA-RCT was a prospective, randomized, controlled trial in 198 patients across 10 centers in the U.S., designed to assess 10 kHz SCS therapy as compared with traditional low frequency SCS51. The study was conducted in compliance with the U.S. Code of Federal Regulations and recommendations guiding physicians in biomedical research by the 18th World Medical Assembly, Helsinki, Finland. The study protocol and informed consent forms were approved by each study site’s institutional review board (Western Institutional Review Board, Puyallup, Washington; Forsyth Medical Center Institutional Review Board, Winston-Salem, North Carolina). Selection bias in the study was addressed by the involvement of independent medical monitors for the recruitment of subjects and randomized assignment of subjects to the 10 kHz SCS group. Patients who signed informed consent forms and were candidates for SCS with chronic, intractable back and/or leg pain, refractory to conservative therapy for a minimum of 3 months with an average back pain intensity of 5 or greater out of 10 cm on the VAS, average leg pain intensity of 5 or greater out of 10 cm on the VAS, an Oswestry Disability Index (ODI) score of 41 to 80 out of 100 were randomized in a 1:1 ratio to receive stimulation with an investigational 10 kHz SCS therapy system (Senza® System; Nevro Corp., Redwood City, USA) or a commercially available SCS system (Precision Plus System; Boston Scientific, USA)51. Patients underwent a percutaneous trial lasting up to 14 days51. Those with ≥40% back pain reduction from baseline proceeded to permanent implantation and subjects with at least 50% pain reduction were considered as ‘responders’51. Subjects were enrolled between June 2012 and December 2012. Following randomization, subjects were implanted with 10 kHz SCS system and followed through 12 months, which concluded in February 2014. Oral analgesics were stabilized from 28 days before enrollment until activation of the implanted SCS system, excluding allowances for perioperative analgesics51. Adjustments were then allowed under the guidance of a study investigator as medically necessary, but the subjects who increased their opioid dose were considered as ‘non-responders’ regardless of their pain relief51. Assessments were performed at scheduled visits (baseline; 1, 3, 6, 9, and 12 months)51. Data from subjects implanted with 10 kHz SCS system was included in the current analysis.
Outcomes
Both studies, SENZA-RCT and SENZA-EU assessed back and leg pain intensity as patient reported VAS (0 to 10 cm, no pain-to-worst pain imaginable) at baseline and 12 months. Opioid medication dose was assessed in both studies at baseline and 12 months as (milligrams morphine equivalent, MME) and the changes in opioid consumption were recorded as “eliminated”, “decreased”, “no change” or “increased”. The assessments were comparable between the studies and therefore it was possible to combine the data from the studies.
Data collection and statistical analysis
Demographic analyses were performed in the implanted patients with data at 12 months and with basic information on opioid usage (Fig. 1). Further analyses such as mean pain intensity (VAS), mean dose of opioids (MME) and categorization of subjects based on opioid dose were performed only in data from patients with opioid dose information. Information on opioid dose was lost to follow-up at 12-month assessment in 7 subjects from SENZA-RCT study. The missing subjects were included in the calculation of mean, SEM and categorization based on opioid dose at baseline and were excluded from analyses at 12-month assessment. Two-tailed paired t-test was used for continuous variables, such as VAS and mean opioid dose and Chi-square test and Fischer’s exact test for comparison of the frequency of proportions between two groups. P-value less than or equal to 5% (p < 0.05) was considered to be statistically significant. Statistical analysis was performed using Microsoft Excel (paired t-test) and Graph Pad (Chi-square test and Fischer’s exact test) software.
Data Availability
All data generated or analysed during this study are included in this published article.
References
Fayaz, A., Croft, P., Langford, R. M., Donaldson, L. J. & Jones, G. T. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 6, e010364, https://doi.org/10.1136/bmjopen-2015-010364 (2016).
Schneiderhan, J., Clauw, D. & Schwenk, T. L. Primary Care of Patients With Chronic Pain. JAMA 317, 2367–2368, https://doi.org/10.1001/jama.2017.5787 (2017).
Elzahaf, R. A., Tashani, O. A., Unsworth, B. A. & Johnson, M. I. The prevalence of chronic pain with an analysis of countries with a Human Development Index less than 0.9: a systematic review without meta-analysis. Curr Med Res Opin 28, 1221–1229, https://doi.org/10.1185/03007995.2012.703132 (2012).
Langley, P. C. The prevalence, correlates and treatment of pain in the European Union. Curr Med Res Opin 27, 463–480, https://doi.org/10.1185/03007995.2010.542136 (2011).
McBeth, J. & Jones, K. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol 21, 403–425, https://doi.org/10.1016/j.berh.2007.03.003 (2007).
Pergolizzi, J. et al. The development of chronic pain: physiological CHANGE necessitates a multidisciplinary approach to treatment. Curr Med Res Opin 29, 1127–1135, https://doi.org/10.1185/03007995.2013.810615 (2013).
Treede, R. D. et al. A classification of chronic pain for ICD-11. Pain 156, 1003–1007, https://doi.org/10.1097/j.pain.0000000000000160 (2015).
Almeida, M., Saragiotto, B., Richards, B. & Maher, C. G. Primary care management of non-specific low back pain: key messages from recent clinical guidelines. Med J Aust 208, 272–275 (2018).
Andersson, G. B. Epidemiological features of chronic low-back pain. Lancet 354, 581–585, https://doi.org/10.1016/S0140-6736(99)01312-4 (1999).
Chou, R. et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 147, 478–491 (2007).
Disease, G. B. D., Injury, I. & Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259, https://doi.org/10.1016/S0140-6736(17)32154-2 (2017).
Kuijpers, T. et al. A systematic review on the effectiveness of pharmacological interventions for chronic non-specific low-back pain. Eur Spine J 20, 40–50, https://doi.org/10.1007/s00586-010-1541-4 (2011).
Qaseem, A., Wilt, T. J., McLean, R. M. & Forciea, M. A. & Clinical Guidelines Committee of the American College of, P. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med 166, 514–530, https://doi.org/10.7326/M16-2367 (2017).
Nadler, S. F. Nonpharmacologic management of pain. J Am Osteopath Assoc 104, S6–12 (2004).
Kea, B., Fu, R., Lowe, R. A. & Sun, B. C. Interpreting the National Hospital Ambulatory Medical Care Survey: United States Emergency Department Opioid Prescribing, 2006-2010. Acad Emerg Med 23, 159–165, https://doi.org/10.1111/acem.12862 (2016).
Knaggs, R. Low back pain clinical guidelines: similarities and divergent views across the pond. Br J Pain 11, 70, https://doi.org/10.1177/2049463717701809 (2017).
Chaparro, L. E. et al. Opioids compared to placebo or other treatments for chronic low-back pain. The Cochrane database of systematic reviews, Cd004959, https://doi.org/10.1002/14651858.CD004959.pub4 (2013).
Jamison, R. N., Raymond, S. A., Slawsby, E. A., Nedeljkovic, S. S. & Katz, N. P. Opioid therapy for chronic noncancer back pain. A randomized prospective study. Spine (Phila Pa 1976) 23, 2591–2600 (1998).
Moulin, D. E. et al. Randomised trial of oral morphine for chronic non-cancer pain. Lancet 347, 143–147 (1996).
Farrar, J. T., Young, J. P. Jr., LaMoreaux, L., Werth, J. L. & Poole, R. M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94, 149–158 (2001).
Duthie, D. J. & Nimmo, W. S. Adverse effects of opioid analgesic drugs. Br J Anaesth 59, 61–77 (1987).
Deyo, R. A., Von Korff, M. & Duhrkoop, D. Opioids for low back pain. BMJ 350, g6380, https://doi.org/10.1136/bmj.g6380 (2015).
Benyamin, R. et al. Opioid complications and side effects. Pain Physician 11, S105–120 (2008).
Fields, H. L. The doctor’s dilemma: opiate analgesics and chronic pain. Neuron 69, 591–594, https://doi.org/10.1016/j.neuron.2011.02.001 (2011).
Mafi, J. N., McCarthy, E. P., Davis, R. B. & Landon, B. E. Worsening trends in the management and treatment of back pain. JAMA Intern Med 173, 1573–1581, https://doi.org/10.1001/jamainternmed.2013.8992 (2013).
Tompkins, D. A. & Campbell, C. M. Opioid-induced hyperalgesia: clinically relevant or extraneous research phenomenon? Curr Pain Headache Rep 15, 129–136, https://doi.org/10.1007/s11916-010-0171-1 (2011).
Chou, R., Ballantyne, J. C., Fanciullo, G. J., Fine, P. G. & Miaskowski, C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 10, 147–159, https://doi.org/10.1016/j.jpain.2008.10.007 (2009).
Ballantyne, J. C. & Mao, J. Opioid therapy for chronic pain. N Engl J Med 349, 1943–1953, https://doi.org/10.1056/NEJMra025411 (2003).
Dowell, D., Haegerich, T. M. & Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep 65, 1–49, https://doi.org/10.15585/mmwr.rr6501e1 (2016).
Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage, https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf.
Verrills, P., Sinclair, C. & Barnard, A. A review of spinal cord stimulation systems for chronic pain. J Pain Res 9, 481–492, https://doi.org/10.2147/JPR.S108884 (2016).
Deer, T. R. et al. The appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation Appropriateness Consensus Committee. Neuromodulation 17, 599–615, discussion 615, https://doi.org/10.1111/ner.12204 (2014).
North, R. B. Neurostimulation for pain of spinal origin. Clin Neurosurg 53, 272–278 (2006).
Jang, H. D. et al. Analysis of failed spinal cord stimulation trials in the treatment of intractable chronic pain. J Korean Neurosurg Soc 43, 85–89, https://doi.org/10.3340/jkns.2008.43.2.85 (2008).
Russo, M. & Van Buyten, J. P. 10-kHz High-Frequency SCS Therapy: A Clinical Summary. Pain Med 16, 934–942, https://doi.org/10.1111/pme.12617 (2015).
North, R. B., Kidd, D., Shipley, J. & Taylor, R. S. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery 61, 361–368, discussion 368–369, https://doi.org/10.1227/01.NEU.0000255522.42579.EA (2007).
North, R. B., Kidd, D. H., Farrokhi, F. & Piantadosi, S. A. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery 56, 98–106, discussion 106–107 (2005).
Kupers, R. C. et al. Spinal cord stimulation in Belgium: a nation-wide survey on the incidence, indications and therapeutic efficacy by the health insurer. Pain 56, 211–216 (1994).
Ohnmeiss, D. D., Rashbaum, R. F. & Bogdanffy, G. M. Prospective outcome evaluation of spinal cord stimulation in patients with intractable leg pain. Spine (Phila Pa 1976) 21, 1344–1350, discussion 1351 (1996).
Alo, K. M., Redko, V. & Charnov, J. Four Year Follow-up of Dual Electrode Spinal Cord Stimulation for Chronic Pain. Neuromodulation 5, 79–88, https://doi.org/10.1046/j.1525-1403.2002.02017.x (2002).
Cameron, T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg 100, 254–267 (2004).
Sears, N. C. et al. Long-term outcomes of spinal cord stimulation with paddle leads in the treatment of complex regional pain syndrome and failed back surgery syndrome. Neuromodulation 14, 312–318, discussion 318, https://doi.org/10.1111/j.1525-1403.2011.00372.x (2011).
Kumar, K., Hunter, G. & Demeria, D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery 58, 481–496, discussion 481–496, https://doi.org/10.1227/01.NEU.0000192162.99567.96 (2006).
Deer, T. et al. Success Using Neuromodulation With BURST (SUNBURST) Study: Results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation 21, 56–66, https://doi.org/10.1111/ner.12698 (2018).
Kapural, L. et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology 123, 851–860, https://doi.org/10.1097/ALN.0000000000000774 (2015).
Tiede, J. et al. Novel spinal cord stimulation parameters in patients with predominant back pain. Neuromodulation 16, 370–375, https://doi.org/10.1111/ner.12032 (2013).
Van Buyten, J. P., Al-Kaisy, A., Smet, I., Palmisani, S. & Smith, T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation 16, 59–65, discussion 65–56, https://doi.org/10.1111/ner.12006 (2013).
Grider, J. S. et al. Effectiveness of Spinal Cord Stimulation in Chronic Spinal Pain: A Systematic Review. Pain Physician 19, E33–54 (2016).
Amirdelfan, K. et al. A proposed definition of remission from chronic pain, based on retrospective evaluation of 24-month outcomes with spinal cord stimulation. Postgrad Med, 1–9, https://doi.org/10.1080/00325481.2019.1592401 (2019).
Al-Kaisy, A. et al. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med 15, 347–354, https://doi.org/10.1111/pme.12294 (2014).
Kapural, L. et al. Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results From a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery 79, 667–677, https://doi.org/10.1227/neu.0000000000001418 (2016).
Amirdelfan, K. et al. Long-term quality of life improvement for chronic intractable back and leg pain patients using spinal cord stimulation: 12-month results from the SENZA-RCT. Qual Life Res 27, 2035–2044, https://doi.org/10.1007/s11136-018-1890-8 (2018).
Deer, T. et al. Spinal cord stimulation as a method of reducing opioids in severe chronic pain: a case report and review of the literature. W V Med J 106, 56–59 (2010).
Taylor, R. S., Van Buyten, J. P. & Buchser, E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factors. Spine (Phila Pa 1976) 30, 152–160, https://doi.org/10.1097/01.brs.0000149199.68381.fe (2005).
Voon, P., Karamouzian, M. & Kerr, T. Chronic pain and opioid misuse: a review of reviews. Subst Abuse Treat Prev Policy 12, 36, https://doi.org/10.1186/s13011-017-0120-7 (2017).
Manchikanti, L. et al. Responsible, Safe, and Effective Prescription of Opioids for Chronic Non-Cancer Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 20, S3–S92 (2017).
Kominek, C. Current and emerging options to combat the opioid epidemic. Am J Manag Care 24, S207–S214 (2018).
Dare, D. ‘I have no quality of life’: Opioid laws have unintended effects, says chronic pain patient, https://www.wate.com/news/investigations/-i-have-no-quality-of-life-opioid-laws-have-unintended-effects-says-chronic-pain-patient/1405787454 (2018).
Philpot, L. M. et al. Controlled Substance Agreements for Opioids in a Primary Care Practice. J Pharm Policy Pract 10, 29, https://doi.org/10.1186/s40545-017-0119-5 (2017).
Mack, K. A., Zhang, K., Paulozzi, L. & Jones, C. Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J Health Care Poor Underserved 26, 182–198, https://doi.org/10.1353/hpu.2015.0009 (2015).
Busse, J. W. et al. Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-analysis. JAMA 320, 2448–2460, https://doi.org/10.1001/jama.2018.18472 (2018).
Els, C. et al. High-dose opioids for chronic non-cancer pain: an overview of Cochrane Reviews. The Cochrane database of systematic reviews 10, CD012299, https://doi.org/10.1002/14651858.CD012299.pub2 (2017).
Els, C. et al. Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: an overview of Cochrane Reviews. The Cochrane database of systematic reviews 10, CD012509, https://doi.org/10.1002/14651858.CD012509.pub2 (2017).
Harbaugh, C. M. et al. Association Between Long-term Opioid Use in Family Members and Persistent Opioid Use After Surgery Among Adolescents and Young Adults. JAMA Surg, e185838, https://doi.org/10.1001/jamasurg.2018.5838 (2019).
Blanco, C. & Volkow, N. D. Management of opioid use disorder in the USA: present status and future directions. Lancet 393, 1760–1772, https://doi.org/10.1016/S0140-6736(18)33078-2 (2019).
Klaess, C. C. et al. Pain Management Pillars for the Clinical Nurse Specialist: Summary of National Association of Clinical Nurse Specialists Opioid Pain Management Task Force. Clin Nurse Spec 33, 136–145, https://doi.org/10.1097/NUR.0000000000000449 (2019).
Hamnvik, O. R., Alford, D. P., Ryan, C. T., Hardesty, I. T. & Drazen, J. M. NEJM Knowledge+ Pain Management and Opioids - A New Adaptive Learning Module. N Engl J Med 380, 1576–1577, https://doi.org/10.1056/NEJMe1903798 (2019).
DiBenedetto, D. J., Wawrzyniak, K. M., Schatman, M. E., Kulich, R. J. & Finkelman, M. 10 kHz spinal cord stimulation: a retrospective analysis of real-world data from a community-based, interdisciplinary pain facility. J Pain Res 11, 2929–2941, https://doi.org/10.2147/JPR.S188795 (2018).
Salmon, J. High Frequency Spinal Cord Stimulation at 10 kHz for Widespread Pain: A Retrospective Survey of Outcomes from Combined Cervical and Thoracic Electrode Placements. Postgrad Med, https://doi.org/10.1080/00325481.2019.1587564 (2019).
Acknowledgements
Authors thank Dr. Madhuri Bhandaru for the help in preparation of the figures for the manuscript.
Author information
Authors and Affiliations
Contributions
Authors A.A., J.V.B., R.C., D.C., B.G., J.S., A.R., L.K., C.P. and K.A. were involved in design, conceptualization, data collection, analysis and writing of the manuscript. All the authors have reviewed and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing Interests
Drs Adnan Al-Kaisy, Jean-Pierre Van Buyten, Kasra Amirdelfan and Leonardo Kapural, are Consultants to Nevro Corp., Redwood City, CA, USA. Dr David Caraway, Mr. Bradford Gliner, Dr. Jeyakumar Subbaroyan, and Dr. Anand Rotte are employees of Nevro Corp., Redwood City, CA, USA. Mr Roy Carganillo has no competing interests. Funding was provided to Dr. Catherine Panwar in her capacity as a medical writer by Nevro Corp., Redwood City, CA, USA, for the preparation of this manuscript.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Kaisy, A., Van Buyten, JP., Carganillo, R. et al. 10 kHz SCS therapy for chronic pain, effects on opioid usage: Post hoc analysis of data from two prospective studies. Sci Rep 9, 11441 (2019). https://doi.org/10.1038/s41598-019-47792-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47792-3
This article is cited by
-
Moderne Rückenmarkstimulation - frühere Limitationen sind Geschichte
Schmerzmedizin (2021)
-
High-frequency spinal cord stimulation at 10 kHz for the treatment of painful diabetic neuropathy: design of a multicenter, randomized controlled trial (SENZA-PDN)
Trials (2020)
-
Improving care of chronic pain patients with spinal cord stimulator therapy amidst the opioid epidemic
Neurological Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.