Abstract
Alzheimer’s disease is associated with the production of Cu rich aβ fibrils. Because monitoring the changes in Cu level of organs has been proposed to follow the evolution of the disease, we analyzed the copper isotopic composition of serum and brain of APPswe/PSEN1dE9 transgenic mice, a model of Alzheimer’s disease, and wild-type (WT) controls. Serum composition of 3, 6, 9 and 12-month-old mice, as well as the composition of 9 brains of 12-month-old mice are reported. In WT mice, brains were ~1‰ isotopically heavier than serum, and the Cu isotopic composition of the serum was isotopically different between males and females. We propose that this effect of sex on the Cu isotopic budget of the serum may be related to a difference of Cu speciation and relative abundance of Cu carriers. Brains of APPswe/PSEN1dE9 mice were slightly lighter than brains of WT mice, while not statistically significant. This trend may reflect an increase of Cu(I) associated with the formation of Aβ fibrils. The Cu isotopic composition of the brains and serum were correlated, implying copper transport between these two reservoirs, in particular a transfer of Cu(I) from the brain to the serum. Altogether, these data suggest that Cu stable isotopic composition of body fluid may have the potential to be used as detection tools for the formation of Aβ fibrils in the brain, but further work has to be done.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is one of the main causes of death in high-income countries and the fifth leading cause of death of US citizen over 651. It is characterized by a loss of brain function affecting reasoning, memory and comportment. The major physiological features of AD are the formation of neurofibrillary tangles by neuronal accumulations of abnormal hyperphosphorylated tau filaments and the formation of senile plaques by extracellular deposits of amyloid β (Aβ) fibrils, mostly the 1 to 42 peptide (Aβ1-42). Early diagnosis of AD is a major challenge and current work focuses on the detection of excess of total tau and Aβ in cerebrospinal fluid, and on using imaging of the brain by positron emission tomography and magnetic resonance imaging (MRI) for Aβ plaques (e.g. refs2,3,4).
The homeostasis of metals, such as Fe, Zn, Al or Cu, is modified in patients with AD (e.g. ref.5). These metals are concentrated in the Aβ plaques and in consequences in the neocortex of AD patients (e.g. refs6,7). The changes in metal homeostatis in AD have been tested as a diagnosis tool as the concentrations in the Aβ plaques should impact the concentration in other organs and fluids (e.g. red blood cells and serum). However, studies focussing on elemental abundance measurements in the serum of AD patients are inconsistent, showing overall Zn abundance decreasing5, increasing8 or unchanged9. Elemental abundance variations in the serum may be affected by factors unrelated to AD, such as intestinal absorption of Zn10, which greatly complicates the interpretation of this data for AD diagnosis.
We and others recently showed that the natural stable isotopic composition of metals such as Zn, Cu and Fe naturally varies between body organs11,12,13. In particular, blood (red blood cells and/or serum) are isotopically distinct in Zn, Cu and Fe from the brain11,12,13). Isotopic fractionation is due to the difference of bonding environments of the elements between different organs12. For example, in normal conditions, Zn is preferentially bound to cysteine-rich protein (metallothionein) in the brain, which preferentially concentrates light isotopes, while in red blood cells (RBCs) Zn is bound to histidine-rich proteins (carbonic anhydrase) that concentrate heavy isotopes12,13. On the other hand, in Aβ plaques Zn is bonded to isotopically heavy amino-acids (histidine and glutamate)14 and therefore should be isotopically heavier in AD patients. Following this logic, Moynier et al.15 studied the Zn isotopic composition of brain and blood samples during the development of transgenic APPswe/PSEN1dE9 and wild type controls mice and found that, as predicted, Zn was isotopically heavier in the brain of AD mice than in age-matched wild types. However, the isotopic variations among serum samples were not large enough to allow for the detection of an isotopic effect.
While Zn is only present under one redox state (Zn2+), Cu is present in both 1+ and 2+ forms. The presence of redox variations of Cu potentially enhances the magnitude of isotopic variations compared to Zn (e.g. see ab initio calculations for various Cu(I) and Cu(II) species in ref.16). In addition, Balter et al.13 showed that Cu is isotopically heavier in the brain of mice than in the whole blood (by about 1 permil for the 65Cu/63Cu ratio) suggesting that, as done with Zn isotopes15, Cu isotopes could be used as tracers of metal dyshomeostasis in the brain. This potential has been confirmed by the discovery of Cu isotopic fractionation in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis compared to control patients17.
In the case of AD, Cu participates in the structure of the Aβ plaques; Cu is enriched in the plaques in mice (e.g. ref.18) and humans19 compared to normal adjacent brain zones. Copper bound to the Aβ plaques could be formed under both +I and +II form20,21. In the brain copper is principally stored in proteins under its reduced form Cu(I) binded to metallothionein and glutathione both cystetine-rich proteins22,23 and a fraction of Cu(II) is present in synapses24. The accumulation of Cu(II) in Aβ plaques changes the speciation of Cu in the brain in the brain which may affect Cu in body fluids such as blood, as suggested from the global Cu level increase observed in the serum of AD patients25. This change may be traceable with Cu stable isotopic composition. Furthermore, formation of Aβ plaques affect the global redox state of Cu in the brain, and can lead to the increase of reactive oxygen species, oxidative stress and loss of cognitive functions (e.g. ref.26).
Here we tested whether Cu stable isotopic composition of serum and brain are modified by the formation of Aβ plaques in mice with late stage AD. We report the Cu isotopic composition of the serum from 23 transgenic APPswe/PSEN1dE927,28 and wild type (WT) mice at various stages (3 month, 6 month, 9 month and 12 month). In addition, we report the Cu isotopic composition of the brains of 9 of the 12-month mice.
Method
Ethical statement
All measurements reported here are analyses of existing samples collected under a protocol approved by the Washington University Animal Studies Committee (ASC). The Procedures were approved by the Washington University Animal Studies Committee (ASC), (Protocol #20120013).
Mice
The animals used for this study correspond to some of the mice reported in Moynier et al.15, for which we still had samples after the analysis of Zn isotopic composition: APPswe/PSEN1dE9 (AD) and wild-type (WT) littermate controls on a C57BL/6 and C3H mixed background. At 3, 6, 9 and 12 months, blood samples were collected. After 12 months, the mice were killed and their brains were collected. All mice were housed under specific pathogen-free conditions in the Washington University animal facilities in accordance with institutional guidelines, and were given the same diet from birth. The diet is the one reported in Moynier et al.15. AD mice were originally obtained from The Jackson Laboratory, then bred and housed in the Washington University animal facilities by crossing to B3C3F1/J (from The Jackson Laboratory).
Sample collection
Samples preparation was described in Moynier et al.15. Mice were anesthetized with ketamine and xylazine. Anesthesia was assessed by the toe pinch method. The thoracic cage was opened and blood was collected in polypropylene microtubes by a cardiac puncture. The vena cava was sectioned under the kidneys, and PBS was injected in the left ventricle for organ perfusion. Death was assessed by cervical dislocation. Serum was separated from red blood cells by centrifugation. After blood collection, mice were perfused by injecting phosphate buffered saline (PBS) through the heart with a dissected hepatic vein to remove blood from organs. Brains were harvested using instruments in stainless steel. All samples were stored in polypropylene microtubes or cryogenic vials (Corning). Harvested organs were snap-frozen and kept frozen until dissolution.
Chemical purification and mass-spectrometry
The Cu purification and isotopic measurements were performed at the Institut de Physique du Globe de Paris (IPGP). The cleaning and dissolution of the samples is similar as the one used previously for Zn isotopic measurements15. Brain were cleaned twice using 18.2 MΩ cm water and the brain and serum were dissolved using a mixture of HNO3 (4 times sub-boiled) and Seastar© optima grade H2O2 in closed Teflon beakers for one to two days. This method provides a complete dissolution of the organs with minimum contamination (as opposed to the combustion of the organs in an oven).
Copper is purified by ion exchange chromatography following a procedure adapted from Maréchal et al.29 and similar to what we used recently30,31. The samples are loaded in 7 N HCl on a 1.6 mL AG-MP1 resin. The resin is further washed by passing 8 mL of 7 N HCl and the Cu is collected in 22 mL of 7 N HCl. This procedure is reproduced twice to ensure a clean Cu fraction. Copper isotopic data were measured using the MC-ICP-MS (Thermo-Finnigan Neptune Plus) located at IPGP following the method used in Savage et al.31. Briefly, copper is injected using a glass spray chamber and a 100 μL/min ESI Teflon micro nebulizer. The MC-ICP-MS was operated in low resolution mode with 65Cu and 63Cu collected in the central and L2 faraday cups, respectively.
The sample dilutions were adjusted (to within ~ ±5%) to match the concentration of the standard. The total yield of Cu is >99%. The blank of the full procedure is ~5 ng which is negligible in comparison to the amount of Cu (>200 ng) present in each samples. Typical external reproducibility estimated from numerous replicates was estimated to be better than 50 ppm (2 standard deviation)31.
The isotopic composition of Cu is reported as parts per 1,000 deviations relative to the NIST SRM 976 standard16.:
We used the standard bracketing method that consists of measuring a standard before and after each sample and use the average of the two standards for the normalizing ratio16. During analysis session the standard utilized was IPGP-Cu and relative to NIST SRM 976, the Cu_IPGP standard has δ65Cu = +0.271 ± 0.006‰ (2sd; n = 55). All the data presented here were converted to δ65Cu relative to NIST SRM 976 by adding 0.271.
Statistical analysis
Statistical analysis was performed using GraphPad Prism. Data are represented as box-and-whiskers graphs, with the box always extending from the 25th to 75th percentiles, the line representing the median, and the whiskers showing the minimal and maximal values. Mann Whitney non-parametric tests were used to compare two groups of unpaired data sets. Two-way ANOVAs were performed to test the effect of various factors (age, sex and genotype), followed by Sidak’s multiple comparison test, or Wilcoxon matched-pairs signed rank test for paired data sets.
Results
Distinct copper isotopic composition of serum and brain in wild-type mice
We measured the Cu isotopic data for 75 serum and 9 brain samples, reported in Table S1. The serum samples represent nineteen 3-month-old, twenty-three 6-month-old, twenty 9-month-old and thirteen 12-month-old mice. The nine brains have been taken from 12-month-old mice. The average of the different groups of samples sorted by ages and sex are reported in Table 1.
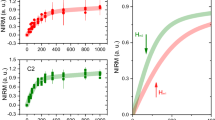
For WT mice, males (δ65Cu = −0.84 ± 0.16, sd) have isotopically lighter serum than females (δ65Cu = 0.44 ± 0.20, sd); the difference is statistically significant (p < 0.0001, Mann Whitney test). This difference is observed at every time point (Fig. 1A,B). The 3-, 6-, and 9-month-old mice have a constant δ65Cu value around −1.1 for males and −0.8 for females. Interestingly, the 12-month old females have a heavier serum isotopic composition (δ65Cu of −0.31 ± 0.19, sd; p = 0.06, Wilcoxon matched-pairs rank test).
Copper isotopic composition of serum and brain in wild type mice. The data are reported as δ65Cu, which is the per mil deviation of the 65Cu/63Cu from the NIST 976 standard. (A) Evolution of δ65Cu in serum of individual mice are shown over time (males are shown in blue and female in red). (B) Pooled data for δ65Cu in serum: boxes extend from the 25th and 75th percentile, the line in the middle of the box represents the median, and the whiskers show the minimum and the maximum. Two-way ANOVA showed a significant effect of sex (p < 0001). p values between males and females are indicated on the graphic for each age (Sidak’s multiple comparison test). Wilcoxon matched-pairs rank test were used to compare females of different ages (p = 0.06 when comparing 3- and 12-month-old females). (C) Comparison of δ65Cu in serum and brain from 12-month-old females. p = 0.06, Wilcoxon matched-pairs rank test.
We next compared the isotopic composition of brain and serum in 12-month-old females. Brains (δ65Cu = 0.63 ± 0.26, sd) are ~1‰ isotopically heavier than serum (p = 0.06, Wilcoxon matched-pairs rank test). Interestingly, the δ65Cu of the brains and associated serum are positively correlated (Figs 1C, 2, r2 = 0.97, slope statistically different from 0 as p = 0.01).
Correlation of the Cu isotopic composition between brain and serum. Data show δ65Cu values from serum of WT and AD 12-month-old female mice. Linear regression showed a significant positive correlation between serum and brain δ65Cu values for WT animals (r2 = 0.97; slope 1.19 ± 0.14, se, p = 0.01), but only a trend for AD animals (r2 = 0.62; slope 0.49 ± 0.44, se, p = 0.38).
Old AD mice have a slightly lighter (but not statistically different) copper isotopic composition in their serum and brain.
We investigated if AD would lead to a different copper isotopic composition in serum and/or brain by analyzing the isotopic composition of organs from the APPswe/PSEN1dE9 transgenic mouse model. The serum of 12-month-old AD females (δ65Cu = −0.59 ± 0.28, sd) was isotopically lighter than the serum of age-matched WT female littermates (δ65Cu of −0.31 ± 0.19, sd), even if the difference is not significant (p = 0.20, Sidak’s multiple comparison test) (Fig. 3B). There was no other significant difference at any age between WT and AD mice in serum. Similarly, the brains of the 12-month-old AD females (δ65Cu = 0.51 ± 0.15, sd) also appear modestly lighter (but not statistically significant) than the brain of the female WT littermate (δ65Cu = 0.63 ± 0.26, sd; see Fig. 3C).
Effect of AD on Cu isotopic composition. (A,B). Data show δ65Cu values from serum of WT and AD mice. Two-way ANOVA showed no significant effect of age or genotype for serum in males (A) or females (B). p values between WT and AD are indicated on the graphic for each age (Sidak’s multiple comparison test). (C) δ65Cu in the brain of 12-month-old females. p = 0.37, Mann-Whitney test.
Discussion
Isotopic difference between brain and serum
Our observation that brains (9 samples) are typically 1‰ heavier than serum (74 samples) in mice is consistent with previous data obtained on a smaller sample pool of samples. Balter et al.13 reported 4 brain samples and 9 whole blood samples from 5-month-old mice with an isotopic difference of ~0.8‰. On the other hand, human serum are heavier than what is reported here for mice by ~0.5‰ (e.g. refs32,33,34,35).
The differences of Zn isotopic composition between organs were previously attributed to the difference of bonding environment12,13. In mice, Zn is isotopically lighter in brain than in serum, which is explained by the fact that Zn is principally bound to cysteine-rich protein in the brain (metallothionein) while it is bound to histidine-rich protein in the serum (albumin)36. Copper shows the reverse isotopic effect (brain heavier than serum). The bonding properties and the associated isotopic fractionation is more complex in the case of Cu than for Zn because of the multiple redox state of Cu (I and II). Copper is principally found as Cu(I) in metallothionein and glutathione in the brain23 and as Cu(II) in synapses24. In human’s serum, Cu is mainly bound to ceruloplasmin (~90%; ref.37) with the rest being bound to β-macroglobulin, albumin, and in some small molecules37,38,39. In ceruloplasmin, Cu is principally found under its +II form and bounds to cysteine and histidine40. It is also found under its +II in β-macroglobulin41, while in albumin it could be under the +I form42. Since the majority of Cu in the brain should be stored under its isotopically light reduced form in metallothionein or glutathione22,23 while it should be mostly found as its isotopically heavy oxidised form in ceruloplasmin in the serum, the clearly isotopically heavier brain is surprising. In the absence of quantitative study on the speciation of Cu in the brain it is not possible to conclude on this topic here, but one possible explanation to this conundrum is that Cu(II) involved in synaptic transmission24 represent a large fraction of the total Cu stored in the brain.
The observation that serum in human (e.g. ref.35) is isotopically heavier than in mouse by ~0.5‰ suggests that the speciation of Cu differs in mice compare to humans. This is supported by previous HPLC-ICPMS measurements on plasma doped with 65Cu37 who found that ceruloplasmin is a more important ligand in human’s plasma compared to mice’s plasma. We can therefore conclude that Cu speciation in mouse serum is different than in human. Therefore, it will be important to test other AD animal models (e.g. minipigs43) as well as human patients.
The correlation that is observed between the δ65Cu of the brain and serum of 12-month old mice (Fig. 2) suggests that the Cu from which we analysed the isotopic composition in the serum has been interacting with the brain and therefore must be mobile. Copper bound to ceruloplasmin is not mobile, as it was demonstrated that Cu homesotatis is unchanged in ceruloplasmin-deficient mice compared to WT44. This implies that ceruloplasmin does not play a role in the transport and transfer of Cu between serum and organs. Therefore, low molecular weight molecules must carry the mobile Cu fraction. This is concordant with the fact that the main Cu transporter, CRT1, bounds both Cu(I) and Cu(II) and can bound large fraction of Cu in the serum45.
Effect of sex
The systematic difference of ~0.4‰ of the δ65Cu between WT male and female mice suggests an effect of sex on the Cu isotopic budget of the serum. Such a sex difference has been previously observed for Cu concentration in mouse plasma46. Our data represent the first example of a significant isotopic difference for mice of different sex, even if the limited data from previous work on Cu isotopes in mice hinted that there was a heavy isotope enrichment in the blood of females compared to males (~0.15‰; ref.13, n = 5 for the females and 4 for the males, compared to n = 19 and n = 13, respectively, in the present study). Such an effect of sex was not observed for Zn isotopes in mice12,13,15,47.
In human, a difference between sexes was previously reported for Fe and Cu isotopes47,48,49. A difference of ~0.20‰ in Cu isotopic composition was also previously seen between men and premenopausal women in the whole blood (−0.52 vs −0.74)50. The origin of the Cu isotopic fractionation between men and premenopausal women is usually attributed to the larger Cu turnover in women due to menstruations (e.g. refs51,52). Such origin is not applicable to male and female mice since the reproductive cycle of mice does not include bleeding53. The sex isotopic difference in mice may be related to a difference of Cu speciation and relative abundance of Cu carrier in serum. However, to our knowledge, there is no study on the effect of sex on the speciation of Cu in mouse blood.
Effect of AD on the Cu isotopic composition of the brain and serum
Twelve-month old AAPswe/PSEN1de9 female mice have slightly isotopically lighter brains in average than WT, even if this difference is not statistically significant. Isotope fractionation in 12-month old AAPswe/PSEN1de9 mice was previsouly observed for Zn15. Aβ plaques starts to develop in AAPswe/PSEN1de9 mice after 6 months with profuse plaques in the hippocampus and cortex at nine months27 and a proliferation until 12 months28. The small enrichment in light Cu isotopes of 12-month AAPswe/PSEN1de9 mice brains compared to WT suggests that the change of speciation associated with the proliferation of Aβ plaques modifies the global Cu isotopic composition of the brain. It is known that the formation of Aβ plaques increases the production of free Cu+ in the brain and therefore induces oxidative damages26. Such increase in Cu+ in the brain should globally decrease the δ65Cu value of the whole brain. On the other hand, copper is primarily bound to metallothionein in normal brain while in Aβ plaques it is bound to histidine54,55. Metallothionein is a cysteine rich protein and at 298 K it has a logarithm of the reduced partition function (lnβ) of ~3.3 (refs16,56) while in the same conditions lnβ of histidine is ~4.4. As a result, Aβ plaques should be isotopically heavier than normal brain. However, it was also shown that Aβ has a bounding site that favors Cu(I)20, which would conversely enrich the Aβ in the light isotope of Cu. If confirmed, the enrichment in the light isotopes of the AD mouse brains would imply that the production and increase of free Cu+ ion in the brain globally, together with the bounding of Cu(I) to Aβ, decrease the 65Cu/63Cu ratio.
Similarly to the brain, the Cu isotopic composition of the serum of the 12-month old AAPswe/PSEN1de9 mice tends to be isotopically different from the WT mice (but again statistically not different), while it is unchanged from 3 to 9 month in both the WT and AD mice. It is enriched in the lighter isotopes compared to WT mouse serum, such that the δ65Cu value in the serum and in the brain correlate (Fig. 2). This correlation between the δ65Cu value of the brain and the serum of the 12-month-old mice suggests that the change of composition of the serum is linked to the change happening in the brain. It was actually shown that the Cu level of the serum is higher in AD patients than in controls25,57. In particular, it was shown that AD serum has an increase of non ceruloplasmin Cu. An increase of Cu(I) species would decrease the total δ65Cu value of the serum. The origin of this non ceruloplasmin Cu was actually suggested to be a transfer of Cu(I) from the brain to the serum20,58 which would explain our observations.
Conclusion
Copper isotopic composition of serum is a potential tool to study the change of homeostasis associated with AD. Here we show that the δ65Cu value of WT males is ~0.4 different from WT females. This suggests an effect of sex on the Cu isotopic budget of the serum, which may be related to a difference of Cu speciation and relative abundance of Cu carrier in serum. We also find a trend that suggests that Aβ plaques induced a change in the Cu isotopic composition of the whole brain and of the serum. This tendency could be explained by the increase of isotopically light Cu(I) species in the brain. The Cu isotopic composition of the brain and of the serum correlate, which can be explained by a transfer of Cu(I) species from the brain to the serum and suggest that monitoring the natural Cu isotopic ratios in the serum may be used to detect the formation of Aβ plaques in the brain, but further work will have to be done to explore this hypothesis. The difference of speciation of Cu in the serum of mice compared to human calls for exploring different animal models (e.g. minipigs) and humans.
Data Availability
All data are available in supplementary materials.
References
Mortality, G. B. D. & Causes of Death, C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171, https://doi.org/10.1016/S0140-6736(14)61682-2 (2015).
Shaw, L. M. et al. Cerebrospinal Fluid Biomarker Signature in Alzheimer’s Disease Neuroimaging Initiative Subjects. Ann. Neurol. 65, 403–413, https://doi.org/10.1002/ana.21610 (2009).
Mattsson, N. et al. CSF Biomarkers and Incipient Alzheimer Disease in Patients With Mild Cognitive Impairment. JAMA-J. Am. Med. Assoc. 302, 385–393 (2009).
Villemagne, V. L. et al. Longitudinal Assessment of A beta and Cognition in Aging and Alzheimer Disease. Ann. Neurol. 69, 181–192, https://doi.org/10.1002/ana.22248 (2011).
Baum, L. et al. Serum zinc is decreased in Alzheimer’s disease and serum arsenic correlates positively with cognitive ability. Biometals 23, 173–179, https://doi.org/10.1007/s10534-009-9277-5 (2010).
Miller, L. M. et al. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer’s disease. J. Struct. Biol. 155, 30–37, https://doi.org/10.1016/jjsb.2005.09.004 (2006).
Religa, D. et al. Elevated cortical zinc in Alzheimer disease. Neurology 67, 69–75, https://doi.org/10.1212/01.wnl.0000223644.08653.b5 (2006).
Rulon, L. L. et al. Serum zinc levels and Alzheimer’s disease. Biological Trace Element Research 75, 79–85, https://doi.org/10.1385/bter:75:1-3:79 (2000).
Haines, A., Iliffe, S., Morgan, P., Dormandy, T. & Wood, B. Serum alumnium and zin and other variables in patients ith and without cognitive impairment in the community. Clin Chim Acta 198, 261–266, https://doi.org/10.1016/0009-8981(91)90360-o (1991).
Vasto, S. et al. Zinc and inflammatory/immune response in aging. Ann N Y Acad Sci. 100, 111–122 (2007).
Balter, V. et al. Bodily variability of zinc natural isotope abundances in sheep. Rapid Commun Mass Spectrom 24, 605–612, https://doi.org/10.1002/rcm.4425 (2010).
Moynier, F., Fujii, T., Shaw, A. & Le Borgne, M. Heterogeneous distribution of natural zinc isotopes in mice. Metallomics 5, 693–699 (2013).
Balter, V. et al. Contrasting Cu, Fe, and Zn isotopic patterns in organs and body fluids of mice and sheep, with emphasis on cellular fractionation. Metallomics 5, 1470–1482, https://doi.org/10.1039/c3mt00151b (2013).
Faller, P. & Hureau, C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-beta peptide. Dalton Trans, 1080–1094, https://doi.org/10.1039/b813398k (2009).
Moynier, F., Foriel, J., Shaw, A. & Le Borgne, M. Zinc isotopic behavior during Alzheimer’s disease. Geochemical Perspective Letters 3, 142–150 (2017).
Moynier, F., Vance, D., Fujii, T. & Savage, P. In Non-traditional stable isotopes Vol. 82 (eds Teng, F-Z, Watkins, J. & Dauphas, N.) 543–600 (Mineralogical Society of America, 2017).
Sauzeat, L. et al. Isotopic Evidence for Disrupted Copper Metabolism in Amyotrophic Lateral Sclerosis. iScience 6, 264–271, https://doi.org/10.1016/j.isci.2018.07.023 (2018).
Rajendran, R. et al. A novel approach to the identification and quantitative elemental analysis of amyloid deposits–insights into the pathology of Alzheimer’s disease. Biochemical and biophysical research communications 382, 91–95, https://doi.org/10.1016/j.bbrc.2009.02.136 (2009).
Lovell, M. A., Robertson, J. D., Teesdale, W. J., Campbell, J. L. & Markesbery, W. R. Copper, iron and zinc in Alzheimer’s disease senile plaques. Journal of the neurological sciences 158, 47–52 (1998).
Barnham, K. J. et al. Structure of the Alzheimer’s disease amyloid precursor protein copper binding domain. A regulator of neuronal copper homeostasis. J Biol Chem 278, 17401–17407, https://doi.org/10.1074/jbc.M300629200 (2003).
Syme, C., Nadal, R., Rygby, S. & Viles, J. Copper Binding to the Amyloid-β (Aβ) Peptide Associated with Alzheimer’s Disease. J Biol Chem 279, 18169–18177 (2004).
Vašák, M. & Meloni, G. Mammalian Metallothionein-3: New Functional and Structural Insights. Int. J. Mol. Sci. 18, 1117, https://doi.org/10.3390/ijms18061117 (2017).
Scheiber, I., Mercer, J. & Dringen, R. Metabolism and functions of copper in brain. Progress in neurobiology 116, 33–57 (2014).
Gaier, E. D., Eipper, B. A. & Mains, R. E. Copper signaling in the mammalian nervous system: synaptic effects. J Neurosci Res 91, 2–19 (2013).
Squitti, R. et al. Excess of serum copper not related to ceruloplasmin in Alzheimer disease. Neurology 64, 1040–1046, https://doi.org/10.1212/01.WNL.0000154531.79362.23 (2005).
Barnham, K. J., Masters, C. L. & Bush, A. I. Neurodegenerative diseases and oxidative stress. Nature reviews. Drug discovery 3, 205–214, https://doi.org/10.1038/nrd1330 (2004).
Jankowsky, J. L. et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13, 159–170, https://doi.org/10.1093/hmg/ddh019 (2004).
Garcia-Alloza, M. et al. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis 24, 516–524, https://doi.org/10.1016/j.nbd.2006.08.017 (2006).
Maréchal, C., Télouk, P. & Albarède, F. Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem. Geol. 156, 251–273 (1999).
Rodovka, Z. et al. Zinc and copper isotope systematics in sediments from the Ries Impact Structure and central European tektites – implications for material sources and loss of volatiles. Meteorit. Planet. Sci. 52, 2178–2192 (2017).
Savage, P. et al. Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation. Geochemical Perspective Letters 1, 53–64 (2015).
Telouk, P. et al. Copper isotope effect in serum of cancer patients. A pilot study. Metallomics 7, 299–308 (2015).
Costas-Rodriguez, M. et al. Isotopic analysis of Cu in blood serum by multi-collector ICP-mass spectrometry: a new approach for the diagnosis and prognosis of liver cirrhosis? Metallomics 7, 491–498, https://doi.org/10.1039/c4mt00319e (2015).
Lauwens, S., Costas-Rodriguez, M., Van Vlierberghe, H. & Vanhaecke, F. Cu isotopic signature in blood serum of liver transplant patients: a follow-up study. Sci Rep 6, 30683, https://doi.org/10.1038/srep30683 (2016).
Balter, V. et al. Natural variations of copper and sulfur stable isotopes in blood of hepatocellular carcinoma patients. Proc Natl Acad Sci USA 112, 982–985, https://doi.org/10.1073/pnas.1415151112 (2015).
Scott, B. J. & Bradwell, A. R. Identification of the Serum Binding-Proteins for Iron, Zinc, Cadmium, Nickel, and Calcium. Clin Chem 29, 629–633 (1983).
Cabrera, A. et al. Copper binding components of blood plasma and organs, and their responses to influx of large doses of (65)Cu, in the mouse. Biometals 21, 525–543, https://doi.org/10.1007/s10534-008-9139-6 (2008).
Cousins, R. J. Absorption, Transport, and Hepatic-Metabolism of Copper and Zinc - Special Reference to Metallothionein and Ceruloplasmin. Physiol Rev 65, 238–309 (1985).
Wirth, P. L. & Linder, M. Distribution of copper among components of human serum. J. Natl. Cancer Inst 75, 277–284 (1985).
Bento, I., Peixoto, C., Zaitsev, V. N. & Lindley, P. F. Ceruloplasmin revisited: structural and functional roles of various metal cation-binding sites. Acta crystallographica. Section D, Biological crystallography 63, 240–248, https://doi.org/10.1107/S090744490604947X (2007).
Moriya, M. et al. Copper is taken up efficiently from albumin and alpha2-macroglobulin by cultured human cells by more than one mechanism. American journal of physiology. Cell physiology 295, C708–721, https://doi.org/10.1152/ajpcell.00029.2008 (2008).
Sendzik, M., Pushie, J., Stefaniak, E. & Haas, K. Structure and Affinity of Cu(I) Bound to Human Serum Albumin. Inorg. Chem. 56, 15057–15065.
Mahan, B., Moynier, F., Jorgensen, A. L., Habekost, M. & Siebert, J. Examining the homeostatic distribution of metals and Zn isotopes in Gottingen minipigs. Metallomics 10, 1264–1281 (2018).
Meyer, L. A., Durley, A. P., Prohaska, J. R. & Harris, Z. L. Copper transport and metabolism are normal in aceruloplasminemic mice. J Biol Chem 276, 36857–36861, https://doi.org/10.1074/jbc.M105361200 (2001).
Lutsenko, S. Copper trafficking to the secretory pathway. Metallomics 8, 840–852 (2016).
Quinn, J. F. et al. Gender effects on plasma and brain copper. Int J Alzheimers Dis 2011, 150916, https://doi.org/10.4061/2011/150916 (2011).
Albarede, F., Telouk, P., Lamboux, A., Jaouen, K. & Balter, V. Isotopic evidence of unaccounted for Fe and Cu erythropoietic pathways. Metallomics 3, 926–933, https://doi.org/10.1039/C1mt00025j (2011).
Walczyk, T. & von Blanckenburg, F. Deciphering the iron isotope message of the human body. Int. J. Mass spectrom. 242, 117–134, https://doi.org/10.1016/J.Ijms.2004.12.028 (2005).
Jaouen, K. et al. Fe and Cu stable isotopes in archeological human bones and their relationship to sex. Am J Phys Anthropol 162, 491–500 (2012).
Jaouen, K. et al. Is aging recorded in blood Cu and Zn isotope compositions? Metallomics 5, 1016 (2013).
Van Heghe, L., Deltombe, O., Delanghe, J., Depypere, H. & Vanhaecke, F. The influence of menstrual blood loss and age on the isotopic composition of Cu, Fe and Zn in human whole blood. J. Anal. At. Spectrom. 29, 478–482 (2014).
Jaouen, K. & Balter, V. Menopause effect on blood Fe and Cu isotope compositions. Am J Phys Anthropol 153, 280–285 (2014).
Byers, S. L., Wiles, M. V., Dunn, S. L. & Taft, R. A. Mouse estrous cycle identification tool and images. Plos One 7, e35538, https://doi.org/10.1371/journal.pone.0035538 (2012).
Tougu, V., Karafin, A. & Palumaa, P. Binding of zinc(II) and copper(II) to the full-length Alzheimer’s amyloid-beta peptide. Journal of neurochemistry 104, 1249–1259, https://doi.org/10.1111/j.1471-4159.2007.05061.x (2008).
Karr, J. W., Akintoye, H., Kaupp, L. J. & Szalai, V. A. N-Terminal deletions modify the Cu2+ binding site in amyloid-beta. Biochemistry-Us 44, 5478–5487 (2005).
Fujii, T., Moynier, F., Blichert-Toft, J. & Albarede, F. Density functional theory estimation of isotope fractionation of Fe, Ni, Cu, and Zn among species relevant to geochemical and biological environments. Geochim. Cosmochim. Acta 140, 553–576, https://doi.org/10.1016/j.gca.2014.05.051 (2014).
Squitti, R. et al. Elevation of serum copper levels in Alzheimer’s disease. Neurology 59, 1153–1161 (2002).
Squitti, R. et al. Excess of nonceruloplasmin serum copper in AD correlates with MMSE, CSF [beta]-amyloid, and h-tau. Neurology 67, 76–82, https://doi.org/10.1212/01.wnl.0000223343.82809.cf (2006).
Acknowledgements
Two anonymous reviewers are thanked for many important comments, especially about the statistically validity of the results. We thank the CNRS via a grant of the mission pour les initiatives transverses et interdisciplinaires (MITI) Defi ISOTOP (grant MAMI) and the Université Sorbonne Paris Cité for a Chaire d’Excellence. Parts of this work were supported by IPGP multidisciplinary program PARI, and by Region île-de-France SESAME Grant No. 12015908.
Author information
Authors and Affiliations
Contributions
F.M. and M.L.B. conceived the study. M.L.B. performed all the animal experienced, J.C., and J.D. performed the isotopic measurements. F.M. wrote the manuscript. M.L.B. and J.C. helped in writing the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moynier, F., Creech, J., Dallas, J. et al. Serum and brain natural copper stable isotopes in a mouse model of Alzheimer’s disease. Sci Rep 9, 11894 (2019). https://doi.org/10.1038/s41598-019-47790-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47790-5
This article is cited by
-
Metal-binding amino acid ligands commonly found in metalloproteins differentially fractionate copper isotopes
Scientific Reports (2024)
-
Distinctive calcium isotopic composition of mice organs and fluids: implications for biological research
Analytical and Bioanalytical Chemistry (2023)
-
Magnesium stable isotope composition, but not concentration, responds to obesity and early insulin-resistant conditions in minipig
Scientific Reports (2022)
-
A novel dual-emission fluorescent probe for ratiometric and visual detection of Cu2+ ions and Ag+ ions
Analytical and Bioanalytical Chemistry (2022)
-
Contribution of Ryugu-like material to Earth’s volatile inventory by Cu and Zn isotopic analysis
Nature Astronomy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.