Abstract
Pythium-induced damping-off disease is a major disease limiting cucumber and tomato production in different parts of the world. The current study investigated the efficiency of Talaromyces variabilis and its bioactive metabolites in suppressing Pythium-induced damping-off of cucumbers and tomatoes. T. variabilis inhibited the in vitro growth of P. aphanidermatum in solid and liquid media. In addition, abnormalities in P. aphanidermatum hyphae were observed as a result of T. variabilis. Extracts from T. variabilis induced cellular leakage and suppressed oospore production of P. aphanidermatum. Biochemical analyses of T. variabilis metabolites showed that T. variabilis produces glucanase, cellulase and siderophores, suggesting the contribution of these metabolites in the inhibition of P. aphandermatum growth and in hyphal abnormalities. Treating cucumber seeds with spore and mycelial suspension of T. variabilis isolates led to a significant improvement in the seedling survival of P. aphanidermatum-inoculated seedlings from 18 to 52% (improvement by 34%) for isolate 48 P and from 30–66% (improvement by 36%) for isolate 28 R. Similarly, treating tomato seeds with spore and mycelial suspension of T. variabilis isolates led to a significant improvement in the seedling survival of P. aphanidermatum-inoculated seedlings from 7 to 36% (improvement by 29%) for isolate 28 R and from 20 to 64% (improvement by 44%) for isolate 48 P. Differences in the percent improvement in seedling survival between experiments may be related to difference in the efficacy of the two different isolates or their interaction with the hosts and pathogen. The use of T. variabilis in the biocontrol of Pythium-induced diseases may offer alternatives to the currently used chemical control.
Similar content being viewed by others
Introduction
Soil-borne pathogens represent a major challenge to crops worldwide. Diseases and loses caused by soil-borne pathogens vary from one place and crop to another depending on the pathogen, environmental conditions and management strategies. Pythium species are a major problem worldwide especially in vegetable crops. Pythium-induced damping-off and root diseases of cucurbits and tomatoes can result in losses of up to 100%1,2. Diseases in these crops are caused by various Pythium species, the most common of which is P. aphanidermatum3,4.
Damping-off and root diseases of vegetable crops can be managed using chemical treatments (e.g. Mefenoxam, Hymexazol and Captan)5,6. Since chemical control has several negative effects on the environment and humans, other environmentally safe methods have been used or developed, including the use of solarization7. Biological control, which depends on microorganisms, including endophytes, is better than using synthetic chemical fungicides, because of their hazards to the environment as well as the potential development of resistance to fungicides8.
Endophytic microorganisms reside inside plant tissues and have multiple benefits to their hosts and environments including mineral solubilization9,10, phytohormones production11, phytoremediation of heavy metals12,13 and disease suppression14,15.
Several endophytic fungi are used as biocontrol agents against plant disease such as Botryosphaeria ribis, Trichoderma sp. and Aspergillus terreus14,16. Talaromyces species have multiple benefits for plants; they have been used as biocontrol agents against several plant pathogen17,18. In Oman, little attention has been given to finding biocontrol agents against soil borne diseases.
There are several mechanisms that microbes use to promote disease stress tolerance in plants including hydrolytic enzymes production19, siderophores production20 and hydrogen cyanide synthesis21. Generally, endophytes reduce pathogen effects in plants immediately after infection by promoting plant stress response systems22,23. Elucidating the ways by which biocontrol agents affect other pathogens is helpful in coming up with effective management strategies for pathogens.
During a recent study in Oman, two Talaromyces isolates with potential biocontrol activities were isolated. This study aimed at investigating the suppressive effects of these isolates against growth, and spore production by Pythium aphanidermatum, the potential metabolites involved in the inhibition, and the potential biocontrol activities of the isolates against Pythium damping-off of cucumbers and tomatoes.
Knowledge in these areas could help come up with effective biocontrol agents against soil borne disease affecting crops in Oman.
Results
Identification of Talaromyces isolates
The combined ITS, TUB and CMD dataset comprises 18 isolates of Talaromyces. Phylogenetic analysis showed that isolates 48 P and 28 R belong to T. variabilis (Fig. 1).
Antagosnistic effect of T. variabilis isolates against P. aphanidermatum
Both T. variabilis isolates showed antagonistic activity against P. aphanidermatum in PDA medium (Table 1). T. variabilis isolates 48 P and 28 R produced inhibition zones of 8.5 mm and 6.25 mm, respectively.
The second experiment was conducted in PDA plates to observe the antagonistic effect of T. variabilis isolates over time. P. aphanidermatum ceased its growth. However, T. variabilis isolates 48 P and 28 R continued to grow towards P. aphanidermatum and filled the plate after 13.5 days and 15.5 days, respectively (Table 1).
Effect of culture filtrates of T. variabilis on P. aphanidermatum growth and oospore production
Treating P. aphanidermatum with culture filtrates of T. variabilis isolates led to considerable inhibition in mycelial growth in all the tested concentrations (Fig. 2). T. variabilis isolates fully suppressed the growth of P. aphanidermatum at 75% concentration. However, the growth was reduced at 50% and 25% concentrations (Fig. 2). Furthermore, oospore production by P. aphanidermatum decreased significantly when it was exposed to 20% culture filtrate of T. variabilis (48 P: 8 oospores; 28 R: 9 oospores) compared to the control (56 oospores) (Table 1).
Extracellular conductivity
Addition of 48 P and 28 R culture filtrates to P. aphanidermatum mycelium resulted in an increase in the extracellular conductivity values to 21.56 mV and 13.66 mV respectively, compared to PDB control (0.86 mV) (Table 1).
Glucanase activity of T. variabilis culture filtrates
The concentration of glucanase enzyme produced by both T. variabilis isolates were similar: 8.09 for 48 P and 8.21 for 28 R (Table 1). The enzyme activity is expressed in nmoles substrate consumed h−1 ml of culture filtrate.
Determination of cellulase activity using filter paper assay (FPA)
Both isolates of T. variabilis strains had cellulase enzyme in their culture filtrates. Isolate 48 P had significantly higher concentration of cellulase activity, 2.12 μmol/min/ml compared to 28 R isolate, 0.43 μmol/min/ml (Table 1).
Siderophore production by T. variabilis isolates
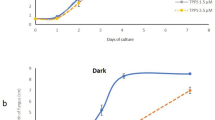
Both isolates of T. variabilis produced siderophore in both media. However, King B medium contains the highest amount of siderophore, 24.25 μM for 48 P and 21.85 μM for 28 R compared to Glucose medium, 15.86 μM for 48 P and 13.56 μM for 28 R (Fig. 3).
Effect of T. variabilis on P. aphanidermatum morphology
Both isolates of T. variabilis induced significant abnormalities in general shape, internal content and the tips of main hyphae and hyphal branches of P. aphanidermatum. Isolate 48 P had a greater impact on hyphal morphology compared to isolate 28 R (Fig. 4).
Effect of T. variabilis isolates 48 P and 28 R on P. aphanidermatum hyphal morphology compared to control. Columns and bars represent means ± CV of SD. Values with the same letters in the same category are not significantly different from each other (Pearson Chi-Square: asymptotic significance, 2-sided; >0.01).
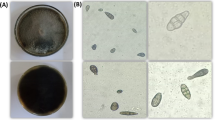
Light microscope examination showed that the general shape of hypha became twisted, bulbous-like, swollen, loss of hyphal content and hyphae have protrusions and narrowings (Fig. 5). Also the internal content became empty or semi-empty, and the tips became wrapped-up, and wavy (Fig. 5).
Abnormalities in hyphae of P. aphanidermatum under the effect of T. variabilis isolates using a light microscope. Normal hyphae of P. aphanidermatum (control; A); Effects of 48 P isolate on P. aphanidermatum hypha: twisted hyphae (B), hyphae with protrusions (C) loss of hyphal content (D), loss of internal content (E), wrapped up tip (F) and hook-like tip (G); Effects of 28 R isolate on P. aphanidermatum hypha: twisted (H), hyphae with protrusions and narrowings (I), hyphal content exit (J), bulbous hyphae (K), swollen hyphae (L), semi-empty internal content (M), wrapped-up tip (N) and hook-like tip (O). Scale bars = 50 μm.
Furthermore, scanning electron microscope showed similar observations such as shrunken and wavy hyphae, hyphal content exits and hyphae have protrusions and narrowings, as compared to the control which had straight, smooth surface and full hyphae (Fig. 6).
Abnormalities in hyphae of P. aphanidermatum under the effect of T. variabilis isolates using scanning electron microscope. Normal hyphae of P. aphanidermatum (control; A,B); Effects of 48 P isolate on P. aphanidermatum hypha: shrunken (C), wavy (D), loss of hyphal content (E) and hyphae with protrusions and narrowings (F); Effects of 28 R isolate on P. aphanidermatum hypha: shrunken (G), wavy (H) and loss of hyphal content and hyphae with protrusions (I).
Biocontrol potential of T. variabilis isolates against damping-off diseases of cucumber and tomato
T. variabilis isolates (48 P and 28 R) did not cause any significantly harmful effects on the length, fresh weight and dry weight of cucumber and tomato seedlings (Tables 2, 3). Both T. variabilis isolates 48 P and 28 R had considerable biocontrol efficacy against damping-off disease in cucumber and tomato (Table 3). Treating cucumber seeds with spores and mycelial suspension of T. variabilis isolate 48 P and P. aphanidermatum led to significant improvement in seedlings survival (51.78%) compared to the control (17.86%). Similar results were observed by T. variabilis isolate 28 R with significant improvement in cucumber survival (66.07%) compared to the control (30.36%) (Table 3).
Similarly, treating tomato seeds with spore and mycelial suspension of 48 P and P. aphanidermatum led to significant improvement in seedling survival (64.28%) compared to the control (19.64%). Also, 28 R significantly improved tomato survival (35.71%) compared to the control (7.14%).
Discussion
Two strains of T. variabilis 48 P and 28 R were isolated from Rhazya stricta and Zygophyllum coccineum, respectively. The two plants are native to Oman. Species of Talaromyces are known endophytes and found on a wide range of plants such as Potentilla fulgens24, Dactylis glomerata25 and Aloe vera26. The current study proved that T. variabilis isolates were not pathogenic to cucumbers and tomatoes plants. This result was compatible with the definition of endophytes as fungi which colonize the stems and leaves of plants without causing any symptoms of disease27.
Our study clearly demonstrated that T. variabilis isolates produced inhibition zones against P. aphanidermatum on PDA media. Many other fungi such as Trichoderma spp.28 and bacteria such as Pseudomonas fluorescens29 are known to be antagosnistic against harmful pathogens such as Aspergillus flavus, Fusarium moniliforme and Rhizoctonia solani. The inhibition activity is mainly due to their ability to secrete bioactive compounds that inhibit plant pathogens30.
The culture filtrates of T. variabilis isolates were effective against P. aphanidermatum growth in liquid media. They significantly decreased P. aphanidermatum dry weight at 25% and 50% concentrations, and fully suppressed its growth at 75% concentration. Previous studies showed that Streptomyces hydrogenans culture filtrates inhibited the growth of Alternaria Brassicicol31. Oospore production was greatly decreased in the presence of culture filtrates of 48 P and 28 R. Similar observation was made by16, where Aspergillus terreus affected spore production by P. aphanidermatum.
Our data showed that both Talaromyces isolates produce cellulase enzyme. Cellulase enzyme can be produced by several fungal genera such as Trichoderma32, Aspergillus33,34 and Talaromyces35. Fungi and bacteria that secrete hydrolytic enzyme have biocontrol ability against plant pathogen. For example chitinase, glucanase and protease enzymes produced by Trichoderma harzianum are antagonistic against some fungi36. Chitinase and β-1,3-glucanase enzymes produced by Clonostachys rosea f. catenulata were responsible for efficient biocontrol against fungal plant pathogens37. About 18% of the P. aphanidermatum cell wall consists of cellulose38. The efficacy of Talaromyces isolates as biocontrol agents against damping-off disease may be in-part due to cellulose production by Talaromyces isolates.
Loss in integrity of P. aphanidermatum cells was observed due to T. variabilis isolates 48 P and 28 R culture filtrates. Consistently, the antifungal metabolites produced by Sporothrix flocculosa led to cellular leakage in several phytopathogens39. Another study by Zhao, et al.40 showed that Streptomyces bikiniensis causes cellular leakage in Fusarium oxysporum.
Our study showed the production of glucanase enzyme by 48 P and 28 R isolates. Several previous studies also documented a role of extracellular enzymes in biocontrol of pathogens. Examples include cellulases produced by Lysinibacillus sphaericus19 and chitinases and glucanases produced by Trichoderma species41. Our results showed that siderophores were produced by 48 P and 28 R isolates in King B and glucose media. Numerous fungi and bacteria could produce siderophores that are effective against pathogens. Siderophores produced by Rhizobium meliloti led to inhibition of Macrophomina phaseolina42. Moreover, cucumbers damping-off disease was controlled by Aspergillus terreus which was able to produce siderophores16. These siderophores may deprive the pathogen of iron, thus limiting essential nutrient43.
The morphology of P. aphanidermatum hyphae were significantly affected by 48 P and 28 R isolates, showing several abnormalities. Getha and Vikineswary44 showed the following abnormalities in Fusarium oxysporum hypha due to antagonistic influence of Streptomyces violaceusniger: swelling, distortion and excessive branching of hyphae, thickened with bulbous-like formation along the ends. Another study by Halo, et al.16 showed abnormalities in hyphae of P. aphanidermatum such as shrunken, semi empty and empty content and wrapped up ends under the effect of Aspergillus terreus.
Our study confirmed the efficient role of 48 P and 28 R isolates against cucumbers damping-off and tomatoes damping-off diseases. A previous study by Sivan, et al.45 showed the suppression P. aphanidermatum by Trichoderma harzianum. Similarly, Gliocladium catenulatum inhibited cucumbers damping-off and root rot diseases caused by P. aphanidermatum46. Also, damping-off of tomatoes disease caused by P. aphanidermatum was inhibited by Trichoderma viride and Pseudomonas fluorescens biocontrol agents47. Furthermore, endophytic actinomycetes were able to supress pathogenic activities of P. aphanidermatum because they produce glucanase enzyme48.
Our study is the first comprehensive report on the efficacy of T. variabilis isolates and their byproducts on P. aphanidermatum and Pythium damping-off of cucumbers and tomatoes.
The efficacy of these endophytes in suppressing P. aphanidermatum in the in vitro and in vivo tests through multiple mechanisms suggests that they may be effective against other harmful phytopathogens, including Pythium species that cause diseases in other plants. Also, using these endophytes provide an efficient alternative to the use of synthetic chemicals because P. aphanidermatum is less likely to develop resistance against these antagonistic fungi because they have multiple modes of action.
Materials and Methods
Talaromyces isolates
Rhazya stricta and Zygophyllum coccineum plants from desert sites in the Sultanate of Oman were selected for the isolation of endophytic fungi. The collections of samples was in May-August 2016 from Adam, 150 km from Muscat, the capital area of Oman. The method of Larran, et al.49 was followed for endophytic fungi isolation, as described by Halo et al.16.
Two fungal isolates (Talaromyces) were identified using sequences of three genes: the internal transcribed spacer region of the ribosomal RNA (ITS), beta-tubulin (TUB) and Calmodulin (CMD). The three genes were amplified using ITS1/ITS4, BT2A/BT2B and CMD5/CMD6 primers, respectively, using previously described reaction mixtures and conditions50,51,52. MEGA V.6 was used for sequence alignment53. Sequences were deposited in GenBank under accession numbers: ITS (48 P: MG957181, 28 R: MH006605), TUB (48 P: MH000341, 28 R: MH006606) and CMD: (48 P: MG979054, 28 R: MH006607).
Antagosnistic effect of T. variabilis isolates against P. aphanidermatum
The antagosnistic acivity of T. variabilis isolates 48 P and 28 R against P. aphanidermatum was investigated using fresh cultures of P. aphanidermatum and T. variabilis as explained in our previous work54, using dual culture assay. There were four replicates in each experiment.
Isolates 48 P and 28 R were grown on PDB media in an incubator shaker at 28 °C for ten days to produce effective metabolites. The culture filtrates were obtained by centrifugation at 10,000 g, filtered through 0.2 µm Minisart filters, transferred to conical flasks and stored at 4 °C for further experiments.
Three concentrations of culture filtrates (75%, 50% and 25%) were used to study their effect on P. aphanidermatum growth in PDB media while the control included only PDB media. The concentration 75% consisted of 75% filtrate and 25% PDB, and so on for the other concentrations. A 3-mm diameter disk of P. aphanidermatum was added to each flask. Flasks were then kept in an incubator shaker at 28 °C and 120 rpm for 7 days. Finally, the liquid was disposed and the mycelium of P. aphanidermatum was dried in an oven at 65 °C for 24 h. The dry weights of the treatments and control were measured using three replicates for each isolate.
Effect of culture filtrates of T. variabilis on extracellular conductivity and oospore production by P. aphanidermatum
The leakage of cellular components from mycelium of P. aphanidermatum was studied using 5 mg of dried mycelium obtained from the liquid culture. 10 ml of T. variabilis culture filtrates was added to dried mycelium and centrifuged. Extracellular conductivity of the culture was measured after 24 h16,31.
The effect of T. variabilis on oospore production of P. aphanidermatum was studied using V8 agar medium with 20% of T. variabilis culture filtrate. There were three replicates per treatment. The V8 agar medium without T. variabilis culture filtrate served as control16.
Glucanase activity of T. variabilis culture filtrates
Glucanase production by T. variabilis isolates was detected using a protocol described by Jackson, et al.55. Samples were analyzed spectrophotometrically at 400 nm using an ELISA spectrophotometer. The final enzyme activity was calculated as per Jackson, et al.55.
Cellulase activity
Two T. variabilis strains were cultivated in basal medium of Mandels and Weber (1969)56 supplemented with 1% cellulose. The flasks were incubated in a rotary shaker at 200 rpm at 28 °C for 10 days. Culture filtrates were obtained through centrifugation and used fresh. Total cellulase activities of fungal strains were determined as described by Mandel and Sternberg (1976)57.
Siderophore production by T. variabilis
Based on our previous experiment16, the highest concentrations of siderophore were obtained using King B medium and glucose medium. The media were inoculated with disks from fresh PDA cultures of 48 P and 28 R and kept in an incubator shaker at 120 rpm at 28 °C for 7 days. The supernatants were obtained by centrifugation followed by filtration through 0.2 µM Minisart filters. ELISA spectrophotometer at 400 nm was used to detect siderophores concentrations using molar extinction coefficient ε = 2000058,59. The experiment had six replicates.
Effect of T. variabilis on morphology of P. aphanidermatum
The antagonistic activity of T. variabilis isolates (48 P and 28 R) was checked against P. aphanidermatum in vitro as detailed in Halo, et al.16. The morphological study focused on main hyphae and their tips. 50 main and hypha tips of P. aphanidermatum near the inhibition zone were examined using a light microscope and scanning electron microscope (SEM, INSTUMENT JSM- 5600). These hyphae were compared with P. aphanidermatum hyphae in PDA control plate. All hyphae were stained with cotton blue.
Biocontrol potential of T. variabilis isolates against damping-off diseases of cucumber and tomato
The effect of T. variabilis isolates (48 P and 28 R) on Pythium damping-off of cucumber and tomato was tested using three treatments and one control. 48 P and 28 R isolates, used in the experiment, were grown in PDB media in an incubator shaker at 28 °C and 120 rpm for 15 days to obtain fungal suspension (spore/mycelial). Also, this test used fresh cultures of P. aphanidermatum which were grown in PDA for 3 days at 28 °C. Seven sterilised seeds of cucumber or tomato were sown in each pot. Pots (12-cm in diameter) were autoclaved once. However, soil used in the pots was autoclaved twice.
Four replicate pots were kept for each treatment or control. The control pots were irrigated with 25 ml PDB media; the pots in first treatment were inoculated with 25 ml of fungal suspension containing spore and/or mycelia of 48 P or 28 R; the second treatment was treated with full plate of fresh PDA culture of P. aphanidermatum, 2 cm below soil surface; the pots in the third treatment was treated with full plate of fresh PDA culture of P. aphanidermatum and 25 ml of 48 P or 28 R fungal suspension60. The experiment was conducted at 28 °C for three weeks at 12–14 hr day length. After that, shoot length, fresh weight and dry weight were determined. Also seedlings survival rate was calculated by dividing the number of surviving seedlings by 7 (total seed sown) and then multiplying them by 100. This experiment was executed twice.
Statistical analysis
Data were analysed by IBM SPSS Statistics 24.0 using Chi-Square test for morphological study to compare performance of the isolates to the control. The Poisson test was used to compare oospore production by P. aphanidermatum treated with each of the culture filtrates. Independent sample t-test, One-way ANOVA and Duncan’s Multiple Range Test were used to compare means of the different treatments. Each test is explained in the caption of each figure/table in the results section.
Data Availability
All data underlying this publication are available in the manuscript.
References
Al-Sa’di, A. M. et al. First report of Pythium splendens associated with severe wilt of muskmelon (Cucumis melo) in Oman. Plant Disease 92, 313–313 (2008).
Stanghellini, M. E. & Phillips, J. M. Pythium aphanidermatum: its occurrence and control with pyroxychlor in the Arabian desert at Abu Dhabi. Plant Disease Reporter 59, 559–563 (1975).
Kipngeno, P., Losenge, T., Maina, N., Kahangi, E. & Juma, P. Efficacy of Bacillus subtilis and Trichoderma asperellum against Pythium aphanidermatum in tomatoes. Biological Control 90, 92–95, https://doi.org/10.1016/j.biocontrol.2015.05.017 (2015).
Lee, S., Garzon, C. D. & Moorman, G. W. Genetic structure and distribution of Pythium aphanidermatum populations in Pennsylvania greenhouses basd on analysis of AFLP and SSR markers. Mycologia 102, 774–784 (2010).
Sharma, P. & Sain, S. Use of biotic agents and abiotic compounds against damping off of cauliflower caused by Pythium aphanidermatum. Indian Phytopathology 58, 395 (2005).
Weiland, J. E., Santamaria, L. & Grünwald, N. J. Sensitivity of Pythium irregulare, P. sylvaticum, and P. ultimum from forest nurseries to mefenoxam and fosetyl-Al, and control of Pythium damping-off. Plant Disease 98, 937–942 (2014).
Deadman, M., Al Maqbali, Y., Al Sa’di, A., Al Hasani, H. & Al Nabhani, M. In Acta Horticulturae Vol. 731, 367–370 (2007).
Al-Sadi, A. M., Al-Masoodi, R. S., Al-Ismaili, M. & Al-Mahmooli, I. H. Population structure and development of resistance to hymexazol among Fusarium solani populations from date palm, citrus and cucumber. Journal of Phytopathology 163, 947–955, https://doi.org/10.1111/jph.12397 (2015).
Xinxian, L. et al. Isolation and characterization endophytic bacteria from hyperaccumulator Sedum alfredii Hance and their potential to promote phytoextraction of zinc polluted soil. World Journal of Microbiology and Biotechnology 27, 1197–1207 (2011).
Nath, R., Sharma, G. & Barooah, M. Plant growth promoting endophytic fungi isolated from tea (Camellia sinensis) shrubs of Assam, India. Appl Ecol. Environ Res 13, 877–891 (2015).
Waqas, M. et al. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17, 10754–10773 (2012).
Khan, A. L. et al. Bacillus amyloliquefaciens BSL16 improves phytoremediation potential of Solanum lycopersicum during copper stress. Journal of Plant Interactions 12, 550–559 (2017).
Li, H.-Y., Wei, D.-Q., Shen, M. & Zhou, Z.-P. Endophytes and their role in phytoremediation. Fungal Diversity 54, 11–18 (2012).
Mejía, L. C. et al. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biological Control 46, 4–14 (2008).
Berg, G. & Hallmann, J. In Microbial root endophytes 53–69 (Springer, 2006).
Halo, B. A., Al-Yahyai, R. & Al-Sadi, A. M. Aspergillus terreus inhibits growth and induces morphological abnormalities in Pythium aphanidermatum and suppresses Pythium-induced damping-off of cucumber. Frontiers in Microbiology 9, 95 (2018).
Stosz, S. K., Fravel, D. R. & Roberts, D. P. In vitro analysis of the role of glucose oxidase from Talaromyces flavus in biocontrol of the plant pathogen Verticillium dahliae. Applied and environmental microbiology 62, 3183–3186 (1996).
Madi, L., Katan, T., Katan, J. & Henis, Y. Biological control of Sclerotium rolfsii and Verticillium dahliae by Talaromyces flavus is mediated by different mechanisms. Phytopathology 87, 1054–1060 (1997).
Naureen, Z. et al. Exploring the potentials of Lysinibacillus sphaericus ZA9 for plant growth promotion and biocontrol activities against phytopathogenic fungi. Frontiers in microbiology 8, 1477 (2017).
Buysens, S., Heungens, K., Poppe, J. & Hofte, M. Involvement of pyochelin and pyoverdin in suppression of Pythium-induced damping-off of tomato by Pseudomonas aeruginosa 7NSK2. Applied and Environmental Microbiology 62, 865–871 (1996).
Maurhofer, M., Keel, C., Haas, D. & Défago, G. Pyoluteorin production by Pseudomonas fluorescens strain CHA0 is involved in the suppression of Pythium damping-off of cress but not of cucumber. European journal of plant pathology 100, 221–232 (1994).
Schulz, B., Boyle, C., Draeger, S., Römmert, A.-K. & Krohn, K. Endophytic fungi: a source of novel biologically active secondary metabolites. Mycological Research 106, 996–1004 (2002).
Singh, L. P., Gill, S. S. & Tuteja, N. Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant signaling & behavior 6, 175–191 (2011).
Bhagobaty, R. & Joshi, S. Fungal endophytes of five medicinal plants prevalent in the traditionally preserved ‘Sacred forests’ of Meghalaya, India. Forest Science and Technology 7, 151–154 (2011).
Márquez, S. S., Bills, G. F. & Zabalgogeazcoa, I. The endophytic mycobiota of the grass Dactylis glomerata. Fungal Divers 27, 171–195 (2007).
Bara, R. et al. Talaromins A and B, new cyclic peptides from the endophytic fungus Talaromyces wortmannii. Tetrahedron Letters 54, 1686–1689 (2013).
Carroll, G. Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecology 69, 2–9 (1988).
Calistru, C., McLean, M. & Berjak, P. In vitro studies on the potential for biological control of Aspergillus flavus and Fusarium moniliforme by Trichoderma species. Mycopathologia 137, 115–124 (1997).
Nagarajkumar, M., Bhaskaran, R. & Velazhahan, R. Involvement of secondary metabolites and extracellular lytic enzymes produced by Pseudomonas fluorescens in inhibition of Rhizoctonia solani, the rice sheath blight pathogen. Microbiological Research 159, 73–81 (2004).
Bernal, G., Illanes, A. & Ciampi, L. Isolation and partial purification of a metabolite from a mutant strain of Bacillus sp. with antibiotic activity against plant pathogenic agents. Electronic Journal of Biotechnology 5, 7–8 (2002).
Manhas, R. K. & Kaur, T. Biocontrol potential of Streptomyces hydrogenans strain DH16 toward Alternaria brassicicola to control damping Off and black leaf spot of Raphanus sativus. Frontiers in plant science 7, 1869 (2016).
de Souza, M. F., da Silva, A. S. A. & Bon, E. P. A novel Trichoderma harzianum strain from the Amazon Forest with high cellulolytic capacity. Biocatalysis and Agricultural. Biotechnology 14, 183–188 (2018).
Mohapatra, S., Padhy, S., Mohapatra, P. K. D. & Thatoi, H. Enhanced reducing sugar production by saccharification of lignocellulosic biomass, Pennisetum species through cellulase from a newly isolated Aspergillus fumigatus. Bioresource Technology (2018).
Zhao, C., Deng, L. & Fang, H. Mixed culture of recombinant Trichoderma reesei and Aspergillus niger for cellulase production to increase the cellulose degrading capability. Biomass and Bioenergy 112, 93–98 (2018).
Jain, L., Kurmi, A. K. & Agrawal, D. Conclusive selection of optimal parameters for cellulase production by Talaromyces verruculosus IIPC 324 under SSF via saccharification of acid-pretreated sugarcane bagasse. Biofuels, 1–9 (2018).
Schirmböck, M. et al. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Applied and Environmental Microbiology 60, 4364–4370 (1994).
Chatterton, S. & Punja, Z. K. Chitinase and β-1, 3-glucanase enzyme production by the mycoparasite Clonostachys rosea f. catenulata against fungal plant pathogens. Canadian journal of microbiology 55, 356–367 (2009).
Blaschek, W., Käsbauer, J., Kraus, J. & Franz, G. Pythium aphanidermatum: culture, cell-wall composition, and isolation and structure of antitumour storage and solubilised cell-wall (1→ 3),(1→ 6)-β-d-glucans. Carbohydrate research 231, 293–307 (1992).
Hajlaou, M., Traquair, J., Jarvis, W. & Bélanger, R. Antifungal activity of extracellular metabolites produced by Sporothrix flocculosa. Biocontrol Science and Technology 4, 229–237 (1994).
Zhao, S., Du, C.-M. & Tian, C.-Y. Suppression of Fusarium oxysporum and induced resistance of plants involved in the biocontrol of Cucumber Fusarium Wilt by Streptomyces bikiniensis HD-087. World Journal of Microbiology and Biotechnology 28, 2919–2927 (2012).
Howell, C. Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant disease 87, 4–10 (2003).
Arora, N., Kang, S. & Maheshwari, D. Isolation of siderophore-producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Current Science, 673–677 (2001).
Dowling, D. N. & O’Gara, F. Metabolites of Pseudomonas involved in the biocontrol of plant disease. Trends in Biotechnology 12, 133–141 (1994).
Getha, K. & Vikineswary, S. Antagonistic effects of Streptomyces violaceusniger strain G10 on Fusarium oxysporum f. sp. cubense race 4: indirect evidence for the role of antibiosis in the antagonistic process. Journal of Industrial Microbiology and Biotechnology 28, 303–310 (2002).
Sivan, A., Elad, Y. & Chet, I. Biological control effects of a new isolate of Trichoderma harzianum on Pythium aphanidermatum. Phytopathology 74, 498–501 (1984).
Punja, Z. K. & Yip, R. Biological control of damping-off and root rot caused by Pythium aphanidermatum on greenhouse cucumbers. Canadian. Journal of Plant Pathology 25, 411–417 (2003).
Manorantitham, S., Prakasam, V. & Rajappan, K. Biocontrol of damping off of tomato caused by Pythium aphanidermatum. Indian Phytopathology 54, 59–61 (2001).
El‐Tarabily, K., Nassar, A., Hardy, G. S. J. & Sivasithamparam, K. Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. Journal of Applied Microbiology 106, 13–26 (2009).
Larran, S., Perello, A., Simon, M. & Moreno, V. Isolation and analysis of endophytic microorganisms in wheat (Triticum aestivum L.) leaves. World Journal of Microbiology and Biotechnology 18, 683–686 (2002).
White, T. J., Bruns, T., Lee, S. & Taylor, J. In PCR protocols: A Guide to Methods and Applications (eds Innis, M. A., Gelfand, D. H., Sninsky, J. J. & White, T. J.) 315–322 (Academic Press, 1990).
Koenraadt, H., Somerville, S. C. & Jones, A. Characterization of mutations in the beta-tubulin gene of benomyl-resistant field strains of Venturia inaequalis and other plant pathogenic fungi. Phytopathology 82, 1348–1354 (1992).
Hong, S.-B., Go, S.-J., Shin, H.-D., Frisvad, J. C. & Samson, R. A. Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 97, 1316–1329 (2005).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30, 2725–2729 (2013).
Halo, B., Al-Yahyai, R. & Al-Sadi, A. M. Aspergillus terreus inhibits growth and induces morphological abnormalities in Pythium aphanidermatum and suppresses Pythium-induced damping-off of cucumber. Frontiers in Microbiology 9, 95 (2018).
Jackson, C. R., Tyler, H. L. & Millar, J. J. Determination of microbial extracellular enzyme activity in waters, soils, and sediments using high throughput microplate assays. Journal of visualized experiments: JoVE 2013, e50399, https://doi.org/10.3791/50399 (2013).
Mandel, M. & Weber, J. Exoglucanase activity by microorganisms. Advance Chemistry 95, 391–414 (1969).
Mandel, M. & Sternberg, D. Recent advances in cellulose technology. J. Ferment. Technol 54, 267–286 (1976).
Meyer, J. a. & Abdallah, M. The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. Microbiology 107, 319–328 (1978).
Rachid, D. & Ahmed, B. Effect of iron and growth inhibitors on siderophores production by Pseudomonas fluorescens. African Journal of Biotechnology 4, 697–702 (2005).
Al-Hinai, A. H. et al. Isolation and characterization of Pseudomonas aeruginosa with antagonistic activity against Pythium aphanidermatum. Journal of Plant Pathology 92, 653–660 (2010).
Acknowledgements
We gratefully acknowledge Sultan Qaboos University (SQU) as well as Oman Animal and Plant Genetic Resources Center for their financial support to this study via the projects IG/AGR/CROP/16/03, EG/AGR/CROP/16/01, and CR/AGR/CROP/19/01.
Author information
Authors and Affiliations
Contributions
B.A.H., R.A.A. and A.M.A. planned the study, B.A.H. processed samples, B.A.H., R.A.A., S.S.M. and A.M.A. analysed results, B.A.H. and A.M.A. wrote the manuscript and B.A.H., R.A.A., S.S.M. and A.M.A. revised and approved the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Halo, B.A., Al-Yahyai, R.A., Maharachchikumbura, S.S.N. et al. Talaromyces variabilis interferes with Pythium aphanidermatum growth and suppresses Pythium-induced damping-off of cucumbers and tomatoes. Sci Rep 9, 11255 (2019). https://doi.org/10.1038/s41598-019-47736-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47736-x
This article is cited by
-
The potential of endophytic bacteria from Prosopis cineraria for the control of Pythium aphanidermatum-induced damping-off in cucumber under saline water irrigation
Journal of Plant Pathology (2022)
-
Chemical genetic approach using β-rubromycin reveals that a RIO kinase-like protein is involved in morphological development in Phytophthora infestans
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.