Abstract
Loss of skeletal muscle mass is common with aging and can cause morbidity and mortality in the elderly. The effects of particulate air pollution on skeletal muscle mass is not known. The study aims to assess the chronic effects of ambient fine particulates (PM2.5) on the body composition of the elderly. From October 2015 to November 2016, a cross-sectional survey on 530 elderly (age > = 65 years) was conducted in the Taipei Basin, Taiwan. The body composition was measured by bioelectrical impedance analysis (InBody 120). One year exposure to air pollution was estimated using the Kriging method at the participant’s residence. Multiple linear regression analysis, after adjustments for demographics and co-pollutants, was used to examine the effects of PM2.5 on body composition indices and force of handgrip. Changes in body composition for an interquartile (1.4 μm/m3) increase in PM2.5 concentration included a 0.4 kg (95% confidence interval (CI): −0.31, −0.58; p < 0.0001) decrease in skeletal muscle mass (2.0%) and a 0.7 kg (95% CI: 0.47, 0.91; p < 0.0001) increase in body fat mass (3.6%). While PM2.5 reduced fat free mass in the upper extremities and trunk, but not in the lower extremities, it increased body fat mass in the three parts. There was no significant effect of PM2.5 on handgrip force. Higher physical activity (versus lower than median) was associated with less detrimental effect of PM2.5 on skeletal muscle mass and body fat mass (p values for interaction term: 0.009 and 0.013, respectively). Long-term PM2.5 exposure is associated with decreased skeletal muscle mass and increased body fat mass in the elderly, which can be ameliorated by physical activity.
Similar content being viewed by others
Introduction
Loss of muscle mass is associated with aging, at a decreasing rate of approximately 6% per decade after mid-life1. Aging is associated with decreased production of several anabolic hormones like growth hormone, insulin-like growth hormone, testosterone, and estrogen, all of which play important roles in maintaining muscle mass and strength2. Secondary causes of muscle loss include inadequate nutrition, physical inactivity, and chronic diseases such as malignancy, organ dysfunction, and neurodegenerative or endocrine diseases3. Extremely low muscle mass with inadequate muscle function in the elderly is referred to as sarcopenia, which is linked to mortality risk4,5. When sarcopenia is accompanied by increased body fat mass, also known as sarcopenic obesity, mortality risk becomes higher comparing to each condition separately6,7,8. Identifying and managing the risk factors for sarcopenia and sarcopenic obesity may help promote healthy aging.
Although the precise cause of sarcopenia and sarcopenic obesity is not known, there are several patho-physiologic mechanisms that affect muscle loss and visceral fat gain. Insulin resistance, a common phenomenon of central obesity, may induce muscle fiber atrophy, intra-muscular lipid accumulation, and mitochondrial dysfunction. These enhance muscle wasting, dysfunction and oxidative stress2,3,9. Sarcopenia further exacerbates obesity-associated insulin resistance and dysglycemia10. Moreover, local or systemic inflammatory states driven by pro-inflammatory cytokines or oxidative stress can enhance proteolysis and inhibit muscle synthesis11,12,13. The over-deposition of adipose tissue, especially in visceral site, augments pro-inflammatory cytokines (i.e. TNFα and IL-6) and have negative effect on muscles14. Loss of muscle mass and functionality may reduce physical activity, which in turn lowers energy expenditure and boosts the development of obesity3. Such mechanisms may set a vicious cycle between sarcopenia and obesity.
Particulate air pollution, especially particulate matter smaller than 2.5 μm (PM2.5), are known to augment systemic inflammation, insulin resistance, and oxidative stress. In a mice study, PM2.5 increases inflammation in adipose tissue and decreases glucose uptake in muscular tissue, resulting in increased systemic insulin resistance15. However, there is paucity of information regarding the effect of ambient PM2.5 on human skeletal muscle and adipose tissue. Previous studies show that cigarette smoke leads to skeletal muscle cell damage, muscle protein breakdown16, skeletal muscle dysfunction17, and central obesity18. Because ambient PM2.5 and cigarette smoke share some common patho-physiologic mechanisms like oxidative stress and inflammation, ambient PM2.5 may have negative effects on muscle and adipose tissue in the elderly, a population susceptible to air pollution.
Materials and Methods
Study design and population
Between October 2015 and November 2016, a cross-sectional study on the elderly (age > = 65 years) was conducted in the Taipei Basin, Taiwan. Those who underwent their annual health exam in two hospitals were invited. Those with malignancy or ambulation and communication difficulties were excluded. The institutional review board of the National Health Research Institutes (EC1040508-E-R2) approved this study. All of the study participants provided a written informed consent. All experiments were performed in accordance with relevant guidelines and regulations.
Questionnaire
A structured questionnaire was designed to collect information on personal habits (e.g. cigarette smoking and alcohol drinking), medical conditions (e.g. underling diseases and corresponding treatments), education, and physical activity. The Chinese edition of the Physical Activity Scale for the Elderly (PASE) questionnaire was used to assess physical activity19,20. Two well-trained interviewers provided the questionnaire. Because of visual impairment and reading difficulties among the elderly, the interviewers helped in reading and explaining the questions and in filling up the answers.
Measurements of body composition and handgrip force
All of the participants underwent measurements of body composition in the morning after >8 hours of fasting to avoid the influence of food and fluid intake21. The participants were in a standing posture and lightly dressed for the examination. Bioelectric impedance analysis devices (BIA, Inbody 120, InBody Co., Ltd. Seoul, Korea) with tetrapolar-8-point-tactile electrodes were used. Each device had two different frequencies (20 and 100 kHz) for impedance measurement in five body segments (the four extremities and the trunk).
The body composition parameters used were skeletal muscle mass (SMM; in kg) of total body, fat free mass (FFM; in kg) of five body segments, and body fat mass (BFM; in kg) of the total body and of the five body segments. The FFM and BFM of the upper or lower extremities were calculated by adding up the component weights of the right and left sides of the arms or legs. Height and weight were measured at the time of body composition measurement.
Handgrip strength was measured using a digital handgrip-dynamometer (TTM-YD, Tokyo, Japan). Three trials for each hand were performed and the best reading was used for data analysis.
Assessment of air pollution exposure
The residential address of each participant was geocoded to estimate the ambient air pollution exposure. The annual mean concentrations of air pollutants, including PM2.5, sulphur dioxide (SO2), ozone (O3), carbon monoxide (CO), and nitrogen dioxide (NO2), in 73 EPA monitoring stations in Taiwan in 2015 were calculated based on hourly measured data. A modified ordinary Kriging adopted from Liao et al.22 was used to approximate the long-term residential exposure. ArcView GIS (version 93) and its Geostatistical Analysts Extension (ESRI Inc., Redland, CA) were used to construct the semi-variogram for spatial estimation of the concentrations of the air pollutants. The cross-validated R2 values of PM2.5, NO2, CO, O3, and SO2 were 0.61, 0.63, 0.28, 0.20, and 0.61, respectively.
Statistical analysis
Linear regression for association between individual variables, muscle strength, and parameters of body composition, and Pearson’s correlation for the relationship between each air pollutant were calculated using the JMP software version 5.0 (SAS Institute, Gary, NC, USA).
Multiple linear regression analysis was used to examine the association between air pollution and health outcomes. The models were adjusted for covariates such as age (years), sex (male/female), body height (cm), body weight (kg), cigarette smoking (never/former/current), alcohol drinking habit (none/less than once per week/more than once per week), education (primary school or less/middle or high school or equivalent/university degree or more), physical activity (total score on the PASE questionnaire), diabetes mellitus (yes/no), asthma (yes/no), COPD (yes/no), stroke history (yes/no), heart diseases (yes/no), chronic renal disease (yes/no), arthritis (yes/no), osteoporosis (yes/no), and ambient temperature and humidity on the date of examination. Personal estimates of long-term exposure to air pollutant at residential the sites were fitted separately into the multiple linear regression model. The two-pollutant model was also applied to adjust for potential confounding effects of co-pollutants. Stratified analysis were done to examine potential modification effect of age (<or> = median), gender, physical activity (PASE score <or> = median), physician diagnosed diabetes mellitus (yes or no), physician diagnosed arthritis (yes or no), and physician diagnosed osteoporosis (yes or no). Interaction term in the full model was used to evaluate the statistical significance of modification effects. A p < 0.05 was considered statistically significant.
Results
The characteristics of participated elders
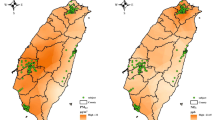
The study population had a mean age of 70.2 years, with male-female ratio of 0.71. Their mean BMI was 24.09 kg/m2 and 36.6% had a BMI > 25 kg/m2. Among them, 5.1% were current smokers and 9.8% were frequent alcohol drinkers (more than once per week). The prevalence of chronic diseases was highest for arthritis (13.8%), followed by diabetes mellitus (12.8%) and osteoporosis (9.1%). The total score of PASE ranged from 0 to 286.2, with mean and median values of 107.4 and 103, respectively. The demographics, body composition indices, and handgrip force of the participants were summarized in Table 1, while their residential locations were shown in Fig. 1.
The distribution of air pollution at residential sites
The distributions of estimated concentrations of five air pollutants among the participants were shown in Table 2. The mean PM2.5 at residential locations was 18.1 μg/m3, which exceeded the National Ambient Air Quality Standards of Taiwan (15 μg/m3). The mean concentrations of NO2, CO, O3, and SO2, were below the national regulated levels. Moreover, NO2 was highly correlated with CO (r = 0.89) but negatively correlated with O3 (r = −0.73) (Table 3).
The effect of air pollution on body composition
The association between air pollution exposure and body composition was shown in Table 4. The 2015 average of PM2.5 at the residential locations was associated with lower skeletal muscle mass and higher body fat mass. An interquartile increase of 1.41 μg/m3 of PM2.5 reduced skeletal muscle mass by 0.4 kg (95% confidence interval (CI): −0.31, −0.58; p < 0.0001), and increased fat mass by 0.7 kg (95% CI: 0.47, 0.91; p < 0.0001), representing 2.0% and 3.6% of change respectively. In terms of the effect of air pollutant on various body parts, PM2.5 reduced the skeletal muscle mass of the upper extremities and trunk by 5.1% and 3.1%, respectively, but not the lower extremities. On the other hand, PM2.5 increased body fat mass in each part (5.7% for the upper extremities, 2.2% for the trunk, and 5.6% for the lower extremities).
Two-pollutant model to clarify the most hazardous air pollutant
In the single pollutant model (Table 4), there were mild effects of CO on skeletal muscle mass and body fat mass, with a similar pattern to the effects of PM2.5. The two-pollutant model was therefore adjusted for the confounding effect of co-pollutants (Table 5). The effect of PM2.5 on body composition was consistent even after adjustments for the co-pollutants. However, the effect of CO became statistically non-significant after adjustments for PM2.5 (Table 5).
Of the 530 relatively healthy elderly who participated in this study, there was no statistically significant effect of ambient air pollution on handgrip force (Table 4).
The modification effect of physical activity
In stratified analysis, we found physical activity significantly modified the PM2.5 effect on body compositions, the lower the physical activity the more the loss of skeletal muscle mass and the increase in body fat mass related to PM2.5 exposure (Table 6).
Discussion
This study demonstrates, for the first time, that exposure to ambient PM2.5 is associated with a reduction in skeletal muscle mass and an increase in body fat mass in the Taiwanese elderly, a population vulnerable to the effects of air pollution and to sarcopenia. Such effects are consistent even after adjusting for co-pollutants. Long-term (average of one year) PM2.5 exposure reduces muscle mass mainly on the upper extremities and trunk, but not for the lower extremities. On the other hand, ambient PM2.5 is also associated with increased body fat mass in the same body parts. Physical activity ameliorates the detrimental effect of PM2.5 on body composition.
In this study, ambient PM2.5 has a detrimental effect on muscle mass, particularly on the non-weight bearing muscles. Compared to previous research, where the average decrease in muscle mass is 6% per decade after mid-life1, the dose effect of PM2.5 in this study is approximately a 2.0% reduction in skeletal muscle mass for every 1.4 μg/m3 increase in PM2.5. This is a noteworthy issue. Although the mechanisms involved in muscle wasting among the elderly are probably multi-factorial and still poorly understood, chronic inflammation and insulin resistance are the probable explanations2. Many experimental and epidemiologic evidences have demonstrated the effects of ambient PM2.5 on exacerbating systemic inflammation and insulin resistance23, providing the biological explanation for the observed effects in this study.
In addition, the non-observed effects of PM2.5 on the lower extremities, the weight-bearing body part, and the protective role of physical activity, imply that exercise may ameliorate the deleterious effects of PM2.5 on muscle mass. Because all of the study participants have full ambulation function, general weight bearing and walking may provide the basic resistance and aerobic training activities for their lower limbs. A previous study applying a short course exercise program for elderly with sarcopenia shows that both resistance and aerobic training can increase muscle mass and strength24, suggesting that exercise may be an effective way to overcome the pathologic process of sarcopenia. Further study with a longitudinal design and exercise intervention on non-weight bearing limbs may help to clarify how body activity modifies the detrimental effect of PM2.5.
There were also significant effects of PM2.5 on increasing body fat mass. Previous animal studies have shown the direct effects of ambient PM2.5 exposure on adipose tissue15,25,26. In mammals, adipocytes are classified into two types: white adipose tissue, or the primary site of energy storage, and brown adipose tissue, which are specialized for fatty acid metabolism, energy expenditure, and heat generation27. In mice studies, long-term exposure to PM2.5 impairs the function of brown adipose tissue and changes the gene expression from brown to white adipocyte specific patterns25,26. Previous literature has linked the reduced functionality of brown adipose tissue to the propensity for obesity28. Such aforementioned evidences may explain how PM2.5 influences the development of obesity. Because fat tissue expansion can further increase insulin resistance and pro-inflammatory states3, leading to more muscle wasting2,11,12,13,14, the differential or possibly even synergetic effects of PM2.5 on muscle and fat tissue established in this study may enhance the pathologic process toward sarcopenia.
Although there was no significant effect of PM2.5 on handgrip force, it may be too premature to conclude that ambient PM2.5 has no effect on muscular function. This study enrolled the elderly mostly living in the Taipei Basin, where the range of long-term PM2.5 exposure is not wide. This may hamper the detectability of PM2.5-associated changes in muscular functionality. Future studies enrolling participants living in areas with higher and wider ranges of air pollution exposure may provide a better picture for examining the dose-response relationship.
There are several limitations to our study. First of all, our study has chosen the Kriging interpolation rather than the satellite-based approach29,30,31 or land-use regression32 for exposure assessments. This may lead to certain degree of non-differential misclassification bias. Yet due to the meteorological conditions and cloud contamination in Northern Taiwan, the satellite-based aerosol optical depth measurements have high missing rates in Taipei. This hampers the utilization of the satellite-based approach in our study. Furthermore, the published land-use regression model33 for PM2.5 in Taiwan was based on data from automatic beta attenuation measurements. However, this method can be interfered by the high humidity in Taiwan34. Accordingly, the Taiwan EPA has decided to report PM2.5 concentration by manual sampling using the US Federal Reference Method as our standard methods since 2014. Moreover, the urbanization similarity and monitoring station density are high in Taipei, the misclassification bias by Kriging method should be small. (There are 16 monitoring stations in the Taipei Basin with approximately area of 243 km2. Most of our study subjects resided in a 15 km * 15 km area with 13 monitoring stations.) Owing to aforementioned reasons, our study finally decided to use the Kriging interpolation for PM2.5 concentration estimation. This method may not totally avoid misclassification bias, but the influence may be trivial and acceptable. Also, a significant exposure response trend between quartile of PM2.5 exposure and body composition parameters is found, which supports the existence of the correlation (see Supplementary Fig. S1).
Secondly, since our study has used a cross-sectional design, precaution has to be taken in determining a causal relationship. Further longitudinal studies are needed to confirm the effect of PM2.5 on declining muscle mass by age. To minimize the potential confounding effect on causal inference, this study statistically controlled several co-variables and chronic disease status related to body composition in previous literature. In particular, physical activity has been adjusted by applying the total score of the Physical Activity Scale for the Elderly, a value indicating daily activity-related caloric expenditure.
Lastly, the limitation of not controlling the nutrition intake should be noted. Because only generally healthy elderly were enrolled, or those who could walk to the hospital on their own to get the regular health exam, it is also likely that they can get their daily food without difficulty. Financial problems in buying daily food are also less likely in this study population because Taiwanese elderly have monthly paid pension, which fulfills their basic daily requirement. Also, there are different effects of PM2.5 on muscle and on fat tissues, and on the upper and lower limb muscles. These are less likely to be explained by nutritional status since malnutrition reduces both muscle and fat mass throughout the entire body.
In conclusion, this study reveals that long-term exposure to urban PM2.5 is associated with reduced skeletal muscle mass and increased body fat mass among healthy elderly living in Taipei Basin, where the annual average of PM2.5 concentration is much higher than the regulated levels suggested by the World Health Organization and where traffic emission is the main source of ambient fine particles. Physical activity ameliorates the detrimental effect of PM2.5 on skeletal muscle mass and body fat mass. Further studies with a longitudinal design and conducted in areas with higher and wider ranges of PM2.5 exposure are warranted to verify the causal relationship and determine the effects on muscle function.
References
Janssen, I. Evolution of sarcopenia research. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 35, 707–712, https://doi.org/10.1139/h10-067 (2010).
Wang, C. & Bai, L. Sarcopenia in the elderly: basic and clinical issues. Geriatrics & gerontology international 12, 388–396, https://doi.org/10.1111/j.1447-0594.2012.00851.x (2012).
Choi, K. M. Sarcopenia and sarcopenic obesity. The Korean journal of internal medicine 31, 1054–1060, https://doi.org/10.3904/kjim.2016.193 (2016).
Hirani, V. et al. Sarcopenia Is Associated With Incident Disability, Institutionalization, and Mortality in Community-Dwelling Older Men: The Concord Health and Ageing in Men Project. Journal of the American Medical Directors Association 16, 607–613, https://doi.org/10.1016/j.jamda.2015.02.006 (2015).
Kim, J. H. et al. Sarcopenia: an independent predictor of mortality in community-dwelling older Korean men. The journals of gerontology. Series A, Biological sciences and medical sciences 69, 1244–1252, https://doi.org/10.1093/gerona/glu050 (2014).
Atkins, J. L. et al. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. Journal of the American Geriatrics Society 62, 253–260, https://doi.org/10.1111/jgs.12652 (2014).
Wannamethee, S. G., Shaper, A. G., Lennon, L. & Whincup, P. H. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. The American journal of clinical nutrition 86, 1339–1346 (2007).
Tian, S. & Xu, Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatrics & gerontology international 16, 155–166, https://doi.org/10.1111/ggi.12579 (2016).
Abbatecola, A. M. et al. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. The journal of nutrition, health & aging 15, 890–895 (2011).
Srikanthan, P., Hevener, A. L. & Karlamangla, A. S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PloS one 5, e10805, https://doi.org/10.1371/journal.pone.0010805 (2010).
Meng, S. J. & Yu, L. J. Oxidative stress, molecular inflammation and sarcopenia. International journal of molecular sciences 11, 1509–1526, https://doi.org/10.3390/ijms11041509 (2010).
Lang, C. H., Frost, R. A., Nairn, A. C., MacLean, D. A. & Vary, T. C. TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. American journal of physiology. Endocrinology and metabolism 282, E336–347, https://doi.org/10.1152/ajpendo.00366.2001 (2002).
Schaap, L. A., Pluijm, S. M., Deeg, D. J. & Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. The American journal of medicine 119, 526 e529–517, https://doi.org/10.1016/j.amjmed.2005.10.049 (2006).
Kalinkovich, A. & Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing research reviews, https://doi.org/10.1016/j.arr.2016.09.008 (2016).
Liu, C. et al. Air pollution-mediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environmental health perspectives 122, 17–26, https://doi.org/10.1289/ehp.1306841 (2014).
Rom, O., Kaisari, S., Aizenbud, D. & Reznick, A. Involvement of E3 Ubiquitin ligases in Cigarette Smoke associated muscle catabolism. Free radical biology & medicine 75(Suppl 1), S5, https://doi.org/10.1016/j.freeradbiomed.2014.10.835 (2014).
Neves, C. D. et al. Oxidative stress and skeletal muscle dysfunction are present in healthy smokers. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 49, e5512, https://doi.org/10.1590/1414-431x20165512 (2016).
Fujiyoshi, A. et al. Lifetime cigarette smoking is associated with abdominal obesity in a community-based sample of Japanese men: The Shiga Epidemiological Study of Subclinical Atherosclerosis (SESSA). Preventive medicine reports 4, 225–232, https://doi.org/10.1016/j.pmedr.2016.06.013 (2016).
Washburn, R. A., Smith, K. W., Jette, A. M. & Janney, C. A. The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of clinical epidemiology 46, 153–162 (1993).
Ku, P.-W., Sun, W.-J., Chang, C.-Y. & Chen, L.-J. Reliability and validity of the Chinese version of the Physical Activity Scale for the Elderly. Sports and Exercise Research 15, 309–319 (2013).
Dehghan, M. & Merchant, A. T. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutrition journal 7, 26, https://doi.org/10.1186/1475-2891-7-26 (2008).
Liao, D. et al. GIS approaches for the estimation of residential-level ambient PM concentrations. Environmental health perspectives 114, 1374–1380 (2006).
EPA, D. Integrated science assessment for particulate matter. US Environmental Protection Agency Washington, DC (2009).
Chen, H. T., Chung, Y. C., Chen, Y. J., Ho, S. Y. & Wu, H. J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. Journal of the American Geriatrics Society 65, 827–832, https://doi.org/10.1111/jgs.14722 (2017).
Xu, X. et al. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicological sciences: an official journal of the Society of Toxicology 124, 88–98, https://doi.org/10.1093/toxsci/kfr211 (2011).
Xu, Z. et al. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Particle and fibre toxicology 8, 20, https://doi.org/10.1186/1743-8977-8-20 (2011).
Spiegelman, B. M. & Flier, J. S. Obesity and the regulation of energy balance. Cell 104, 531–543 (2001).
Marcelin, G. & Chua, S. Jr. Contributions of adipocyte lipid metabolism to body fat content and implications for the treatment of obesity. Current opinion in pharmacology 10, 588–593, https://doi.org/10.1016/j.coph.2010.05.008 (2010).
Zou, B. et al. High-resolution satellite mapping of fine particulates based on geographically weighted regression. IEEE Geoscience and Remote Sensing Letters 13, 495–499 (2016).
Zou, B. et al. Air pollution intervention and life-saving effect in China. Environment international 125, 529–541, https://doi.org/10.1016/j.envint.2018.10.045 (2019).
van Donkelaar, A. et al. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: development and application. Environmental health perspectives 118, 847–855, https://doi.org/10.1289/ehp.0901623 (2010).
Di, Q. et al. Assessing PM2.5 Exposures with High Spatiotemporal Resolution across the Continental United States. Environmental science & technology 50, 4712–4721, https://doi.org/10.1021/acs.est.5b06121 (2016).
Wu, C. D. et al. Land-use regression with long-term satellite-based greenness index and culture-specific sources to model PM2.5 spatial-temporal variability. Environmental pollution (Barking, Essex: 1987) 224, 148–157, https://doi.org/10.1016/j.envpol.2017.01.074 (2017).
Huang, C.-H. Field comparison of real-time PM2. 5 readings from a beta gauge monitor and a light scattering method. Aerosol and Air Quality Research 7, 239–250 (2007).
Acknowledgements
The authors thank the field workers for their assistance in the data collection, as well as the elderly who participated in this study. This study was supported by grants from the Environmental Protection Administration Executive Yuan, R.O.C. (Taiwan) (Grant NHRI-105-EMSP08), partially by the Ministry of Science and Technology (Taiwan) (MOST 106-2621-M-002-007, and MOST 107-2621-M-002-006), and partially by the National Taiwan University Hospital, Hsin-Chu Branch (Taiwan) (108-HCH075).
Author information
Authors and Affiliations
Contributions
C.H.C., H.C.C. and Y.L.G. designed the study. L.Y.H. and K.Y.L. recruited study subjects and collected data. C.D.W. performed exposure assessment. L.Y.H., B.Y.C., W.S.C. and S.C.P. involved in the interpretation of the results. C.H.C. and Y.L.G. performed the analyses and wrote the manuscript. All authors proof-read the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, CH., Huang, LY., Lee, KY. et al. Effects of PM2.5 on Skeletal Muscle Mass and Body Fat Mass of the Elderly in Taipei, Taiwan. Sci Rep 9, 11176 (2019). https://doi.org/10.1038/s41598-019-47576-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47576-9
This article is cited by
-
Review: myogenic and muscle toxicity targets of environmental methylmercury exposure
Archives of Toxicology (2024)
-
Mediating effect of body fat percentage in the association between ambient particulate matter exposure and hypertension: a subset analysis of China hypertension survey
BMC Public Health (2023)
-
Body composition modify the association between ambient particulate matter and lung function among asthma patients
Environmental Science and Pollution Research (2023)
-
Wie hängen Luftverschmutzung und Adipositas zusammen?
Info Diabetologie (2023)
-
Dynamic changes in ambient PM2.5 and body mass index among old adults: a nationwide cohort study
Environmental Science and Pollution Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.