Abstract
Carex buekii is a tall sedge, forming large stands in wetlands, particularly in river floodplains across Central Europe and thus on many sites determining the typical appearance of riverine habitats. Our paper aims at increasing the knowledge on ecology of C. buekii and its role in the wetlands. Field data were collected in Poland, the Czech Republic, Slovakia, Hungary, and Italy. Carex buekii usually occurs in nutrient rich habitats, but is also capable of colonising relatively nutrient-poor ones; it grows on both acidic and alkaline soils (pH 3.3–7.4) with diverse concentrations of assimilable elements (Ca, Mg, P, K). One of the most important ecological characteristics of C. buekii is its relationship to the floodplains of watercourses. It seems to be dependent on, or at least very tolerant to regular disturbances by streaming, floods and transport of sediments. Carex buekii usually forms relatively uniform stands of its own association, Caricetum buekii. The species most frequently accompanying C. buekii are Urtica dioica, Calystegia sepium, Galium aparine, Rubus caesius, Phalaris arundinacea, and Cirsium arvense. The sedge also occurs in the understorey of forests with e.g. Alnus glutinosa, Salix fragilis, Padus avium, and Quercus robur. Carex buekii is able to colonise man-made or man-changed habitats such as railway embankments and roadsides or regulated river banks. Taking into account the IUCN Red List Criteria we propose to regard C. buekii as a least-concern (LC).
Similar content being viewed by others
Introduction
Carex buekii belongs to the section Phacocystis, one of the largest sections of the genus Carex, with about 90 species worldwide1, including about 16 species in Europe2. The section is taxonomically very difficult due to indistinct morphological differentiation between taxa and frequent hybridisation3. Consequently, Carex buekii is frequently confused with other members of the section Phacocystis, usually with C. acuta, C. elata and C. randalpina4,5. In spite of these facts, Carex buekii is relatively well distinguishable on account of its dark reddish-brown basal leaf sheaths which display a characteristic reticulate-fibrous structure, the shiny upper side of the leaves and its nerveless or indistinctly nerved utricles with very short beaks2,6.
The sedge occurs in central-eastern Europe, in the northern part of the Balkan Peninsula and in south-eastern Asia7. Carex buekii is included in the group of “river corridor plants”8. It usually grows on river banks along large and medium-sized river beds as well as on floodplains and valley slopes9,10,11. Carex buekii is also found in man-made habitats, such as ditch and canal banks, bridgeheads and river flood embankments, and roadsides12,13.
Carex buekii usually forms dense patches of plant communities classified in the phytosociological literature as Caricetum buekii Hejný et Kopecký in Kopecký et Hejný 1965 within the class Phragmito-Magnocaricetea Klika in Klika et Novák 194114. This association has been recorded in Austria15, Croatia16, the Czech Republic17,18, Germany19,20, Hungary10,21, Poland22, and Slovakia12,23. The sedge has also been encountered as an accompanying species in wet meadow communities (Calthion Tüxen 1937, Filipendulion Segal 1966) as well as an understorey species in floodplain forests (Alno-Ulmion Br.-Bl. et R.Tx. 1943, Salicion albae R. Tx. 1955). For example, the association Filipendulo ulmariae-Caricetum buekii Háberová ex Balátová-Tuláčková in Rybníček et al. 1984 has been described from stream alluvia in the Slovenský kras Mts (southern part of Central Slovakia)24,25. Milenović and Ranđelović26 distinguished a forest association, Carici buekii-Alnetum glutinosae, which was invalidly described, as no type relevé was selected (see art. 5 of the International Code of Phytosociological Nomenclature (IPCN)27), from the upstream section of the River Sokobanjska Moravica (Serbia, the right-bank tributary of the South Morava, in the River Danube basin).
The ecology of C. buekii is rather poorly known and data on habitats with the occurrence of the species have been obtained from a few European countries only12,28. Therefore, it is difficult to evaluate its sensitivity to the recent global environmental changes, e.g. the climate change, land use change or overall eutrophication. On the other hand, it is likely that either retreat or spread of this species might have important implications for riverine wetland biodiversity, intensity of erosions, nutrient loadings and other processes.
Since the literature contains scant information on the ecology of the sedge, the present study was aimed at: (i) analysis of habitat requirements of C. buekii and its role in wetlands, (ii) exploring relationships between species composition in C. buekii-containing assemblages and habitat condition, (iii) identifying current threats and conservation needs of C. buekii in Central Europe.
Material and Methods
Field sampling
Field data were collected from May through July 2016 in Poland (20 plots), the Czech Republic (20 plots), Slovakia (21 plots), and Hungary (19 plots); in addition, the data set was supplemented by information from northern Italy (4 plots) (Fig. 1; Supplementary Tables S1 and S2). Only stands with a dominance of Carex buekii were recorded. The area of vegetation plots was usually in the range 30–100 m2. In order to obtain better insight into the ecology of C. buekii we decided to include in our research a few small plots from Poland (4 plots, 20–25 m2) and the Czech Republic (6 plots, 12–24 m2). The species cover in each plot was estimated using a nine-grade scale29. For each plot, three samples of soil were collected with Egner’s soil sampler from the plant root zone (0–25 cm). After mixing, they formed a single soil sample representing a given plot and intended for chemical analyses. The plant nomenclature follows Mirek et al.30. The nomenclature of the communities was not unified according to a single source and therefore at least the first use of each syntaxon is accompanied by the author’s name and year of the description. The names have been checked for validity according to IPCN27.
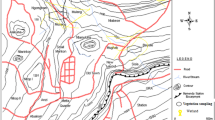
Locations of Carex buekii sampling sites and global distribution of Carex buekii (in the bottom left corner; according to Koopman et al.7; black circles – herbarium data, white circles – data from literature).
Sources of climatic data and soil analyses
Climatic data for the studied regions have been taken from the national climate/environmental atlases or databases (Supplementary Table S1).
Soil samples were dried at room temperature and sieved to remove fractions larger than 1 mm. The following properties were determined: organic matter content (as loss on ignition at 550 °C), pH (potentiometrically in 1 M KCl), concentrations of assimilable forms of: phosphorous (P), potassium (K), magnesium (Mg), and calcium (Ca) using the American Society of Agronomy method, electrolytic conductivity of the saturated soil extract (ECe, conductometrically), total organic carbon (C), and nitrogen (N) (CHNS analyser, Costech Analytical Technologies Inc.)31. The last assay provided data for calculation of the ratio between C and N (C/N). A granulometric analysis was carried out according to Bouyoucos’s areometric method in the Casagrande and Prószyński modification32.
Data analysis
The basic statistical metrics (arithmetic mean, minimum and maximum values) were determined for each of the studied soil properties, separately for data from Poland, the Czech Republic, Slovakia, Hungary, and Italy. The Shannon’s diversity index and the evenness were calculated for each relevé, using the MVSP package33. Relations between plot size and species number, evenness and Shannon’s diversity index were assessed on the basis of Spearman’s correlation coefficient. Statistical significance of differences between empirical distributions of the data analysed and the theoretical normal distribution was examined using the Shapiro–Wilk test. Since distributions of most data sets deviated from normal, the non-parametric Kruskal–Wallis test and Dunn’s multiple comparison test were used to examine whether differences between sites of C. buekii and between the diversity and evenness indices were significant. Calculations and graphs were prepared using the software package Statistica v. 12.0 for Windows34.
Plant species distribution patterns in relation to environmental variables were analysed by the canonical correspondence analysis (CCA), after the detrended correspondence analysis (DCA) had detected a unimodal structure of the species data (the gradient length represented by the first ordination axis higher than 3 SD). The environmental data were not transformed.
Tests of significance of the first and all canonical axes were performed for the statistical assessment of the relationship between the plant species composition and environmental variables (Monte Carlo test: 499 permutations under reduced model).
The Monte Carlo permutation test was further applied to test the statistical significance of environmental variables in explaining the plant species composition. For this purpose, stepwise “forward selection” of explanatory variables was used. The software package CANOCO v. 4.5 was used for the analyses35.
Evaluation of threat level
The status of C. buekii was assessed using the IUCN Red List Criteria36, which are the world’s most widely accepted system for measuring extinction risk. The assessment has followed the Guidelines for Application of IUCN Red List Criteria at Regional Level37. The criteria for evaluating the level of endangerment of plant communities (i.e. current status, past trend and prognosis) has followed Berg et al.38.
Results
Habitat conditions
Carex buekii is a lowland species; most of the localities in this study were situated at elevations of 95–480 (−616) m (Fig. 2a; Supplementary Table S1).
Ranges of environmental variables at C. buekii sites. Large boxes indicate 25–75% of the interquartile ranges; small boxes - medians; white circles - outliers; asterisks - extreme values. (a) Elevation; (b) Ratio between organic carbon and nitrogen concentration; (c) Organic matter content; (d) Soil pH; (e) Calcium concentration; (f) Magnesium concentration; (g) Phosphorus concentration; (h) Potassium concentration; (i) Electrolytic conductivity of the saturated soil extract.
The mean annual temperature at C. buekii sites usually ranged from 7 °C to 12 °C and the annual sum of precipitation from 500 mm to 800 mm (Supplementary Table S1).
The C/N mean for all samples was 12.8 and ranged from 9.8 to 18.5 (−23.3) (Table 1; Fig. 2b). Most soil samples can be described as mineral-humic, except for some samples from Poland, being organic-mineral.
The mean organic matter content (about 10.0%) was similar for most of the samples. Outlying values were determined for the Slovak and Hungarian sites (2.6 and 22.2%, respectively) (Table 1; Fig. 2c).
The soil pH was found to span a wide range, from strongly acidic (16%) to alkaline (21%). Most soil samples (63%), however, were acidic or slightly acidic (pH 4.5–6.5, mean 5.5). The Slovak samples were distinct in their relatively high pH values, with a maximum of 7.6 (Table 1; Fig. 2d).
The mean concentrations of assimilable forms of elements of C. buekii sites were: 3299.9 mg/kg (range 34.2–33633.3) for Ca, 176.9 mg/kg (range 12.3–2056.0) for Mg, 288.7 mg/kg (range 31.0–985.0) for P and 331.3 mg/kg (range 41.8–974.0) for K (Fig. 2e–h; Table 1). In terms of concentrations of assimilable elements in the soil samples from countries examined, (i) the highest mean concentrations of Ca and Mg were detected in the Slovak and Hungarian samples (some of which showed outlying values); (ii) the mean P concentration was high in the samples collected in Poland compared to the other countries (there were two outliers with the highest P concentrations among the samples); (iii) the highest K concentration was recorded in the Slovak samples, the lowest being found in samples from Hungary and Italy (Table 1; Fig. 2e–h).
The soil conductivity expressed as conductivity of the soil saturated extract was very low; all the samples showed a values below 1 dS·m−1 (Table 1; Fig. 2i).
The soil of all habitats examined consisted mainly of muddy and clayey silts (50%), the remainder fractions representing sand and different clays (Fig. 3). The highest content of clay was found in the Polish and Hungarian samples, while the highest content of silts in the Slovak and Hungarian samples. Significant differences in clay content were revealed between samples from Poland and the Czech Republic as well as between samples from Poland and Slovakia (Supplementary Table S3). Significant differences in silts content were found between samples from Hungary and the Czech Republic as well as between samples from Slovakia and the Czech Republic.
The results of the Kruskal–Wallis test and post hoc Dunn’s multiple comparisons test indicated that the C. buekii stands in the investigated countries vary significantly in eleven environmental variables (elevation, precipitation, temperature, soil pH, soil fractions, C/N ratio, and the concentrations of K, Ca and Mg) (Supplementary Table S3). The highest number of statistically significant differences was found between Polish and Slovak samples as well as between those collected in Poland and Hungary.
Diversity of plant communities with Carex buekii
Carex buekii is as a dominant species within the Caricetum buekii association. The vegetation samples examined were found to contain a total of 178 species; 83 species occurred in less than 3 plots (47% of the species turned sporadic). The species most frequently accompanying C. buekii were Urtica dioica (61% of relevés), Calystegia sepium (45%), Galium aparine (40%), Rubus caesius (36%), Phalaris arundinacea (35%), and Cirsium arvense (32%) (Supplementary Table S2). In addition, more than 50% of the Polish, Slovak, and Hungarian vegetation samples contained Vicia cracca, Galium rivale and Solidago gigantea, respectively. The phytocoenoses with C. buekii were relatively species poor; overall, an average of 10 species per phytosociological relevé was recorded (Supplementary Table S4). The value of the Shannon’s diversity index ranged from 0, in single-species patches (relevés 22, 23 and 36) up to 4.25 (mean 2.82) in patches composed of more than 20 species (e.g. relevé 71) (Supplementary Tables S1 and S4).
The Hungarian, Polish and Italian vegetation samples showed a higher species richness (mean number of species 14, 12 and 11, respectively), a higher biodiversity index (equal or higher than 3.20) and a more even distribution of species between assemblages (evenness equal or higher than 0.90), compared to the plots sampled in the Czech Republic and Slovakia (Supplementary Table S4). The Polish and Czech relevés differed significantly in terms of species richness, diversity and evenness. Low correlations between plot size and species number, evenness and Shannon’s diversity index were detected (Spearman correlation coefficients = 0.4006, 0.2172 and 0.3917, respectively).
Most of the phytocoenoses were growing in open-terrain, rush-vegetated areas. Moist forests in which C. buekii was the understorey dominant, yielded 14 relevés (13 in tree stands with Alnus glutinosa, Padus avium, Salix fragilis, and Salix alba in Hungary; one relevé in a tree stand with Quercus robur in Poland). In addition, 3 vegetation samples in Italy showed a single occurrence of the tree species mentioned above. Some other relevés, e.g. from the rivers Svratka, Svitava and Orlice in the Czech Republic, have been situated on the forest margins, thus being influenced by shading (Supplementary Table S1) and by the transition of forest species such as Aegopodium podagraria, Impatiens parviflora and Stellaria nemorum.
Distribution of plots and species along environmental gradients
All the variables included in CCA accounted for 18.23% of the total variance in the species data (Table 2). All the canonical axes were significant as tested by the unrestricted Monte Carlo permutation test (the first axis: p = 0.021; all axes: p = 0.005).
The results of the forward selection of variables revealed seven variables (precipitation, pH, organic matter content, elevation, temperature, K and P) to be statistically significant, and accounting for 13.4% of the total variance in the species composition (Table 3).
The results of CCA showed different position of samples in ordination space depending on the studied region (Fig. 4). The lower right quarter of the diagram contains Hungarian forest samples formed by Alnus glutinosa, Salix alba and Salix fragilis, related to relatively high values of precipitation and temperature, moderate values of organic matter content and elevation, and relatively low and moderate values of pH, and K and P concentrations. This part of the diagram additionally features one Polish forest sample formed by Quercus robur in a tree layer. The upper right quarter of the diagram contains samples from Italy, including three phytocoenoses with scattered occurrence of trees, Quercus robur, Alnus glutinosa and Salix alba, related to relatively high values of precipitation, temperature and elevation, moderate values of pH and P concentrations, and relatively low values of organic matter content and K concentrations. Five forest samples placed in the lower left quarter of the diagram are obtained from Hungarian forests with occurrence of Padus avium in the tree layer, related to relatively low values of precipitation and temperature, moderate values of organic matter content and pH, and relatively high values of K and P concentrations. Most of the phytocoenoses with C. buekii growing in open-terrain, rush-vegetated areas, are located in the left part of the ordination space. The upper left quarter of the diagram contains non-forest samples mainly from the Czech Republic and Slovakia, related to relatively low values of precipitation and temperature, low and moderate values of organic matter content, relatively high and moderate values of pH and elevation, and relatively high values of P and K concentrations. Most samples collected in Poland are located in the lower left quarter of the diagram, including non-forest phytocoenoses related to relatively low values of precipitation and temperature, high and moderate values of organic matter content, P and K concentration, and relatively low and moderate values of pH and elevation (Fig. 4).
Ordination diagram of vegetation samples and environmental variables along the first two CCA axes. Poland – boxes, the Czech Republic – circles, Slovakia – asterisks, Hungary – up-triangles, Italy – left-triangles; grey symbols – open-terrain samples, black symbols – forest and shrub samples; elevat – elevation; temp – mean annual temperature; precip – annual sum of precipitation; *statistically significant variables. For abbreviations of soil properties, see Table 1.
As shown by the ordination diagram of species and environmental variables, the maximum abundance of C. buekii, together with a group of most frequently accompanying species located in the central part of the diagram, is related to moderate values of all statistically significant variables (Fig. 5).
Ordination diagram of species and environmental variables along the first two CCA axes. Herb layer – black triangles; shrub layer – black squares, tree layer – black circles; precip – annual sum of precipitation; elevat – elevation; temp – mean annual temperature; *statistically significant variables. For abbreviations of soil properties, see Table 1. Abbreviations (96 species, without 83 sporadic species): Aeg.pod - Aegopodium podagraria, All.pet - Alliaria petiolata, Aln.gluA - Alnus glutinosa (tree layer), Alo.pra - Alopecurus pratensis, Ane.nem - Anemone nemorosa, Ant.syl - Anthriscus sylvestris, Arr.ela - Arrhenatherum elatius, Art.vul - Artemisia vulgaris, Bid.fro - Bidens frondosa, Cal.epi - Calamagrostis epigeios, Cal.sep - Calystegia sepium, Car.acu - Carex acutiformis, Car.bri - Carex brizoides, Car.bue - Carex buekii, Car.hir - Carex hirta, Car.ela - Carex elata, Car.pra - Carex praecox, Che.maj - Chelidonium majus, Cir.arv - Cirsium arvense, Con.arv - Convolvulus arvensis, Cor.sanC - Cornus sanguinea (herb layer), Cra.monB - Crataegus monogyna (shrub layer), Cra.monC - Crataegus monogyna (herb layer), Dac.glo - Dactylis glomerata, Des.cae - Deschampsia caespitosa, Equ.arv - Equisetum arvense, Equ.pal - Equisteum palustre, Euo.eur - Euonymus europaeus, Eup.can - Eupatorium cannabinum, Fil.ulm - Filipendula ulmaria, Fil.vul - Filipendula vulgaris, Fra.ves - Fragaria vesca, Gal.bif - Galeopsis bifida, Gal.spe - Galeopsis speciosa, Gal.tet - Galeopsis tetrahit, Gal.apa - Galium aparine, Gal.bor - Galium boreale, Gal.mol - Galium mollugo, Gal.riv - Galium rivale, Gal.ver - Galium verum, Geu.urb - Geum urbanum, Her.sph - Heracleum sphondylium, Hol.lan - Holcus lanatus, Hum.lup - Humulus lupulus, Hyp.per - Hypericum perforatum, Imp.nol - Impatiens noli-tangere, Imp.par - Impatiens parviflora, Jun.effJuncus - effusus, Lam.mac - Lamium maculatum, Lat.pra - Lathyrus pratensis, Lyc.flo - Lychnis flos-cuculi, Lys.vul - Lysimachia vulgaris, Lyt.sal - Lythrum salicaria, Men.lon - Mentha longifolia, Myo.pal - Myosotis palustris, Myo.aqu - Myososton aquaticum, Pad.aviA - Padus avium (tree layer), Pet.hyb - Petasites hybridus, Pha.aru - Phalaris arundinacea, Phr.aus - Phragmites australis, Poa.pra - Poa pratensis, Poa.tri - Poa trivialis, Pol.amp - Polygonum amphibium f. terrestre, Pol.hyd - Polygonum hydropiper, Pot.rep - Potentilla reptans, Pru.spiC - Prunus spinosa (herb layer), Que.robA - Quercus robur (tree layer), Ran.acr - Ranunculus acris, Rub.caeC - Rubus caesius (herb layer), Rub.ida -Rubus idaeus, Rub.fruC - Rubus fruticosus agg. (herb layer), Rum.ace - Rumex acetosa, Rum.thy - Rumex thyrsiflorus, Sal.albA - Salix alba (tree layer), Sal.fraA –Salix fragilis (tree layer), Sam.nigC - Sambucus nigra (herb layer), San.off - Sanguisorba officinalis, Sci.syl - Scirpus sylvaticus, Scr.nod - Scrophularia nodosa, Scu.gal - Scutellaria galericulata, Sel.cer - Selinum carvifolia, Sil.alb - Silene alba, Sol.dul - Solanum dulcamara, Sol.gig - Solidago gigantea, Sta.pal - Stachys palustris, Sta.syl - Stachys sylvatica, Ste.gra - Stellaria graminea, Ste.med - Stellaria media, Sym.off - Symphytum officinale, Tan.vul - Tanacetum vulgare, Tha.luc - Thalictrum lucidum, Urt.dio - Urtica dioica, Ver.lon - Veronica longifolia, Vib.opu - Viburnum opulusC (herb layer), Vic.cra - Vicia cracca.
Threat level of Carex buekii
The current evaluation of the species situation concerning Central Europe with the use of IUCN guidelines at regional level37 indicates that the category LC (Least Concern) is suitable for C. buekii. This sedge does not meet any of the A-E criteria defined in relation to the categories CR (critically endangered), EN (endangered) and VU (vulnerable). Although, C. buekii is classified as a river corridor plant and its habitats are severely fragmented, the extent of occurrence (EOO) and/or area of occupancy (AOO) are larger than the threshold given for the NT category36. Moreover, this sedge is able to colonise man-made or man-changed habitats such as railway embankments and roadsides or regulated river banks. These facts prove the adaptation abilities of this species.
The threat category of vegetation predominated by C. buekii was determined as LC (Least Concern). This category includes rare or infrequent plant communities, currently not threatened by human activities nor declining. The current status, i.e. the present number, distribution and size of the stands in the studied area, was estimated as infrequent (total area of stands moderately large and occurrence in 11–33% of the geographically defined units). The trend in the past, as constant (more or less constant situation or only minor local loss, ±10% range) and the prognosis, i.e. the properties of existing or foreseeable, directly or indirectly, effects the survival of a plant community type, as low negative direct or indirect impacts.
Discussion
Ecology of Carex buekii
According to Ellenberg et al.39, C. buekii requires sunny (Ellenberg indicator value, EIV for light = 8), moist (EIV = 8), nutrient rich (EIV = 6), and alkaline (EIV = 8) sites. Studies conducted by Heerde et al.28 revealed a need to verify the indicators shown above. The authors demonstrated that, in Germany, C. buekii can grow in habitats with a varying degree of insolation (the sedge occurred also at shaded sites), showing a wide range of humidity (even at considerably drier habitats) and diverse pH values. In the countries we obtained our data from, the sedge occurs both at open and sunny sites and in moderately shaded forest localities. Carex buekii is also highly tolerant to habitat moisture variation. For example, it grew, in a good condition, on inundated meadows as well as overdried soil of dykes and embankments13. The tolerance to variable moisture is most probably related to the sedge’s root length, as the roots can grow to as much as 3 m down in the soil14. The sedge usually occurred in nutrient rich habitats, but was also capable of colonising relatively nutrient-poor ones. Moreover, it seems that the occurrence of C. buekii is not limited by the soil pH. At the sites we investigated, the species grew on both acidic and alkaline soils; only in Slovakia the sedge was more frequently encountered on higher-pH soils12, however, this may be related to a generally rare occurrence of acidic soils in this country. Similarly, diverse concentrations of assimilable elements (Ca, Mg, P, K) in the soil did not limit the species’ occurrence. We observed diverse soil conditions both at open-terrain rush-covered C. buekii habitats and in forested ones. Among the types of soil which are probably avoided by C. buekii, are saline soils, representing extreme conditions for lots of plant species. All the samples have shown very low conductivity of the saturated soil extract which is typical of soils with low concentrations of soluble salts40. On the other hand, this sedge avoids peaty substrates, probably due to an extremely low amount of accessible nutrients. These relationships are also obvious from the distribution of C. buekii in all the countries; it grows neither in the regions with frequent occurrence of salt marshes nor in peatland areas41. More detailed ecological studies involving a larger data set from the entire distribution range of this sedge are doubtlessly required in the future. With data from these regions it will be possible to comprehensively define the ecological range of C. buekii on a global scale.
One of the most important ecological characteristics of C. buekii is its very strong relationship to the floodplains of watercourses, particularly of large rivers8. It seems to be dependent on, or at least very tolerant to regular disturbances by streaming, floods and transport of sediments42. Although it forms large stands, also on the sites relatively far away from active river beds and without regular floods, it is not clear if these occurrences may be stable from the long-term perspective (i.e. dozens of years). The stands on man-made (e.g. dykes) as well as natural and near-nature habitats subjected to recent land use and environmental changes (e.g. abandoned meadows, terrestralised floodplain pools and river arms) have not occurred in the landscape for sufficiently long time to enable any reliable conclusions. On the one hand, a lot of the populations have been repeatedly mapped on the same sites during the last 10–20 years. On the other hand, it cannot be excluded that very long sedimentation of a thick litter layer produced by C. buekii might finally lead to the lowering of vitality of its stands, similarly as in many other reed bed and tall sedge species43.
Our study sites are situated on the climatic gradient from south-western Poland (with the northernmost locality in the River Kaczawa valley at Kwiatkowice) to north-western Italy (with the southernmost locality in the River Malone valley at Front) well representing the climatic conditions in which C. buekii is able to grow44. Although the climate certainly has important implications for the development of any vegetation type, it seems to be relatively less important in some types of wetlands, particularly in river floodplains that usually exhibit a specific local climate compared to the surrounding landscape outside the floodplains. In this study, we have used the data available on macro-climatic conditions of the regions where the relevés were recorded. A better insight into the local climatic conditions directly on the study sites could be obtained by continual measurements using temperature sensors. Additionally, floodplains contribute not only to the buffering of climate extremes but also their soils usually originate in mixing soil particles from the broad surroundings. Therefore, it may be supposed that interactions between climate and soil properties (e.g. the amount of nutrients, calcium and soil pH), frequently modifying species’ distribution and habitat ecology on a large scale, are not very important in floodplains. Most common among the temperate European species and communities is the preference of nutrient and/or calcium-rich habitats in the regions with relatively cold and humid climate and either avoidance of these conditions, or widening of the ecological niche towards nutrient and calcium poorer habitats in relatively warm and dry climates18,45. Our data do not indicate any important relationship in this sense. Therefore, we suggest that habitat type, surrounding vegetation, management and partly also soil properties are the main forces shaping the overall appearance, dynamics and species composition of the vegetation predominated by C. buekii. Climatic factors, on the other hand, form the limits for the occurrence or non-occurrence of the species and its stands.
Vegetation characteristic of Carex buekii
Despite differences in Shannon’s diversity and evenness, the floristic composition in stands with C. buekii dominance is generally similar in all the studied countries. In Europe, the species listed as those accompanying C. buekii most often include Calystegia sepium, Urtica dioica, Phalaris arundinacea, Galium aparine, Cirsium arvense, Filipendula ulmaria, Rubus caesius, and Symphytum officinale16,20,21. Thus, it seems that the vegetation dominated by C. buekii is relatively uniform within its distribution range which we confirmed also in this study. This result is not surprising because C. buekii is very strongly dominant, not allowing co-occurrence of many other species. Although the cover of herb layer usually does not reach 100%, a very dense root system and dense and thick litter layer form conditions unsuitable for the establishment of other species and thus monocoenoses of C. buekii are not rare. Whenever other species occur, these are usually common wetland, grassland and nitrophilous species, spreading from the surrounding vegetation. Most of the accompanying species are perennials with dispersal by vegetative propagules. Annual plant species are much less common, as the thick litter layer prevents seed germination and seedling recruitment46. Therefore, annuals such as Bidens frondosa, Myosoton aquaticum or Galeopsis spp. usually occur only in the stands where the continual litter layer is somewhat disturbed, e.g. by water or on resting sites of game. Galium aparine is the only frequent annual because, according to our experiences, it is able to germinate even on a thick layer of undecomposed biomass.
Despite the relatively low species diversity of its stands and unstable overall species composition (i.e. most of the species are treated just as accompanying ones, without any syntaxonomical value), Caricetum buekii has been placed in different higher syntaxa16,21,47. Kopecký and Hejný14 described the association Caricetum buekii and assigned it to the alliance Phalaridion arundinaceae Kopecký 1961 within the order Nasturio-Glycerietalia Pignatti 1953 and the class Phragmito-Magnocaricetea Klika in Klika et Novák 1941. However, the alliance Phalaridion arundinacae has not been accepted by many authors due to its poor floristic differenciation. Philippi48 placed Caricetum buekii in the alliance Magnocaricion Koch 1926, the order Phragmitetalia Koch 1926 and the class Phragmito-Magnocaricetea. Further, Ellmauer and Mucina15 assigned this plant community to the alliance Calthion Tüxen 1937, the order Molinietalia Koch 1926 and the class Molinio-Arrhenatheretea Tüxen 1937. Kopecký and Hejný14 recorded the presence of C. buekii under the canopy of trees and shrubs representing the genera Salix and Alnus. Similarly, Alegro and Markovič9 listed C. buekii as an understorey component of floodplain forests, but did not provide any clear indication of the syntaxonomic affiliation. Further, Milenović and Ranđelović26 identified a forest association Carici buekii-Alnetum glutinosae (invalid description according to IPCN, art. 5) and placed it in the class Alnetea glutinosae Br.-Bl. et Tüxen ex Westhoff et al. 1946. Also our own research shows C. buekii to be capable of growing in the understorey of floodplain forests formed by Alnus glutinosa, Salix fragilis, Quercus robur, Padus avium, and Salix alba. In summary, it should be emphasised that C. buekii is most often mentioned as a dominant species within the Caricetum buekii association, which, according to a majority of literature sources, is included in the Phragmito-Magnocaricetea class.
Threats, conservation recommendations and ecosystem functions
Carex buekii’s capability of colonising anthropogenically altered sites such as dykes, banks of ditches and roadsides, may indicate the species’ tolerance to anthropogenic pressure. Direct destruction of phytocoenoses and habitats, as a result of changes in land use (disturbance of river valleys, earthworks associated with river regulation, embankment uplift, floodplain use as arable land, lowering of the groundwater level, etc.) seems to be the major threat for the sedge as well as for other species from the group of “river corridor plants”, e.g. Allium schoenoprasum (LC category according to IUCN Red List), Alisma lanceolatum (LC), Cyperus michelianus (LC), Mentha pulegium (LC), Limosella aquatica (LC), Lindernia procumbens (LC), Salvinia natans (LC), Veronica catenata (LC)8,42. In addition, construction of roads and associated infrastructure leads to habitat fragmentation and overdrying. Wet meadows in river valleys with a mass occurrence of C. buekii are generally subjected to overgrowth of shrubs and woody species. Although C. buekii is also found in forests, it occurs there in plots with scattered trees rather than with a compact tree canopy. Alien species, including those regarded as invasive, e.g. Solidago gigantea, often grow in communities predominated by C. buekii, shown by our data from Hungary, Poland and Slovakia. Most likely, however, anthropophytes do not pose a threat to C. buekii as these rather competitive species are mainly unable to compete with this perennial, tuft-forming sedge with its long and thick rhizomes.
Conservation of C. buekii-supporting communities requires elimination of activities which directly contribute to destruction of the species and its habitat. Although the stands of C. buekii do not seem to be very important for preservation of other plant species, its overall ecosystem function, e.g. its relationships to various groups of animals, fungi and micro-organisms, is still poorly known. The scarce studies suggest that C. buekii may serve as an important host plant for some groups of insects49. It should be emphasised that destructive anthropogenic activities represent a highly negative pressure also on the whole range of other species and plant communities related to floodplains. Some of them, for instance floodplain wet meadows, are very valuable from the point of view of wetland biodiversity conservation50,51,52 and they are even more threatened than Caricetum buekii. In contrast, they suffer from the spread of Caricetum buekii in floodplains after abandonment of mowing16,18. Thus, the optimal management of floodplain habitats should lead to a mosaic of various plant communities, offering suitable conditions for a high number of plant and animal species. For instance, floodplains with various non-forest vegetation types are known as biodiversity hot spots for Odonata53. Functions of typical floodplain communities in the landscape are indispensable; they do not only maintain unique organisms and a high level of biodiversity, but they also contribute to the protection against erosion and floods of large areas54,55.
Conservation status
Schnittler and Günther56, who analysed national Red Lists and distribution maps in European countries, concluded that C. buekii is a species threatened in Europe, and placed it in the category vulnerable (VU). However, this sedge was not taken into account on the red list of vascular plants in Europe, published in 201157.
In Poland, C. buekii has been placed both in the Red Book of Plants13 and on the Red List58 as a near-threatened (NT) species, although earlier Kopeć and Michalska-Hejduk59 suggested category CR (critically endangered). Most records of this sedge are from the south-western part of the country, from the Odra river valley in the vicinity of Wrocław13,22. Carex buekii is scattered throughout the Czech Republic41 and, like in Poland, it is regarded as a near-threatened (NT) species60. In Slovakia, the sedge is known from localities in almost the whole country; numerous data are known from basins and hilly regions in Central Slovakia and a more scattered occurrence from lowlands, basins and mountains in Western and Eastern Slovakia12,61. In Slovakia, the sedge is regarded as a least-concern (LC) species62. Carex buekii is widespread in SW and NE Hungary at higher altitudes63 and does not feature on the Hungarian Red List of vascular plants64. In Italy, the species is confirmed in Northern Piedmont only7. Carex buekii does not feature on the Red List of the Italian flora65, but as it is currently only known from a few isolated Italian sites, it should be granted the threatened species status7. In other European countries within the species’ range (Austria, Bulgaria, Germany, Romania, SW Russia, Serbia and Ukraine), the sedge has not been listed as threatened, except for Slovenia and Croatia where it is regarded as vulnerable (VU) and near-threatened (NT), respectively7,66. The occurrence of C. buekii in Bosnia and Herzegovina, Latvia and Lithuania is questionable and needs confirmation. Recently, a few old vouchers of C. buekii have been found in Greece (one record), Macedonia (two records) and Switzerland (three records)7. Outside Europe, the sedge grows in Azerbaijan, Georgia, Lebanon (only two records) and Turkey; the presence in Kazakhstan must be considered doubtful and needs confirmation7.
It should be emphasised that the occurrence of C. buekii is closely related to river floodplains, and as a river corridor plant8 this species should be considered as vulnerable to any human activity directed to transformation of European and Asian river valley habitats. However, taking into account the IUCN Red List Criteria, as well as its ability to colonise man-made or man-changed habitats, we propose to regard C. buekii currently as least-concern species (LC) within the centre of its occurrence.
References
Standley, L. A., Cayouette, J. & Bruederle, L. Carex L. sect. Phacocystis Dumort. (eds Ball, P. W., Reznicek, A. A. & Murray, D. F. Flora of North America north of Mexico, 23. Magnoliophyta: Commelinidae (in part): Cyperaceae) 379–401 (Oxford University Press, 2002).
Chater, A. O. Carex L. (eds Tutin, T. G. et al. Flora Europaea, 5. Alismataceae to Orchidaceae (Monocotyledones) 290–323 (Cambridge University Press, 1980).
Cayouette, J. & Morisset, P. Chromosome studies of natural hybrids between maritime species of Carex (section Phacocystis and Cryptocarpae) in northeastern North America and their taxonomical implication. Canad. J. Bot. 63, 1957–1982 (1985).
Wallnöfer, B. Die Entdeckungsgeschichte von C. randalpina B. Wallnöfer spec. nov. (=“C. oenensis“) und deren Hybriden. Linzer Biol. Beitr. 25(2), 709–744 (1993).
Stoeva, M., Uzunova, K., Popova, E. & Stoyanova, K. Patterns and levels of variation within section Phacocystis of genus Carex (Cyperaceae) in Bulgaria. Phytologia Balcanica 11, 45–62 (2005).
Egorova, T. V. The sedges (Carex L.) of Russia and adjacent states (within the limits of the former USSR). St. Petersburg State Chemical-Pharmaceutical Academy, St. Petersburg, Missouri Botanical Garden Press, Saint-Lous (1999).
Koopman, J. et al. Global distribution of Carex buekii (Cyperaceae) reappraised. Phytotaxa 358(2), 139–161 (2018).
Burkart, M. River corridor plants (Stromtalpflanzen) in Central European lowland: a review of poorly understood plant distribution pattern. Global. Ecol. Biogeogr. 10, 449–468 (2001).
Alegro, A. L. & Markovič, L. Carex buekii Wimm. (Cyperaceae) in the flora of Croatia. Nat. Croat. 8(2), 101–107 (1999).
Hrivnák, R., Oťaheľova, H., Valachovič, M., Cvachová, A. & Balázs, P. Aquatic and marsh plant communities of an inundation area of the Ipeľ River (rkm 96–119). Kitaibelia 6(2), 267–279 (2001).
Smoczyk, M. & Čejková, A. Rozšíření Carex buekii Wimm. na horním toku Divoké Orlice. Acta Mus. Richnov., sect. natur. 20(1-2), 7–18 (2013).
Hrivnák, R. Caricetum melanostachyae Balász 1943 a Caricetum buekii Hejný et Kopecký 1965 na strednom Slovensku. Bull. Slov. Bot. Spoloč. 22, 215–227 (2000).
Dajdok, Z. et al. (Polska Czerwona Księga Roślin) 729-731 (Instytut Ochrony Przyrody PAN, Instytut Botaniki im. W. Szafera PAN, 2014).
Kopecký, K. & Hejný, S. Allgemeine Charakteristik der Pflanzengesellschaften des Phalaridion arundinaceae – Verbandes. Preslia 37, 53–78 (1965).
Ellmauer, T. & Mucina, L. Molinio-Arrhenatheretea (eds Mucina, L., Grabherr, G. & Ellmauer, T. Die Pflanzengesellschaften Österreichs. Teil 1. Anthropogene Vegetation) 297–401 (Gustav Fischer Verlag, 1993).
Stančić, Z. New plant community (Caricetum buekii Hejný et Kopecký in Kopecký et Hejný 1965) from Croatia. Nat. Croat. 17(1), 15–26 (2008).
Hanáková, P. & Duchoslav, M. Vegetace rákosin a vysokých ostřic (tř. Phragmito-Magnocaricetea) nivy Moravy v Hornomoravském úvalu II. vegetace ř. Oenanthetalia aquaticae a Nasturtio-Glycerietalia. Čas. Slez. Muz. Opava (A) 52, 75–87 (2003).
Šumberová, K. et al. Vegetace rákopis a vysokých ostřic (Phragmito-Magno-Caricetea) (ed. Chytrý, M. Vegetace České republiky 3. Vodní a mokřadní vegetace) 385–579 (Academia, Praha, 2011).
Walentowski, H., Raab, B. & Zahlmeister, W. A. Vorläufige Rote Liste der in Bayern nachgewiesenen oder zu erwartenden Pflanzengesellschaften. Ber. Bayer. Bot. Ges. Beih. 7, 1170 (1992).
Warthemann, G. & Reichhoff, L. Die Banater Segge (Carex buekii Wimm.) und das Caricetum buekii Kopecký et Hejný 1965 in Sachsen-Anhalt im Vergleich mit anderen Regionen Mitteleuropas. Mitt florist Kart Sachsen-Anhalt (Halle) 9, 3–14 (2004).
Lájer, K. A. Caricetum buekii, Caricetum cespitosae, Caricetum paniceo-nigrae, Cirsietum rivularis és Sagittario-Sparganietum emersi hazai előfordulásáról. Kitaibelia 8, 35–42 (2003).
Dajdok, Z., Więcław, H., Bosiacka, B., Koopman, J. & Wójcik, G. Caricetum buekii Kopecký et Hejný 1965 in south-western Poland: present distribution and habitat conditions (eds Szczuka, E., Szymczak, G., Śmigała, M., Marciniec, R. Botanika – tradycja i nowoczesność, 57 Zjazd Polskiego Towarzystwa Botanicznego, 2016).
Oťaheľová, H., Hrivnák, R. & Valachovič, M. Phragmito-Magnocaricetea Klika in Klika et Novák 1941 (ed Valachovič, M. Rastlinné spoločenstvá Slovenska 3. Vegetácia mokradí) 51–183 (Veda, 2001).
Háberová, I. Rastlinné spoločenstvá alúvií Silickej planiny. Acta Bot. Slov Ser A 4, 123–135 (1978).
Rybníček, K., Balátová-Tuláčková, E. & Neuhäusl, R. Přehled rostlinných společenstev rašelinišť a mokřadních luk Československa. [Synopsis of plant communities of mires and wetland meadows in Czechoslovakia; in Czech]. Studie Československé akademie věd 8, 1–123 (1984).
Milenović, V. & Randelović, N. A socijacija Carici buekii-Alnetum glutinosae R. Randj. et V. Milenović 2007 u gornjemtokareke Moravice i njeneindikatorskevrednosti. Proceedings of the 9th Symposium on Flora of Southeastern Serbia and Neighbouring Regions, Niš. 127–131 (2007).
Weber, H. E., Moravec, J. & Theurillat, J. P. International Code of Phytosociologica Nomenclature. 3rd edition. J. Veg. Sci. 11, 739–768 (2000).
Heerde, A., Müller, F. & Gnüchtel, A. Verbreitung, Soziologie und Ökologie von Carex buekii Wimm in Sachsen. Tuexenia 26, 339–352 (2006).
van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effect on community similarity. Vegetatio 39, 97–114 (1979).
Mirek, Z., Piękoś-Mirkowa, H., Zając, A. & Zając, M. Flowering plants and pteridophytes of Poland. A checklist. W. Szafer Institute of Botany, Polish Academy of Sciences (2002).
Sparks, D.L., Page, A. L., Helmke, P. A. & Loeppert, R. H. editors.Methods of soil analysis part 3 - chemical methods. SSSA Book Ser. 5.3. Madison: Soil Science Society of America, American Society of Agronomy (1996).
Ryzak, M., Bartminski, P. & Bieganowski, A. Methods for determination of particle size distribution of mineral soils. Acta Agrophysica 175, 1–84 (2009).
Kovach, W. L. MVSP PLUS version 3.1. Pentraeth (1985–1999).
Statsoft Inc. Statistica (data analysis software system), v. 12.0. http://www.statsoft.com (2016).
ter Braak, C. J. F. & Šmilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordinantion (version 4.5). Microcomputer Power. Ithaca, NY, USA (2002).
IUCN [International Union for Conservation of Nature and Natural Resources] Standards and Petitions Subcommittee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 13. Prepared by the Standards and Petitions Subcommittee. Downloadable from http://www.iucnredlist.org/documents/RedListGuidelines.pdf (2017).
IUCN [International Union for Conservation of Nature and Natural Resources]. Guidelines for Application of IUCN Red List Criteria at Regional and National Levels: Version 4.0. Prepared by the IUCN Species Survival Commission. Downloadable from http://www.iucnredlist.org/technical-documents/categories-and-criteria (2012).
Berg, C. et al. Red Lists and conservation prioritization of plant communities – a methodological framework. Applied Vegetation Science 17, 504–515 (2014).
Ellenberg, H. et al. Indicator values of plants in Central. Europe. Scripta Geobotanica 18, 1–248 (1991).
Richards, L. A. Diagnosis and improvement of saline and alkali soils. USDA. Agriculture Handbook 60, Washington D. C. (1954).
Řepka, R. & Grulich, V. Ostřice České republiky: terénní obrazový průvodce. Lesnická Práce, Kostelec nad Černými lesy (2014).
Nobis, A. & Skórka, P. River corridor plants revisited: What drives their unique distribution patterns? Plant Biosystems 150(2), 244–253 (2014).
Brzosko, E. The life history of Carex cespitosa: consequences for population dynamics and vegetation succession. V. The life history and role of a C. cespitosa population in the process of succession on abandoned meadows – general discussion. Pol. Bot. Stud. 14, 53–60 (1999).
Meusel, H., Jager, E. & Weinert, E. Vergleichende Chorologie der Zentraleuropäischen Flora. 1. (Gustav Fischer Verlag, 1965).
Hejný, S. Ökologische Charakteristik der Wasser- und Sumpfpflanzen in den slowakischen Tiefebenen (Donau- und Theissgebiet). (Vydavateľstvo SAV, 1960).
Xiong, S. & Nilsson, C. The effects of plant litter on vegetation: a meta-analysis. J. Ecol. 87, 984–994 (1999).
Sádlo, J. & Bufková, I. Vegetation of the Vltava river alluvial plain in the Šumava Mts (Czech Republic) and the problem of relict primary meadows. Preslia 74, 67–83 (2002).
Philippi, G. Klasse Phragmitetea Tx. et Prsg. (ed. Oberdorfer, E. 1998: Süddeutsche Pflanzengesellschaften. Teil I. Fels- und Mauergesellschaften, alpine Fluren, Wasser-, Verlandungs- und Moorgeselschaften. 4th ed.) 119–165 (Gustav Fischer Verlag, 1974).
Novotný, V. Relationships between life histories of leafhoppers (Auchenorrhyncha - Hemiptera) and their host plants (Juncaceae, Cyperaceae, Poaceae). Oikos 73, 33–42 (1995).
Myśliwy, M. & Bosiacka, B. Disappearance of Molinio-Arrhenatheretea Meadows Diagnostic Species in the Upper Płonia River Valley (NW Poland). Pol. J. Environ. Stud. 18(3), 513–519 (2009).
Zelnik, I. & Čarni, A. Plant species diversity and composition of wet grasslands in relation to environmental factors. Biodivers. Conserv. 22, 2179–2192 (2013).
Dengler, J., Janišová, M., Török, P. & Wellstein, C. Biodiversity of Palearctic grasslands: a synthesis. Agr. Ecosyst. Environ. 182, 1–14 (2014).
Godreau, V. et al. Biodiversity in the floodplain of Saône: a global approach. Biodivers. Conserv. 8, 839–864 (1999).
Tabacchi, E. et al. Development, maintenance and role of riparian vegetation in the river landscape. Freshwater Biol. 40, 497–516 (1998).
Tabacchi, E. et al. Impacts of riparian vegetation on hydrological processes. Hydrol. Process 14(16–17), 2959–2976 (2000).
Schnittler, M. & Günther, K. F. Central European vascular plants requiring priority conservation measures - an analysis from national Red Lists and distribution maps. Biodivers. Conserv. 8, 891–925 (1999).
Bilz, M., Kell, S.P., Maxted, N. & Lansdown, R.V. European red list of vascular plants. Publications Office of the European Union, Luxembourg (2011).
Kaźmierczakowa, R. et al. Polish red list of pteridophytes and flowering plants. (Instytut Ochrony Przyrody Polskiej Akademii Nauk, 2016).
Kopeć, D. & Michalska-Hejduk, D. How threatened is the Polish wetland flora? Oceanol. Hydrobiol. St. 41(3), 79–89 (2012).
Grulich, V. Red List of vascular plants of the Czech Republic: 3rd edition. Preslia 84, 631–645 (2012).
Hájková, P. Calthion palustris (eds Hegedüšová Vantarová, K. & Škodová, I. Rastlinné spoločenstvá Slovenska. 5. Travinno-bylinná vegetácia) 272–304 (Veda, 2014).
Eliáš, P. jun, Dítě, D., Kliment, J., Hrivnák, R. & Feráková, V. Red list of ferns and flowering plants of Slovakia, 5th edition. Biologia 70, 218–228 (2015).
Bartha, D. et. al. Magyarország edényes növényfajainak elterjedési atlasza. Nyugat-magyarországi. (Egyetem Kiadó, 2015).
Barina, Z. et. al. Vörös Lista – A magyarországi edényes flóra veszélyeztetett fajai. (Saját Kiadás, 2007).
Pignatti, S., Menegoni, P. & Giacanelli, V. Liste rosse e blu della flora italiana. ANPA (2001).
Nikolić, T. & Topić, J. Crvena knjiga vaskularne flore Republike Hrvatske: kategorije EX, RE, CR, EN i VU. Državni zavod za zaštitu prirode, Ministarstvo kulture (2005).
Acknowledgements
The authors are grateful to Marek Podlasiński (West Pomeranian University of Technology, Szczecin, Poland) for analysis of soil samples. The research and elaboration of this paper was supported by grants nr. 14-36079G (Centre of Excellence PLADIAS, Czech Science Foundation to KŠ) and RVO67985939 (The Czech Academy of Sciences to KŠ).
Author information
Authors and Affiliations
Contributions
Z.D., H.W., J.K. conducted fieldwork in Poland; K.Š. conducted fieldwork in the Czech Republic; R.H. conducted fieldwork in Slovakia; A.M. conducted fieldwork in Hungary; C.h.M., E.M. conducted fieldwork in Italy; H.W., K.Š., B.B. analysed the data and wrote the first version of the manuscript, which was further modified, commented and finally approved by all the authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Więcław, H., Šumberová, K., Bosiacka, B. et al. Ecology, threats and conservation status of Carex buekii (Cyperaceae) in Central Europe. Sci Rep 9, 11162 (2019). https://doi.org/10.1038/s41598-019-47563-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47563-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.