Abstract
Recent evidence has indicated that the lymphatic vessel endothelial hyaluronan receptor (LYVE-1) is implicated in chronic inflammation and the lymphatic immune response. The soluble form of LYVE-1 (sLYVE-1) is produced by ectodomain shedding of LYVE-1 under pathological conditions including cancer and chronic inflammation. In this study, 1014 consecutive patients who underwent coronary angiography from May 2015 to September 2015 were included to investigate whether serum sLYVE-1 is associated with coronary artery disease (CAD) and its concomitant diseases includes chronic kidney disease (CKD). Results showed that there was no significant difference in sLYVE-1 levels between patients with CAD and without. However, a significantly higher level of sLYVE-1 was seen in patients with renal dysfunction compared to those with a normal eGFR. Results were validated in a separate cohort of 259 patients who were divided into four groups based on their kidney function assessed by estimated glomerular filtration rate (eGFR). Simple bivariate correlation analysis revealed that Lg[sLYVE-1] was negatively correlated with eGFR (r = −0.358, p < 0.001) and cystatin C (r = 0.303, p < 0.001). Multivariable logistic regression analysis revealed that the increase in Lg[sLYVE-1] was an independent determinant of renal dysfunction (odds ratio = 1.633, p = 0.007). Therefore, renal function should be considered when serum sLYVE-1 is used as a biomarker for the detection of pathological conditions such as chronic inflammation and cancer. Further study is required to elucidate the exact role of sLYVE-1 in renal function.
Similar content being viewed by others
Introduction
The lymphatic system, known as the second circulatory system, is indispensable in draining interstitial fluid from tissues and returning it to blood circulation1. Studies have shown that acute inflammatory reactions and chronic inflammatory diseases are accompanied by both the expansion of preexisting lymphatic vessels (lymphatic hyperplasia) and the growth of new lymphatic vessels (lymphangiogenesis), indicating a role for the lymphatic system in immune surveillance and the inflammatory process, including that which arises in the context of coronary artery disease (CAD)2,3,4,5,6.
LYVE-1, a major receptor for hyaluronic acid (HA) on the lymph vessel wall, has been widely used as a uniquely powerful marker for lymph vessels7. During the last decade, accumulating evidence has provided insight into the role of LYVE-1 in lymphangiogenesis and immune responses. LYVE-1 has been reported as a mediator of lymphangiogenesis induced by low-molecular weight HA8. Additionally, it has been shown that, in cultured primary lymphatic endothelial cells, surface expression of LYVE-1 is rapidly and reversibly lost after exposure to tumor necrosis factor-α (TNF-α) and tumor necrosis factor-β (TNF-β), occurring via internalization and lysosomic degradation of LYVE-1 coupled with a shutdown in gene expression9.
Recently, ectodomain shedding of LYVE-1 has been found to occur in lymphatic vessels during chronic inflammation10. A potential role for soluble LYVE-1 (sLYVE-1), which contains the intact binding domain for its ligands, has been reported in the inhibition of LYVE-1 signaling11. Although the exact function of sLYVE-1 in normal physiology and pathological conditions is not yet clear, the association between sLYVE-1 and several diseases, including rheumatoid arthritis and cancer, has been explored12,13.
Given the important role of the lymphatic system in reversing cholesterol transport during the development of atherosclerosis, an association between serum sLYVE-1 levels and CAD severity was hypothesized14,15. In this study, we aimed to investigate whether serum sLYVE-1 is related to CAD as well as its concomitant diseases including hypertension, dyslipidemia, diabetes, and chronic renal dysfunction, all of which are known to be conditions associated with chronic inflammation16,17,18,19,20.
Methods
The study protocols for cohort 1 and cohort 2 were approved by the Ethics Committee of Rui Jin Hospital, Shanghai Jiaotong University School of Medicine. Written informed consent was obtained from all participants. All experiments and methods were performed in accordance with relevant guidelines and regulations.
Study population
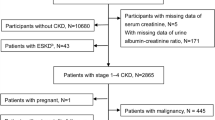
1014 consecutive patients subjected to coronary angiography (CAG) from May 2015 to September 2015 were included in this study (referred to as cohort 1 below). To avoid confounding data, patients with acute myocardial infarction, acute and chronic viral or bacterial infection, tumors, type 1 diabetes mellitus, or rheumatoid arthritis and other connective tissue diseases were excluded. Results were validated in a separate cohort of 259 patients with or without renal disfunction who subjected to coronary angiography for the first time from February 2017 to November 2017 (referred to as cohort 2 below). Patients with acute myocardial infarction, acute and chronic viral or bacterial infection, tumors, type 1 diabetes mellitus, connective tissue diseases, radiographic contrast nephropathy and hemodynamic instability were excluded. Patients in cohort 2 were divided into four groups according to estimated glomerular filtration rate (eGFR) (eGFR < 30 mL/min/1.73 m2, n = 37; 30 mL/min/1.73 m2 ≤ eGFR < 60 mL/min/1.73 m2, n = 74;60 mL/min/1.73 m2 ≤ eGFR < 90 mL/min/1.73 m2, n = 74; 90 mL/min/1.73 m2 ≤ eGFR, n = 74). Data recorded for each patient included a detailed medical and family history, results of routine laboratory tests, and details regarding concomitant diseases.
Definitions
Type 2 diabetes mellitus (T2DM) was identified if two fasting plasma glucose levels were ≥7.0 mmol/L, if two 2 h postprandial plasma glucose readings were ≥11.1 mmol/L after a glucose load of 75 g, if two casual glucose readings were ≥11.1 mmol/L, or if the patient was taking oral hypoglycemic drugs or parenteral insulin21. CAD patients were documented based on angiographic examination (luminal diameter narrowing ≥50%)22. Patients with significant CAD were further classified according to the number of diseased coronary arteries (1-, 2-, or 3-vessel disease). eGFR was calculated by using a modified CKD-EPI equation as follows: eGFRCKD-KPI = 141*0.993^age*[serum creatinine/79.56]^(–0.411)(−1.209 if serum creatinine ≥79.56μ mol/L) for men, and eGFRCKD-KPI = 141*0.993^age*[serum creatinine/61.88]^(–0.329)(−1.209 if serum creatinine ≥61.88 μmol/L) for women. Preserved and impaired renal function were defined as eGFRCKD-KPI ≥ 60 mL/min/1.73 m2 and eGFRCKD-KPI < 60 mL/min/1.73 m2, respectively.
Coronary angiography and gensini score
Selective coronary angiography was performed via the femoral or radial artery by interventional cardiologists blinded to the study protocol. Significant CAD was diagnosed visually if narrowing of the luminal diameter ≥50% was present in a major epicardial coronary artery, and left main coronary artery narrowing ≥50% was considered 2-vessel disease22. Gensini score was calculated according to the published method23.
Biochemical investigation
Blood samples were collected after overnight fasting from all patients. Serum cystatin-c, as well as creatinine, blood urea nitrogen, uric acid, total cholesterol, Triglyceride, low-density lipoprotein-cholesterol, high-density lipoprotein cholesterol and hsCRP were measured with standard laboratory techniques in our hospital laboratory. Serum sLYVE-1 level was determined with a commercially available ELISA kit (Human LYVE-1 DuoSet ELISA, DY2089, R&D System, Minneapolis, MN) according to the instructions. Once collected, the samples were immediately immersed in ice and centrifuged within 30 minutes at 12,000 rpm for 10 min to obtain platelet-poor serum. All samples were stored at −80 °C before analysis.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD), while discrete data were presented as median with a 25–75% range. Categorical data were summarized as frequency with percentage. For categorical clinical variables, we evaluated differences between groups with the chi-square test followed by Bonferroni’s correction to account for multiple comparisons. For continuous variables, we evaluated the presence of a normal distribution with the Kolmolgorov–Smirnov test, and applied logarithmic transformations on continuous variables displaying a non-normal distribution of sLYVE-1 levels. Continuous variables were compared using independent t-test between two groups. And we analyzed differences among patient groups by one-way analysis of variance (ANOVA) or the Kruskal–Wallis analysis, followed by post-hoc analysis. The correlation between variables was determined by the Pearson correlation tests or Spearman correlation tests as appropriate. In multivariable stepwise logistic regression analysis, conventional risk factors and Lg[sLYVE-1] were adjusted for the assessment of renal dysfunction. A 2-tailed p < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS 22.0 for Windows (SPSS, Inc., Chicago, IL, USA). G*Power 3.124 was used to conduct post hoc power analysis.
Results
Clinical characteristics and biochemical measurements
The baseline clinical characteristics and biochemical measurements of 1014 patients are presented in Table 1. Patients were further trisected into 3 groups according to serum sLYVE-1 levels (Table 2). As shown, patients with high sLYVE-1 levels were much older, and the prevalence of diabetes mellitus and chronic kidney disease (CKD) were significantly higher in this group. Fasting glucose, glycosylated hemoglobin, and creatinine concentration consistently increased in patients with higher sLYVE-1 levels, while eGFR decreased. Notably, the prevalence of hypertension and dyslipidemia showed no significant difference among the three groups.
sLYVE-1 was not associated with CAD
Serum sLYVE-1 concentrations showed no significant differences between subjects with hypertension and those without, with dyslipidemia and without, or between female and male subjects. Notably, the sLYVE-1 concentration was higher in subjects with diabetes mellitus. Contrary to our expectations there was no significant difference between patients with CAD and those without (Fig. 1). Patients with CAD were further classified according to the number of diseased coronary arteries. No significant differences of serum sLYVE-1 levels were found among four groups (p = 0.147 for ANOVA) (Fig. 2A). Partial correlation analysis showed that serum sLYVE-1 concentration was not significantly related to the number of diseased coronary arteries (Partial correlation coefficient = −0.044, p = 0.216, gender, age, BMI, SBP, Fast glucose, HbA1c, LVEF, hsCRP, HBP and DM were listed as covariables). However, a significantly higher sLYVE-1 level was seen in patients with CKD compared to those without (639.9 ± 348.2 vs. 445.9 ± 203.0, p = 0.003) (Fig. 1).
Serum sLYVE-1 levels in subgroups of cohort 1 patients. Comparison of sLYVE-1 levels were made between males and females (test power 0.282), and between subjects with hypertension and without (test power 0.297), with diabetes mellitus and without (test power 0.624), with dyslipidemia and without (test power 0.061), with CAD or without (test power 0.424), with CKD and without (test power 0.974). Results are presented as mean ± SD. Independent t-test was performed. *p < 0.05, ***p < 0.001. CAD, coronary artery disease; CKD, chronic kidney disease; DM, diabetes mellitus; HBP, high blood pressure.
Serum sLYVE-1 levels in relation to the number of diseased coronary arteries and eGFR. Results are presented as mean ± SD. One-way ANOVA was performed followed by Turkey’s multiple comparisons tests. (A) According to angiography, patients in cohort 1 were divided into 4 groups: 0-vessel disease (n = 235), 1-vessel disease (n = 262), 2-vessel disease (n = 219), and 3-vessel disease (n = 298). Serum sLYVE-1 concentrations were not significantly different among groups. (B) Serum sLYVE-1 based on eGFR levels in cohort 2 patients. The serum level of sLYVE-1 was negatively associated with eGFR. *p < 0.05, **p < 0.01, ***p < 0.001. ns = not significant.
sLYVE-1 was associated with renal dysfunction
An additional 259 patients were involved to further validate the association between sLYVE-1 level and renal function. Subjects were divided into four groups according to eGFR (Table 3). Significant differences with respect to gender existed amongst the four groups. The prevalence of hypertension and diabetes mellitus was higher in patients with lower eGFR, as was higher systolic blood pressure and hsCRP level. Notably, serum sLYVE-1 concentration increased as eGFR decreased (Table 3, Fig. 2B).
sLYVE-1 was an independent risk factor for renal dysfunction
To explore the correlation between sLYVE-1 and renal dysfunction, we analyzed sLYVE-1 as a log-transformed standardized continuous variable, Lg[sLYVE-1]. Simple bivariate correlation analysis revealed that Lg[sLYVE-1] negatively correlated with eGFR (r = −0.358, p < 0.001) and positively correlated with cystatin C (r = 0.303, p < 0.001). Moreover, an independent correlation between Lg[sLYVE-1] and eGFR (r = −0.274, p < 0.001) or cystatin C (r = 0.249, p < 0.001) was demonstrated in partial correlation analysis when adjusted for gender, age, BMI, SBP, fasting glucose, HbA1c, LVEF, hsCRP, HBP and DM (Table 4).
To further establish the correlation between sLYVE-1 and renal dysfunction, patients were classified into two groups based on eGFR (eGFR < 60 mL/min/1.73 m2 and eGFR > 60 mL/min/1.73 m2). Univariate and multiple logistic regression analysis confirmed that Lg[sLYVE-1] was an independent predictor for the presence of renal dysfunction, both adjusted for age and gender and adjusted for the full model including age, gender, BMI, SBP, fasting glucose, HbA1c, LVEF, hsCRP, HBP and DM (Table 5). However, subgroup analysis by using a logistic regression model revealed Lg[sLYVE-1] to be a better predictor for diminished renal function in younger patients or those with HBP (Fig. 3).
Forrest plots for renal dysfunction. Forrest plots (unadjusted) to analyze the predictive value of sLYVE-1 for eGFR < 60 mL/min/1.73 m2 in different subgroups of patients in cohort 2. sLYVE-1 is entered as a log-transformed continuous variable. DM, diabetes mellitus; HBP, high blood pressure; CAD, coronary artery disease.
Discussion
The role of LYVE-1 in pathological conditions has not been fully identified
LYVE-1, which has 41% sequence homology to the HA receptor CD44, has been proved to play an important role in HA clearance and HA-mediated leukocyte adhesion7. However, a number of studies have showed that LYVE-1 is also expressed in certain bone marrow-derived monocytic lineages, which probably function in lymphangiogenesis in pathological processes including chronic inflammatory diseases, transplant rejection, and tumorigenesis25,26,27,28.
The results of studies in which the LYVE-1 gene was deleted in mice have been controversial. Nicholas et al.29 and Mai et al.30 showed that LYVE-1 knock out (LYVE-1−/−) mice exhibited an clearly normal phenotype, without any detected significant changes in lymphatic vessel ultrastructure or function. In addition, LYVE-1−/− mice also showed typical trafficking of dermal CD11c+ dendritic cells to draining lymph nodes via afferent lymphatics and normal regression of drug-induced skin inflammation. Conversely, Johnson et al.31 reported that LYVE1 gene deletion not only impeded lymphatic delivery of cutaneous dendritic cells but also impaired their ability to trigger CD8+ T cell responses in skin-draining lymph nodes. These results suggest that LYVE-1 might not be essential for normal lymphatic development and function, but do not rule out a role for LYVE-1 in pathological conditions such as chronic inflammation.
LYVE-1 ectodomain shedding
The ectodomain of LYVE-1 is known to undergo proteolytic cleavage, producing the soluble form of LYVE-1. This process can be provoked by vascular endothelial growth factor (VEGF-A), which is an important growth factor promoting lymphangiogenesis and angiogenesis in pathological conditions. Surprisingly, wild-type LYVE-1, but not uncleavable LYVE-1(generated by a mutation at the site of proteolytic cleavage), facilitated migration of lymphatic endothelial cells in response to VEGF-A, indicating a potential role of ectodomain shedding of LYVE-1 in accelerating pathological lymphatic vessel growth10. Furthermore, Hoi Leong Xavier et al. reported that LYVE-1-mediated lymphangiogenic responses can be inhibited by membrane type 1-matrix metalloproteinase (MT1-MMP), an endogenous suppressor of lymphangiogenesis, which directly cleaves LYVE-1 on lymphatic endothelial cells32. Consistently, it has been revealed that sLYVE-1 impairs fibroblast growth factor 2 (FGF2)/LYVE-1 signaling and functions11. These data suggest an antagonistic effect of sLYVE-1 on LYVE-1 signaling in lymphatic endothelial cells.
The value of sLYVE-1 in diagnostics
The association of sLYVE-1 with several pathological conditions has been reported, and an increase in sLYVE-1 levels during inflammation and tumorigenesis has been observed12,13. However, in the current study, serum sLYVE-1 concentrations showed no significant differences between subjects with chronic pathological conditions including hypertension and dyslipidemia and those without. Unexpectedly, a patient’s sLYVE-1 level was not associated with the occurrence and severity of CAD. Although the interpretation of the negative results should be carefully made because of small test powers, these data suggest that changes in sLYVE-1 level do not occur in all pathological conditions. Significant changes might only occur when the balance of activators and inhibitors of LYVE-1 ectodomain shedding is completely disturbed.
The role of lymph system in CAD remains an interesting area worth exploring
Although sLYVE-1 levels were not associated with the occurrence and severity of CAD, the role of the lymphatic system in CAD remains an interesting area. Recently, lymphatic vessels have been reported to play an important role in reverse cholesterol transport and in the regression of atherosclerosis in experimental animal models. Mouse strains with lymphatic insufficiency have higher plasma cholesterol levels in comparison with control mice fed both a western-type high fat diet and a normal diet15. Thus, therapies aimed at reversing atherosclerosis may benefit from cholesterol clearance mediated by lymphatic transport function14.
It has been reported that ApoE−/− mice display an increase in lymphangiogenesis in progressive atherosclerotic lesions characterized by calcium deposits and cholesterol crystals, and in atherosclerotic iliac arteries5,33. A change of adventitial lymphatics in the progression of atherosclerosis has also been reported34,35. It is intuitive that deficient lymphatic drainage could aggravate atherosclerosis by impeding the clearance of excessive amounts of lipids, inflammatory cytokines, and immune cells. Thus, an abnormal angiogenesis and lymphangiogenesis might be a part involved in the sustained inflammatory response during human atherogenesis.
The effect of sLYVE-1 on renal dysfunction remains unidentified
In the present study, a significant association of sLYVE-1 and renal dysfunction was, for the first time, observed. An increased sLYVE-1 level was an independent risk factor for lower eGFR. These data imply changes in the lymphatic system in patients with renal dysfunction.
The expression pattern of LYVE-1 in mouse kidney has been illustrated by Hyun-Wook and his colleagues36. In the adult mouse kidney, LYVE-1 was predominantly located in the lymphatic endothelial cells and in certain endothelial cells in the glomerular capillaries. Under pathological conditions in a mouse model of renal interstitial fibrosis, an increase of lymphangiogenesis in the fibrotic kidney has been observed37,38. Meng et al.39 reported that after renal ischemia and reperfusion, LYVE-1 was localized in the endothelial cells of the glomerular region. Taking all these together, the roles of LYVE-1 and sLYVE-1 appear to important and are not yet completely understood.
In conclusion, our data show the serum sLYVE-1 level in different pathological conditions, and reveal a positive association with renal dysfunction. Although the exact role of sLYVE-1 in disease progression needs to be further elucidated, renal function should considered when sLYVE-1 is used as a biomarker when diagnosing pathological conditions in the future.
References
Adamczyk, L. A. et al. Lymph vessels: the forgotten second circulation in health and disease. Virchows Arch. Int. J. Pathol. 469, 3–17 (2016).
Hardavella, G. et al. Lymphangiogenesis in COPD: another link in the pathogenesis of the disease. Resp. Med. 106, 687–693 (2012).
Reis-Filho, J. S. & Schmitt, F. C. Lymphangiogenesis in tumors: what do we know? Microsc. Res. Techniq. 60, 171–180 (2003).
Xu, H. Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann. Rheum. Dis. 62, 1227–1229 (2003).
Kholova, I. et al. Lymphatic vasculature is increased in heart valves, ischaemic and inflamed hearts and in cholesterol-rich and calcified atherosclerotic lesions. Eur. J. Clin. Invest. 41, 487–497 (2011).
Bellingan, G. J., Caldwell, H., Howie, S. E., Dransfield, I. & Haslett, C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J. Immunol. 157, 2577–2585 (1996).
Banerji, S. N. J. & Wang, S. X. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144, 789–801 (1999).
Wu, M. et al. Low molecular weight hyaluronan induces lymphangiogenesis through LYVE-1-mediated signaling pathways. PLoS One 9, e92857 (2014).
Johnson, L. A., Prevo, R., Clasper, S. & Jackson, D. G. Inflammation-induced uptake and degradation of the lymphatic endothelial hyaluronan receptor LYVE-1. J. Biol. Chem. 282, 33671–33680 (2007).
Nishida-Fukuda, H. et al. Ectodomain Shedding of Lymphatic Vessel Endothelial Hyaluronan Receptor 1 (LYVE-1) Is Induced by Vascular Endothelial Growth Factor A (VEGF-A). J. Biol. Chem. 291, 10490–10500 (2016).
Platonova, N. et al. Evidence for the interaction of fibroblast growth factor-2 with the lymphatic endothelial cell marker LYVE-1. Blood (2012).
Yoo, J. et al. Exosomal amyloid A and lymphatic vessel endothelial hyaluronic acid receptor-1 proteins are associated with disease activity in rheumatoid arthritis. Arthritis Res. Ther. 19, 119 (2017).
Nunomiya, K. et al. Relationship between Serum Level of Lymphatic Vessel Endothelial Hyaluronan Receptor-1 and Prognosis in Patients with Lung Cancer. J. Cancer 5, 242–247 (2014).
Martel, C. et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J. Clin. Invest. 123, 1571–1579 (2013).
Vuorio, T. et al. Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler. Thromb. Vasc. Biol. 34, 1162–1170 (2014).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002).
Shah, S. V., Shukla, A. M., Bose, C., Basnakian, A. G. & Rajapurkar, M. Recent advances in understanding the pathogenesis of atherosclerosis in CKD patients. J. Ren. Nutr. 25, 205–208 (2015).
Lontchi-Yimagou, E., Sobngwi, E., Matsha, T. E. & Kengne, A. P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 13, 435–444 (2013).
Byrne, C. J., Khurana, S., Kumar, A. & Tai, T. C. Inflammatory Signaling in Hypertension: Regulation of Adrenal Catecholamine Biosynthesis. Front. Endocrinol (Lausanne) 9, 343 (2018).
Lira, F. S., Rosa, N. J. C., Antunes, B. M. & Fernandes, R. A. The relationship between inflammation, dyslipidemia and physical exercise: from the epidemiological to molecular approach. Curr. Diabetes Rev. 10, 391–396 (2014).
Ryden, L. et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur. Heart J. 34, 3035–3087 (2013).
Mack, W. J., Azen, S. P., Dunn, M. & Hodis, H. N. A comparison of quantitative computerized and human panel coronary endpoint measures: implications for the design of angiographic trials. Control. Clin. Trials 18, 168–179 (1997).
Gensini, G. G. A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 606 (1983).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Chen, L., Cursiefen, C., Barabino, S., Zhang, Q. & Dana, M. R. Novel expression and characterization of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1) by conjunctival cells. Invest. Ophthalmol. Vis. Sci. 46, 4536–4540 (2005).
Jeon, B. H. et al. Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res. 68, 1100–1109 (2008).
Maruyama, K. et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 115, 2363–2372 (2005).
Schledzewski, K. et al. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J. Pathol. 209, 67–77 (2006).
Gale, N. W. et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol. Cell. Biol. 27, 595–604 (2007).
Luong, M. X. et al. Lack of lymphatic vessel phenotype in LYVE-1/CD44 double knockout mice. J. Cell. Physiol. 219, 430–437 (2009).
Johnson, L. A. et al. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat. Immunol. 18, 762–770 (2017).
Wong, H. L. et al. MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis. Nat. Commun. 7, 10824 (2016).
Grzegorek, I. et al. Arterial wall lymphangiogenesis is increased in the human iliac atherosclerotic arteries: involvement of CCR7. receptor. Lymphat. Res. Biol. 12, 222–231 (2014).
Taher, M. et al. Phenotypic transformation of intimal and adventitial lymphatics in atherosclerosis: a regulatory role for soluble VEGF receptor 2. FASEB J. 30, 2490–2499 (2016).
Nakano, T. et al. Angiogenesis and lymphangiogenesis and expression of lymphangiogenic factors in the atherosclerotic intima of human coronary arteries. Hum. Pathol. 36, 330–340 (2005).
Lee, H. W. et al. Expression of lymphatic endothelium-specific hyaluronan receptor LYVE-1 in the developing mouse kidney. Cell. Tissue Res. 343, 429–444 (2011).
Lee, A. S. et al. Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int. 83, 50–62 (2013).
Jung, Y. J. et al. Hyaluronan-induced VEGF-C promotes fibrosis-induced lymphangiogenesis via Toll-like receptor 4-dependent signal pathway. Biochem. Biophys. Res. Commun. 466, 339–345 (2015).
Meng, F. A novel role of HIF-1alpha/PROX-1/LYVE-1 axis on tissue regeneration after renal ischaemia/reperfusion in mice. Arch. Physiol. Biochem. 1–11 (2018).
Acknowledgements
The authors thank the National Natural Science Foundation of China (81770249 to Ruiyan Zhang and 81670389 to Jinzhou Zhu) for the support. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the work presented in the paper and to the preparation of the paper itself. D.P.D., J.Z.Z. and R.Y.Z. contributed to all stages including conception and design of the study, as well as acquisition, analysis and interpretation of the data. C.K.H., H.H. assisted with data acquisition, analysis and interpretation of the data. Z.B.Z. and J.W.N. performed coronary angiography. All authors have approved the final article being submitted.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dai, D., Huang, C., Ni, J. et al. Serum sLYVE-1 is not associated with coronary disease but with renal dysfunction: a retrospective study. Sci Rep 9, 10816 (2019). https://doi.org/10.1038/s41598-019-47367-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47367-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.