Abstract
Retrospective studies have found that left upper lobectomy (LUL) may be a new risk factor for stroke, and the potential mechanism is pulmonary vein thrombosis, which more likely develops in the left superior pulmonary vein (LSPV) stump. The LSPV remaining after left pneumonectomy is similar to that remaining after LUL. However, the association between left pneumonectomy, LUL, and postoperative stroke remains unclear. Thus, we sought to analyze whether both LUL and left pneumonectomy are risk factors for postoperative stroke. We prospectively included consecutive patients who underwent resection between November 2016 and March 2018 at our institution with 6 months of follow-up. Baseline demographic and clinical data were taken. A logistic regression model was used to determine independent predictors of postoperative stroke. In our study, 756 patients who underwent an isolated pulmonary lobectomy procedure were screened; of these, 637 patients who completed the 6-month follow-up were included in the analysis. Multivariable logistic regression analysis adjusted for common risk factors showed that the LUL and left pneumonectomy were independent predictors of stroke (odds ratio, 18.12; 95% confidence interval, 2.12–155.24; P = 0.008). Moreover, diabetes mellitus also was a predictor of postoperative stroke. In conclusion, both LUL and left pneumonectomy are significant risk factors for postoperative stroke.
Similar content being viewed by others
Introduction
Stroke is one of the most feared complications of surgery, which occurs in 0.08–0.7% and 0.6% of general and thoracic surgery patients, respectively1,2,3. The mortality is approximately 15% after the first stroke in the general population, whereas the acute mortality following a perioperative stroke is approximately 26%4. Long-term neurological disabilities following postoperative stroke result in a substantial social and financial burden to the family and the community. According to large-scale database studies, advanced age, ischemic heart disease, history of cerebrovascular disease, atrial fibrillation, and congestive heart failure are the common risk factors for perioperative stroke5.
Major cardiovascular surgery around a relatively high incidence of perioperative stroke6,7. Recent studies found that left upper lobectomy (LUL) may be a new risk factor for stroke in cancer patients8,9, and the potential mechanism is pulmonary vein thrombosis, which more likely forms in the left superior pulmonary vein (LSPV) stump9,10. Interestingly, a giant thrombus in the LSPV after left pneumonectomy has been reported11. It is known that the LSPV remaining after left pneumonectomy is the same as that after LUL. Thus, we speculate that left pneumonectomy may also be a risk factor for postoperative stroke. However, there is only one case report that mentions the relationship between left pneumonectomy and stroke12. In addition, these studies are retrospective in nature and are only performed in patients with lung cancer, and whether this conclusion is applicable to patients other than lung cancer remains relatively unknown.
In this prospective cohort study, we sought to determine whether both LUL and left pneumonectomy are risk factors for postoperative stroke in patients with lung cancer, inflammatory pseudotumor, bronchiectasis, and other pathological diseases who underwent lobectomy.
Methods
Study design and patient selection
This prospective cohort study was designed to provide data on postoperative stroke risk factors of patients who underwent pulmonary lobectomy in our institution. We used the STROBE checklist. Eligible patients were enrolled consecutively from November 2016 to March 2018 at the First Affiliated Hospital of Zhengzhou University. The follow-up time of this study was 6 months after surgery. LUL and left pneumonectomy were compared with other operative procedures in these patients.
The study protocol was approved by the institutional review committee of the First Affiliated Hospital of Zhengzhou University (project identification code: 2018-LW-043), and informed consent was obtained from the patients prior to study participation. The study was performed according to the institution’s ethical guideline and the principles of the Helsinki Declaration.
We prospectively and consecutively selected patients. Inclusion criteria were as follows: (1) age between 18 and 80 years, (2) pulmonary lobectomy, bilobectomy, or pneumonectomy was planned at our hospital, and (3) signed the informed consent form. Exclusion criteria were as follows: (1) life expectancy was less than 6 months, (2) a patient who had a surgical history of lobectomy, bilobectomy, or pneumonectomy, (3) formerly known abnormalities of any coagulation factor, (4) use of unfractionated heparin, low molecular weight heparin (LMWH), and any form of anticoagulant and antiplatelet drug in the past 30 days, (5) history of previous cardiovascular surgery (coronary artery bypass surgery or percutaneous coronary intervention), (6) history of previous stroke, (7) pregnant, lactating, or potentially pregnant, (8) uncontrollable infectious disease, autoimmune disease, or other severe comorbidities, (9) psychiatric disease, and (10) inaccessible to follow-up.
Surgery and pathology
All patients presented to our hospital for a routine preoperative check-up and underwent computed tomography (CT) of the chest and abdomen and magnetic resonance imaging (MRI) of the brain. For all patients (except for large tumor size, lymph node metastases, bronchial invasion, chest wall invasion or pulmonary vessel invasion on preoperative images), video-assisted thoracoscopic surgery (VATS) with a 7-cm small thoracotomy, 3-cm window, and three ports were executed. For the other patients, a thoracotomy of 13 to 20 cm was performed. All patients underwent a standard procedure for anatomical pulmonary surgery. The pulmonary vein, pulmonary artery, and bronchus were all divided by a linear stapler. Tumor pathological typing was according to the 2015 World Health Organization Classification of Lung Tumors13.
Postoperative venous thrombosis prevention
For all patients, hemagglutination test was performed within 24 hours after surgery and followed with venous thrombosis preventive measures.
According to the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) for the prevention of venous thromboembolism14, we selected the following postoperative venous thrombosis preventive procedures: (1) routine thromboprophylaxis with LMWH [international normalized ratio (INR) target, 2.5; range, 2.0 to 3.0] was administered for patients who underwent major thoracic surgery; and (2) mechanical thromboprophylaxis with properly fitted graduated compression stockings (GCS) was utilized for thoracic surgery patients who had a high risk of bleeding.
Outcome of interest
The outcome of interest was incident ischemic stroke during the 6-month follow-up period. Ischemic stroke was defined as a sudden onset of focal neurological deficit that lasts for more than 24 hours and is not due to non-ischemic causes; if the neurological deficit lasts less than 24 hours, evidence of acute cerebral infarction must appear in the neuroimaging examination15.
Suspected ischemic strokes, which were assessed by trained and research neurologists, were reported via telephone follow-up with participants every month within 6 months after surgery. Medical records were requested for ischemic stroke events and reviewed by two experienced neurologists, both blinded to the study design and baseline characteristics, who also validated potential strokes. Differences were resolved by discussion and a consensus was reached between the two neurologists. If consensus couldn’t be reached, the third reviewer was involved in resolving any differences. All patients who might have an incident stroke during follow-up underwent head magnetic resonance imaging (MRI) or computed tomography (CT).
Statistical analysis
SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Frequencies were tabulated for each patient’s risk factor. The results are expressed as percentages for categorical variables and as mean (standard deviation, SD) for continuous variables. A univariable analysis was performed using Chi-squared or Fisher’s exact tests for categorical variables or continuous variables. Student’s t test or Mann-Whitney U test was used to compare patients with and without postoperative stroke. All variables showing significance in the nominal two-tailed test (P < 0.10) and traditional cerebral infarction risk factors [age, sex, smoking index (smoking index = the number of cigarettes smoked per day (CPD) × years of tobacco use16), drinking history, hypertension, diabetes mellitus, hyperlipidemia, arrhythmia, coronary heart disease, hypercholesterolemia, type of procedure, operative approach (thoracotomy/VATS)] were entered into a binary logistic regression model using an enter selection method. We report the unadjusted and adjusted values, with 95% confidence intervals (CIs) to indicate statistical accuracy. A P < 0.05 (two-sided) was considered statistically significant.
Results
Study participants and baseline characteristics
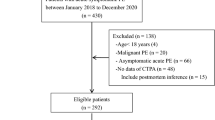
From November 2016 to March 2018, a total of 914 patients were screened consecutively; of these, 756 patients met the inclusion criteria, whereas 119 patients were excluded (19 patients died within 6 months after surgery, 4 had undergone pulmonary surgery, 11 had coagulation factor abnormalities, 47 used anticoagulant and antiplatelet drug in the past 30 days, 5 had undergone heart surgery, 1 had an autoimmune disease, 27 was lost to follow-up, and 5 withdrew). In this study, 637 patients were enrolled for analysis (Fig. 1). The median age of patients included in this study was 60 [interquartile range (IQR), 53–66) years, and 61.07% of the patients were male. The baseline characteristics of the 637 patients who had an isolated pulmonary lobectomy procedure and completed the 6-month follow-up were described in Table 1.
Clinical characteristics of postoperative stroke patients
Throughout the study population, the incidence of postoperative stroke was 1.57%. Among the 10 patients with postoperative ischemic stroke, seven had LUL, two had left pneumonectomy, and one had right upper lobectomy (RUL). The patients with postoperative stroke accounted for 5.17% of all patients who underwent LUL and left pneumonectomy. One of these 10 cases had a stroke only after the first postoperative day. Five patients had thrombosis at the LSPV stump as detected by contrast-enhanced CT (CECT). No thrombi were found on CECT of three patients. Two patients refused to undergo chest CECT within 6 months after surgery. Patient details are shown in Table 2.
Predictors of postoperative stroke
The clinical factors of the 10 patients with postoperative stroke were compared with those of the other 627 patients without postoperative stroke. Univariable analysis revealed that LUL and left pneumonectomy (p < 0.001), diabetes mellitus (p = 0.020), and thoracotomy (p = 0.016) were higher in patients with postoperative stroke. Univariable logistic regression analysis demonstrated that LUL and left pneumonectomy (odds ratio, 25.20; 95% CI, 3.17–200.42; P = 0.002) were significant predictors of postoperative stroke. After adjusting for confounders [age, sex, smoking index, drinking history, hypertension, diabetes mellitus, hyperlipidemia, arrhythmia, coronary heart disease, hypercholesterolemia, type of procedure, operative approach (thoracotomy/VATS], LUL, and left pneumonectomy independently predicted stroke (odds ratio, 18.12; 95% CI, 2.12–155.24; P = 0.008). In addition, diabetes mellitus (odds ratio, 6.43; 95% CI, 1.37–30.21; P = 0.019) was also an independent predictor of postoperative stroke (Table 3).
Discussion
Our study demonstrated that patients who underwent LUL and left pneumonectomy more frequently developed postoperative stroke compared with patients who underwent other operative procedures. In the multivariable-adjusted models, LUL, left pneumonectomy, and diabetes mellitus were independently associated with postoperative stroke. We found that the LSPV thrombosis can be detected in most postoperative stroke patients who underwent LUL and left pneumonectomy, indicating that the LSPV thrombosis may be associated with postoperative stroke.
One retrospective study reported that the LUL procedure was identified as the only risk factor for postoperative stroke8, which is partially consistent with our findings. Some reports concluded that thrombosis of the pulmonary vein stump after pulmonary lobectomy may be a new risk factor for embolism17. There are two possible explanations for the mechanism of thrombosis. Malm et al. reported that the mechanism of thrombosis may be related to endothelial damage during operation and blood stasis caused by blind pulmonary vein stumps18. Kwek et al. reported that longer pulmonary vein stumps are responsible for thrombosis development19. The length of the LSPV is longer than others, and the LSPV has the longest intrapericardial segment10,20,21. It was reported that thrombosis in the pulmonary vein stump was detected in 3.3%-3.6% of the patients who underwent lobectomy and in 13.5%-17.9% of those who underwent LUL9,19. In our study, among the 8 postoperative stroke patients who underwent CECT after surgery, 5 patients had thrombosis at the LSPV stump, whereas 3 patients had no left upper pulmonary vein thrombosis. Acute renal arterial thrombosis diagnosed 3 days after LUL has been previously reported, which strongly suggests that infarction was associated with surgery, but CECT failed to detect pulmonary vein thrombosis22. Therefore, we suspect that the thrombosis moved before the examination and reached the infarct site blocking blood circulation. Even if no thrombus is observed in the pulmonary vein stump, symptomatic embolism within a few days after LUL or left pneumonectomy should still be suspected.
In our research, diabetes mellitus also was a significant predictor of postoperative stroke. Not surprisingly, diabetes mellitus is a well-established independent risk factor for stroke and is associated with poor outcome after stroke, and the mechanism of stroke development secondary to diabetes may be due to cerebrovascular atherosclerosis, cardiac embolism, or rheologic abnormalities23. It has been confirmed that diabetes is a risk factor for postoperative stroke in major cardiovascular surgery24,25.
Postoperative atrial fibrillation (AF) is generally considered to be associated with postoperative stroke26. It was reported that left lobectomy may be a risk factor for AF following pulmonary lobectomy27. However, in our study, ten patients with postoperative cerebral infarction did not have a history of AF or postoperative AF. A retrospective study of postoperative stroke and several case reports of postoperative vital organ infarction support our results8,28,29,30. In these reports, LUL was performed leaving the pulmonary vein stump, but no postoperative AF occurred. The other typical stroke risk factors (such as age, smoking, hypertension, hyperlipidemia, and so on) were not found to be predictive of stroke in this study. We consider that it is related to the small number of postoperative stroke patients. Therefore, we are preparing to expand the sample size to confirm the relationship between these risk factors and postoperative stroke in a future study.
The results of our study highlight the importance of taking measures to curtail this risk. If the length of the venous stump is the main cause of thrombosis, the LSPV should be shortened as much as possible to prevent thrombosis. It is reported that the LSPV should be divided in the pericardium to shorten the length of the PV stump8,9. However, this method may not be suitable for all patients because it is highly invasive and has technical issues. If the stroke is caused by a thrombosis of the LSPV, we suggest that anticoagulant therapy should be performed before surgery or prolonged postoperative anticoagulant therapy should be considered as thromboembolic prophylaxis to prevent thrombosis especially for patients undergoing LUL or left lung resection. In addition, it is best to perform CECT for routine imaging after surgery. For patients with PV stump thrombosis, anticoagulant therapy should be performed immediately. It is necessary to assess pulmonary vein thrombosis based on the history of surgery for patients with cryptogenic stroke. However, in most instances, the pulmonary vein stump was not checked as a potential origin embolism. Consequently, this relationship might be ignored.
The cause of thrombosis is controversial. Aggressive glucose control through effective drug treatment and modification of other associated risk factors such as blood pressure are critical steps toward effective stroke prevention. In short, the findings might provide useful information for preventing postoperative stroke and guiding clinical decision making in a patient cohort of pulmonary lobectomy surgical patients with postoperative stroke.
Although this is the first relevant study with the largest sample size, there are still several limitations. Firstly, not all patients had routine head MRI or CT examination after surgery; thus, the incidence of asymptomatic postoperative stroke might be underestimated. Secondly, not all patients had postoperative chest CECT. Two patients with postoperative stroke did not undergo postoperative CT examination. We were unable to determine whether the patient had LSPV stump thrombus. Thirdly, the patients who died during the study period were excluded; therefore, we cannot conclude that some deaths were due to postoperative stroke. Finally, the incidence of postoperative stroke is low, and the number of postoperative stroke patients was small in this study. Therefore, a multicenter prospective cohort study with a larger sample size is needed to further determine the relationship between LUL and left pneumonectomy and postoperative cerebral infarction.
Conclusions
In summary, LUL, left pneumonectomy, and diabetes mellitus are significant risk factors for postoperative stroke. We recommend that further studies should be carried out with respect to the mechanism of postoperative stroke. If it is possible to elucidate this, it is of great significance for the prevention and treatment of postoperative stroke.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bateman, B. T., Schumacher, H. C., Wang, S., Shaefi, S. & Berman, M. F. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology 110, 231–238, https://doi.org/10.1097/ALN.0b013e318194b5ff (2009).
Kam, P. C. & Calcroft, R. M. Peri-operative stroke in general surgical patients. Anaesthesia 52, 879–883 (1997).
Mashour, G. A., Shanks, A. M. & Kheterpal, S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. The Journal of the American Society of Anesthesiologists 114, 1289–1296 (2011).
Parikh, S. & Cohen, J. R. Perioperative stroke after general surgical procedures. New York state journal of medicine 93, 162–165 (1993).
Vlisides, P. & Mashour, G. A. Perioperative Stroke. Canadian journal of anaesthesia = Journal canadien d’anesthesie 63, 193–204, https://doi.org/10.1007/s12630-015-0494-9 (2016).
Goodney, P. P., Likosky, D. S. & Cronenwett, J. L. Factors associated with stroke or death after carotid endarterectomy in Northern New England. Journal of vascular surgery 48, 1139–1145, https://doi.org/10.1016/j.jvs.2008.05.013 (2008).
Bucerius, J. et al. Stroke after cardiac surgery: a risk factor analysis of 16,184 consecutive adult patients. The Annals of thoracic surgery 75, 472–478 (2003).
Yamamoto, T. et al. Is left upper lobectomy for lung cancer a risk factor for cerebral infarction? Surgery Today 46, 780–784, https://doi.org/10.1007/s00595-015-1233-0 (2016).
Ohtaka, K. et al. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. The Annals of thoracic surgery 95, 1924–1928, https://doi.org/10.1016/j.athoracsur.2013.03.005 (2013).
Miyoshi, R. et al. Pulmonary vein thrombosis after lobectomy with vein stump closure by ligation. Asian cardiovascular & thoracic annals 26, 546–551, https://doi.org/10.1177/0218492318802141 (2018).
Kamori, T. et al. Pulmonary vein stump thrombosis after left pneumonectomy, diagnosed based on a high plasma D-dimer level: a case report. Journal of Thoracic Disease 9, E210–E214, https://doi.org/10.21037/jtd.2017.02.58 (2017).
S, S., M, Y., K, N. & T, T. Mechanical Thrombectomy for Acute Ischemic Stroke Arising from Thrombus of the Left Superior Pulmonary Vein Stump after Left Pneumonectomy: A Case Report. NMC case report journal 6, 17–20 (2019).
Travis, W. D. et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. Journal of Thoracic Oncology 10, 1243–1260, https://doi.org/10.1097/JTO.0000000000000630 (2015).
Geerts, W. H. et al. Prevention of Venous Thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133, 381S–453S, https://doi.org/10.1378/chest.08-0656 (2008).
Wang, Y. et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. The New England journal of medicine 369, 11–19, https://doi.org/10.1056/NEJMoa1215340 (2013).
Feng, X. et al. Number of Cigarettes Smoked Per Day, Smoking Index, and Intracranial Aneurysm Rupture: A Case-Control Study. Frontiers in neurology 9, 380–380, https://doi.org/10.3389/fneur.2018.00380 (2018).
Gual-Capllonch, F., Teis, A. & Palomeras, E. Pulmonary vein spontaneous echocontrast and stroke after pulmonary lobectomy. Journal of clinical ultrasound: JCU 41, 321–322, https://doi.org/10.1002/jcu.21913 (2013).
Malm, B., Hull, S. & Jadbabaie, F. Left Upper Pulmonary Vein Thrombus in a Patient with Atrial Fibrillation and Prior Lobectomy. The American Journal of Medicine 127, e7–e8, https://doi.org/10.1016/j.amjmed.2014.07.029 (2014).
Ohtaka, K. et al. Left upper lobectomy can be a risk factor for thrombosis in the pulmonary vein stump. Journal of cardiothoracic surgery 9, 5 (2014).
Ohira, S., Doi, K., Okawa, K., Matsushiro, T. & Yaku, H. Surgical Removal of Extensive Left Pulmonary Vein Stump Thrombus After Pulmonary Lobectomy: A Rare Cause of Acute Cerebral Embolism. The Annals of thoracic surgery 96, e135–e136, https://doi.org/10.1016/j.athoracsur.2013.07.028 (2013).
Porres, D. V. et al. Learning from the Pulmonary Veins. RadioGraphics 33, 999–1022, https://doi.org/10.1148/rg.334125043 (2013).
Asteriou, C., Barbetakis, N., Efstathiou, A., Kleontas, A. & Tsilikas, C. Renal Artery Thrombosis following Lobectomy for Lung Cancer. Case Reports in Oncology 3, 208–211, https://doi.org/10.1159/000314838 (2010).
Biller, J. & Love, B. B. Diabetes and stroke. The Medical Clinics of North America 77, 95–110 (1993).
Nystrom, T., Holzmann, M. J. & Sartipy, U. Long-Term Risk of Stroke in Patients With Type 1 and Type 2 Diabetes Following Coronary Artery Bypass Grafting. Journal of the American Heart Association 4, https://doi.org/10.1161/jaha.115.002411 (2015).
Miyazaki, S. et al. Risk factors of stroke and delirium after off-pump coronary artery bypass surgery. Interactive cardiovascular and thoracic surgery 12, 379–383, https://doi.org/10.1510/icvts.2010.248872 (2011).
Lahtinen, J. et al. Postoperative atrial fibrillation is a major cause of stroke after on-pump coronary artery bypass surgery. The Annals of thoracic surgery 77, 1241–1244, https://doi.org/10.1016/j.athoracsur.2003.09.077 (2004).
Xin, Y. et al. Left lobectomy might be a risk factor for atrial fibrillation following pulmonary lobectomy. European Journal of Cardio-Thoracic Surgery 45, 247–250 (2013).
Schwalm, S., Ward, R. P. & Spencer, K. T. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography 17, 487–488, https://doi.org/10.1016/j.echo.2004.02.003 (2004).
Oura, H., Hirose, M., Aikawa, H. & Ishiki, M. Abdominal organ infarction encountered immediately after surgery of primary lung cancer. Kyobu geka. The Japanese journal of thoracic surgery 58, 137–142 (2005).
Manabe, S. et al. Renal infarction in a patient with pulmonary vein thrombosis after left upper lobectomy. Case Reports in Nephrology and Dialysis 4, 103–108 (2014).
Acknowledgements
This work was financially supported by grants from National Natural Science Foundation of China (No. 81571260, 81701272, 81701287).
Author information
Authors and Affiliations
Contributions
N.X., X.M. and C.W. conceived and designed the research; X.M., Y.L., and Y.W. collected the data; X.M. and M.Y. analyzed the data; Y.L. and C.W. reviewed the data and manuscript; Y.L. supervised all steps of the research project. X.M. wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, N., Meng, X., Wu, C. et al. Both left upper lobectomy and left pneumonectomy are risk factors for postoperative stroke. Sci Rep 9, 10432 (2019). https://doi.org/10.1038/s41598-019-46989-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46989-w
This article is cited by
-
Risk factors for postoperative cerebral infarction in Lung Cancer patients: a retrospective study
Journal of Cardiothoracic Surgery (2023)
-
Dyspnea after discharge from hospital due to pulmonary vein thrombosis after video-assisted left upper lobectomy: a case report
JA Clinical Reports (2022)
-
Endovascular recanalization of acute ischemic stroke patients exhibiting large vessel occlusion after pulmonary lobectomy: case series
BMC Neurology (2022)
-
Efficacy of direct oral anticoagulant for renal infarction due to pulmonary vein stump thrombosis after left pneumonectomy
Surgical Case Reports (2022)
-
Left Upper Lobectomy for Lung Cancer as a Risk Factor for Cerebral Infarction: A Systematic Review and Meta-Analysis
Lung (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.