Abstract
The TATA-box binding protein associated factor 1 (TAF1) protein is a key unit of the transcription factor II D complex that serves a vital function during transcription initiation. Variants of TAF1 have been associated with neurodevelopmental disorders, but TAF1’s molecular functions remain elusive. In this study, we present a five-generation family affected with X-linked intellectual disability that co-segregated with a TAF1 c.3568C>T, p.(Arg1190Cys) variant. All affected males presented with intellectual disability and dysmorphic features, while heterozygous females were asymptomatic and had completely skewed X-chromosome inactivation. We investigated the role of TAF1 and its association to neurodevelopment by creating the first complete knockout model of the TAF1 orthologue in zebrafish. A crucial function of human TAF1 during embryogenesis can be inferred from the model, demonstrating that intact taf1 is essential for embryonic development. Transcriptome analysis of taf1 zebrafish knockout revealed enrichment for genes associated with neurodevelopmental processes. In conclusion, we propose that functional TAF1 is essential for embryonic development and specifically neurodevelopmental processes.
Similar content being viewed by others
Introduction
Transcription factor II D (TFIID) consists of the TATA-box binding protein (TBP) and 12–14 TBP-associated factors (TAFs), and is part of the preinitiation complex that initiates transcription of RNA polymerase II transcription dependent genes1,2,3. Variants in several TFIID components, TBP4, TAF25, TAF66 and TAF137, have been associated with neurodevelopmental disorders, proposing a fundamental function of TFIID during embryonic development and especially neurodevelopment. TAF1 is the largest TAF unit of the TFIID complex and plays a key role in the preinitiation complex by facilitating binding to promoter regions2. The TAF1 gene (GRCh37/hg19, chrX:70586114–70685855, NM_004606.4) includes 39 exons and encodes more than 20 coding and non-coding transcripts expressed in various tissues, including the central nervous system8.
Coding germline variants of TAF1 have been reported to cause intellectual disability (ID). Two TAF1 missense variants were identified in two families within a cohort study including 405 unresolved families with X-linked ID (XLID)9, in which two individuals from the family reported here were included (clinical data was not presented). The cohort study was followed by a comprehensive report by O’Rawe et al. of 14 males with syndromic XLID (MIM: 300966) from eleven unrelated families who presented with nine different single nucleotide variants (eight missense variants and one splice site variant) and two duplications including TAF1. They also demonstrated that asymptomatic female carriers with pathogenic missense variants in TAF1 from two independent families displayed skewed X-chromosome inactivation (XCI)10, which was recently confirmed by Hurst et al. in a third case, a mother carrying a TAF1 p.Ser1600Gly substitution that caused XLID in her son11. A non-coding 2.6 kb insertion of a SINE-VNTR-Alu (SVA)-type retrotransposon in intron 328 of TAF1 causes the neurological disorder X-linked dystonia-parkinsonism (XDP; MIM: 313650). XDP is a progressive neurodegenerative disorder characterized by involuntary movements (dystonia), most often developing in adult life in combination with parkinsonism12,13,14. Previous mapping9 of protein networks have demonstrated TAF1’s association with the RNA polymerase II complex together with proteins associated with ID, such as ATN115, MLL216, RBM1017, USP27X9, TBP4, TAF25, TAF66 and TAF137. A central role of TAF1 during embryonic development has been proposed after encountering elevated Taf1 expression during early fetal development in mice18. However, to our knowledge, no extensive functional assessment of TAF1’s role during embryonic development has been made and the molecular context of TAF1’s association to disease remains elusive.
Here, we report clinical and genetic findings of a large family with a likely pathogenic variant in TAF1 and demonstrate TAF1’s association to neurogenesis by creating the first complete taf1 knockout model. All affected patients were males who had syndromic XLID with phenotypes overlapping with previously published cases, and asymptomatic female carriers revealed completely skewed XCI as described before10,11. The taf1 zebrafish knockout model showed lethality during embryonic development, establishing a crucial function of taf1 during embryogenesis. Moreover, transcriptome analysis of taf1 mutants three days post fertilization (dpf) showed enrichment for genes associated with neurodevelopmental processes, providing the first assessments of taf1 function during embryonic development.
Results
Clinical report
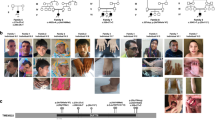
We clinically assessed all affected males from the family (Fig. 1A). They presented with severe (III:2 and III:5), moderate (IV:5 and V:4), or mild (III:8 and IV:1) ID. All affected males had a long face, pointed chin, large hands, prominent forehead, short neck, and low-set, protruding, large ears. A long philtrum, prominent supraorbital ridge, deep-set eyes, and large feet were observed for the majority of males (Table 1). III:2 and III:5 had cataract and died from pneumonia at the ages of 65 and 25 years, respectively. III:8 died from diphtheria at the age of six years. Comprehensive clinical assessments of IV:5 and V:4 revealed additional clinical features, such as oral-pharyngeal dysphagia, a high palate, generalized muscle hypotonia, joint hypermobility, and kyphosis. IV:5 also presented with down-slanted palpebral fissures, scoliosis, and generalized hirsutism. V:4 presented with an asymmetric intergluteal cleft with a sacral dimple (Fig. 1B; Table 1). No hearing impairments or other malformations of the auditory system were noted. Brain magnetic resonance imaging (MRI) of V:4 at two years of age identified no pathological deviations.
The five-generation family affected with syndromic X-linked intellectual disability (XLID) and the architecture of the TAF1 gene with missense variants associated with intellectual disability. (A) Six males, in the same five-generation family, were initially diagnosed with syndromic intellectual disability. Seventeen family members were genotyped (wt = wild-type, *=variant carrier). (B) Photographs show clinical features of the six affected males who presented with a long face, pointed chin, prominent forehead, long philtrum, prominent supraorbital ridge, deep-set eyes, and low-set, protruding, large ears. (C) Schematic view of TAF1 gene and corresponding protein with domains. Germline missense variants associated with intellectual disability are shown above the schematic (black). The likely pathogenic missense variant presented in this report, TAF1 c.3568C>T, p.(Arg1190Cys), is marked in bold. Light grey bars mark somatic variants reported in the Cosmic cancer database. Domains of TAF1 are kinase domain 1 (amino acid residues 1–414, red) and 2 (residues 1,425–1,872, red), TBP-binding domain (residues 1–140, purple), HAT domain (residues 600–1,009, blue), RAP74-interacting domain (residues 1,110–1,236, pink), and bromodomains 1 and 2 (residues 1,359–1,638, green)34,59.
Segregation analysis and bioinformatic predictions propose that the TAF1 c.3568C>T, p.(Arg1190Cys) variant is likely pathogenic
Linkage analysis of 14 family members using 44 polymorphic microsatellite markers, highlighted a candidate region of 28.3 Mb on Xq11.1–Xq21.32 (Supplementary Data S1). A TAF1 c.3568C>T, p.(Arg1190Cys) variant was identified within the linked region as part of a cohort X-exome study in which DNA samples from IV:5 and V:4 were included without clinical details9. Segregation analysis of 17 family members confirmed the X-linked segregation pattern. Two affected males were hemizygous, five carrier females were heterozygous and ten asymptomatic family members did not carry the TAF1 variant (Figs. 1A and S1).
The TAF1 c.3568C>T variant is located in the RAP74-interacting domain (Fig. 1C) and predicted to be disease-causing (MutationTaster19), deleterious (SIFT20, 0.000) and to affect a conserved base (PhyloP21, 3.47 and GERP++22, 4.91). Population data in gnomAD reveal an underrepresentation of missense variants in TAF1 (gnomAD: Z = 5.49, o/e = 0.44,)23. Furthermore, no hemizygous or homozygous loss-of-function variants have been reported in SweGen databases24. Similarly, gnomAD reports depletion of loss-of-function variants in TAF1, (pLI = 1, o/e = 0.00) with only one hemizygous individual harboring an early p.(Ala17Glyfs) loss-of-function variant and one individual hemizygous for a predicted splice acceptor site in the 3′ UTR. In addition, they report one homozygous and 14 hemizygous individuals with an rs200964328 loss-of-function variant; however, this variant only affects a 450 nucleotides short 498 base pair (bp) TAF1 isoform23 and not the canonical protein. In summary, population data demonstrate a strong underrepresentation of missense variants and lack of complete loss-of-function variants in the general population.
Skewed X-chromosome inactivation in carrier females
The X-chromosome inactivation (XCI) pattern was investigated in five carrier females and three non-carrier females by screening polymorphic microsatellites within the X-linked RP2 and AR genes as previously described25,26. The assay revealed a 100% XCI of the mutant allele in all heterozygous carrier females, but normal XCI pattern in the three non-carrier females (Fig. 2A and 2B). Inactivation of the mutant allele was validated by Sanger sequencing of reversed transcribed PCR products derived from carrier females’ RNA, expression of only the wild-type (wt) allele (Fig. 2D).
Skewed X-chromosome inactivation in carrier females. (A) Fragment length analysis on DNA confirmed biallelic expression of microsatellites (androgen receptor [AR] and retinitis pigmentosa 2 [RP2]) in all females. (B) DNA from carrier females digested with the methylation-sensitive HpaII restriction enzyme showed monoallelic detection, indicating completely skewed X-chromosome inactivation. (C) Sanger sequencing of DNA confirmed heterozygosity for the disease-causing variant in carrier females and wild-type sequence for non-carrier females. (D) Sequencing of reversed transcribed RNA showed the expression of only wild-type allele in all carrier females and non-carrier females.
taf1 knockout zebrafish embryos display lethal malformations
To investigate taf1’s role during embryogenesis, two mutant lines were successfully bred using Clustered Regulatory Interspaced Short Palindromic Repeats (CRISPR) CRISPR-associated protein 9 (Cas9): taf1uu1931, with an 8 bp deletion in taf1 (GRCz10/danRer10, NM_001044785.1) exon 7, resulting in a frameshift of one amino acid followed by a premature stop codon, c.855_862del, p.(Gly296Leufs*2) (Supplementary Fig. S2A), and taf1uu1941, with a 10 bp deletion in exon 8, resulting in a frameshift of eight amino acids and a premature stop codon, c.1082_1091del, p.Glu362Alafs*9 (Fig. 3A), referred to as mutants or knockouts. The phenotype was assessed in offspring originating from the same clutch of crossing two taf1uu1941/+ adults and two taf1uu1931/+ adults. Three dpf, homozygous taf1 uu1941/uu1941 (n = 9) and taf1 uu1931/uu1931 (n = 11) mutants (mut) displayed reduced length, underdeveloped cartilage, eyes and ears, short pectoral fins, heart edema, blood-filled cavities and a dorsally bent body axis compared to siblings (sib; n = 34 for both groups; Fig. 3B and Supplementary Fig. S2). The tectum was underdeveloped in taf1uu1941/uu1941 (n = 7) compared to siblings (n = 22; Fig. 3C). Measurement of the tectum was obstructed in taf1uu1931/uu1931, but tectum underdevelopment is likely since similar reduction in head size of about 15% was found in both taf1uu1931/uu1931 (mut n = 9, sib n = 33) and taf1uu1941/uu1941 (n = 7, sib n = 22; (Supplementary Figs S3Q–T). At 3 dpf, taf1uu1941/uu1941 (n = 10) displayed reduced heart beat compared to siblings (n = 35), but taf1uu1931/uu1931 did not (mut n = 11, sib n = 33; Supplementary Figs. S3O and S3P). Neither gross gastrointestinal malformations nor musculature malformations were observed. At 5 dpf the phenotype was profound and taf1uu1941/uu1941 (n = 11) displayed severe cranial cartilage defects and a reduced or absent heart beat implied embryonic lethality27. Heterozygous embryos (taf1uu1931/+ and taf1uu1941/+) displayed no deviations compared to wt (taf1+/+) zebrafish embryos for any of the observed or quantified phenotypes (Supplementary Fig. S3 and Table S4).
Zebrafish taf1 knockout model revealed an essential function of taf1 during embryogenesis and neurodevelopment. (A) Sequencing results of taf1uu1941/uu1941 F2 zebrafish embryos confirmed a 10 bp deletion in exon 8, which results in a frameshift and a stop codon, p.Glu362Alafs*9. (B) Three days post fertilization (dpf) mutant taf1uu1941/uu1941 (right) zebrafish embryos showed heart and ventricle edema, blood filled cavities, bent body axis, and general underdevelopment (UD) including short pectoral fins, reduced length and underdeveloped cartilage, eyes, and ears. (C) Phenotype quantification demonstrated reduced length, underdevelopment of eyes, ears and tectum when comparing wild-type taf1+/+ and heterozygous taf1uu1941/+ (siblings; sib) with taf1uu1941/uu1941 mutants. Adjusted p-values were generated using Student’s t-test, adjusted with Bonferroni correction. (D) Gene set enrichment analysis of all differentially expressed genes revealed four major themes associated with loss of taf1. Chromatin and DNA assembly as well as muscle development pathways were upregulated (red) and pathways of ion transport as well as sensory and visual perception pathways were downregulated (blue) in taf1uu1941/uu1941 zebrafish embryos.
Transcriptome analysis revealed enrichment of genes important for neurodevelopment processes
Transcriptome sequencing was performed using RNA from pooled mutant (taf1uu1941/uu1941) and sibling (taf1+/+ and taf1uu1941/+) zebrafish embryos of the same clutch to investigate taf1’s function during embryogenesis. In sample correlation analysis, the three replicates of mutant and sibling embryos were divided into two groups: mutants and siblings (Supplementary Fig. S4). The 10 bp deletion in taf1uu1941/uu1941 mutants was confirmed by Sanger sequencing of cDNA and investigation of transcriptome reads in the Integrative Genomics Viewer28. All reads from mutant samples contained the 10 bp deletion. As expected, a few alleles in each of the three sibling pools contained the 10 bp deletion, originating from the heterozygous zebrafish embryos.
A paired differential gene expression analysis reported 6,628 out of 23,919 detected genes as differentially expressed (padj <0.01; Supplementary Table S5). PANTHER overrepresentation test29,30 on all differentially expressed genes revealed 2.2 enrichment for genes of neuron-neuron synaptic transmission (padj <1.3E-04). Out of the 6,628 differentially expressed genes, 612 genes were more than 4-fold downregulated (log2 <−2) and 258 genes were more than 4-fold upregulated (log2 >2) in mutants. PANTHER overrepresentation test revealed that the 612 most downregulated genes had strongest association to neuromuscular synaptic transmission (7-fold enriched, padj < 6.2E-05), specifically by downregulation of GABA receptor genes. The top 258 upregulated genes had strongest association to chromatin assembly (55-fold enriched, padj <1.4E-10), specifically by upregulation of histone genes (Supplementary Table S6). In line with PANTHER analysis, an additional gene set enrichment analysis (GSEA; Supplementary Table S5) of all differentially expressed genes, visualized with enrichment map, proposed upregulation of genes associated with chromatin and DNA assembly and muscle development, and downregulation of genes associated with ion transport, and sensory and visual perception (Fig. 3D).
Discussion
TAF1 is a central protein of the TFIID complex that plays a key role during the initiation of transcription. Several genes encoding proteins of the complex have been associated with ID4,5,6,7, including TAF1 that recently was added to the list of about 150 genes linked to XLID9,31. In 2015, two missense variants in TAF1, p.(Arg1190Cys) and p.(Asn493Asp), were identified in patients with syndromic XLID by investigating 405 families with unresolved XLID9. To date, a total of 13 missense variants, one splice site variant and two duplications including TAF110 have been reported to cause ID in 19 patients9,10,11,32. However, no functional investigations of TAF1’s role during embryogenesis and neurodevelopment have been performed. In this study, we investigated the clinical, genetic, and molecular patterns of a five-generation family with a TAF1 p.(Arg1190Cys) variant, here determined as likely pathogenic according to ACMG guidelines33. Moreover, we also explored TAF1’s function by creating the first taf1 knockout model that presented a lethal phenotype during embryogenesis and dysregulation of biological processes associated with neurodevelopment.
The male patients investigated in this study presented with XLID, distinct facial features, and additional malformations. In general, the clinical features overlapped with symptoms in previously described patients with TAF1 missense germline variants. For example, ID, facial dysmorphology and V:4 presented with a peculiar symptom of an asymmetric intergluteal cleft with a sacral dimple, which has been observed in 12 previously reported probands10. Further, our report broadens the clinical spectrum of XLID associated with TAF1 with features such as delayed gross motor development, a prominent forehead, short neck, large hands and feet, and deep-set eyes.
Additional to the clinical comparison with previous cases and segregation analysis, the TAF1 c.3568C>T, p.(Arg1190Cys) variant was assessed by various bioinformatic prediction tools combined with evaluation of TAF1’s susceptibility towards variance using publicly available population databases. GnomAD and SweGen demonstrated a lack of complete loss-of-function variants and underrepresentation of missense variants. The p.(Arg1190Cys) variant is located in the RAP74-interacting domain (amino acid residues 1,110–1,236), where an underrepresentation of germline missense variants in the general population is displayed (14 observed versus 24 expected). The RAP74-interacting domain interacts with TAF734 and supposedly, variants in this domain could interfere with the TAF1–TAF7 interaction, thereby contributing to disease. Further, DECIPHER35 reported several affected individuals with TAF1 variants predicted as pathogenic or likely pathogenic. Collectively, this indicates that some TAF1 missense variants might be disease-causing and that complete lack of TAF1 is not compatible with life.
Somatic variants in TAF1 have been proposed to play a role in tumorigenesis by phosphorylation of p5336, which may inhibit overall p53 tumor suppressor activity. In addition, TAF1 has been proposed as a driver gene for several cancers, such as mammary cancer37, colorectal cancer38, and clear cell endometrial cancer39. The Cosmic database40 reports >400 somatic missense variants spread throughout the different domains of the protein (Fig. 1C). Interestingly, the p.(Arg1190Cys) variant (NP_004597.2) investigated in this study has been reported twice in cancer patients as p.(Arg1169Cys) variant (NP_620278.1). Also, one previously reported germline p.(Ile1337Thr) variant (NP_004597.2) causing ID10 has been reported in two cancer patients p.(Ile1316Thr), (NP_620278.1). Further, a somatic variant, p.(Arg1225Gln), (NP_620278.1), affecting the same amino acid previously associated with syndromic XLID p.(Arg1246Trp), (NP_ 004597.2), has been reported in two cancer patients. It is well established that somatically mutated genes associated with cancer can be associated with ID when mutated in the germline41,42,43.
The pathogenicity of the TAF1 p.(Arg1190Cys) variant was further strengthened by the observed 100% skewed XCI in five carrier females, and normal XCI in three non-carrier females of the same family. Skewed XCI in females carrying pathogenic XLID variants is a recognized phenomenon44 and has been reported in females heterozygous for pathogenic TAF1 variants in three independent families before10,11. The linkage analysis of the family presented here revealed recombination of the maternal X-chromosome in female IV:11. The recombination resulted in carriership of 65%–79% of the affected X-chromosome and 21%–35% of the wt X-chromosome (Supplementary Fig. S5). The 21%–35% of the wt X-chromosome included TAF1 and IV:11 was thus found not to be a carrier of the disease-causing variant. In accordance with the other two non-carrier females, IV:11 did not show skewed XCI. The fact that IV:11 carried 65%–79% of the disease-causing X-chromosome but not the disease-causing variant, strengthens the hypothesis that the skewed XCI is caused by the p.(Arg1190Cys) variant. Generally, skewed XCI is thought to arise because of selective advantage of wt cells or disadvantage of mutant cells45. However, the mechanism leading to skewed XCI in females carrying pathogenic TAF1 variants is unclear and, even though it is not within the scope of this article, further studies are needed to clarify the impact of TAF1 variants on cell survival.
Full knockout models of TAF1 orthologues to investigate TAF1 function have so far been missing. However, a study in mice reported that Taf1 expression is drastically elevated during embryonic development and is then decreased and maintained at stable levels from post-natal week 3 and onwards18, proposing an essential function of Taf1 during embryogenesis. The absence of hemi- and homozygous loss-of-function variants in the protein coding part of the canonical TAF1 isoform in human population databases proposes that the complete loss of TAF1 is lethal. To further illuminate taf1 function during embryonic development we established the first adequate taf1 knockout model by creating two CRISPR/Cas9 edited zebrafish lines. The reduced taf1 RNA expression suggested that the frameshift variants, resulting in premature stop codons, led to nonsense-mediated decay of mutant RNA. Both lines presented with a similar phenotype and demonstrated that germline loss of taf1 resulted in general developmental delay and was embryonically lethal. The utilization of two independent single guide RNAs (sgRNAs) excluded off-target effects as the cause for the lethal phenotype. The minor phenotypical differences between taf1uu1931/uu1931 and taf1uu1941/uu1941 could be explained by the differential functionality of the truncated protein, line-specific genetic variation or minor guide-specific off-target effects. Quantification of tectum size was performed as a proxy for brain malformations previously reported in patients with TAF1 variants. At 3 dpf, a reduction of notably 32% was measured in the mutants (taf1uu1941/uu1941) compared to siblings (taf1uu1941/+ and taf1+/+). This is in line with a prior study that assessed the effect of taf1 knockdown by morpholino, which displayed a 10% decrease in tectum size10. The increased reduction observed in our model is likely linked to the advantage of examining animals with complete loss of taf1. The absence of malformations in heterozygous zebrafish mutants in our study confirms that monoallelic expression of taf1 is sufficient for normal embryonic development and suggests why previous attempts did not reveal measurable phenotypic deviations in F0 taf1 mutants10.
To illuminate pathways affected by loss of taf1, we investigated the enrichment for genes of certain biological processes in our set of differentially expressed genes. Overrepresentation analysis of all differentially expressed genes (n = 6,628) revealed a 2.2 overrepresentation of genes associated with neuron-neuron synaptic transmission. Genes more than 4-fold downregulated (n = 612) were 7-fold enriched for neuromuscular synaptic transmission and the genes more than 4-fold upregulated (n = 258) were 55-fold enriched for chromatin assembly, proposing that taf1 regulates genes important for neurodevelopmental processes (Supplementary Table S6). More specifically, histone genes were particularly upregulated and GABA receptor genes were particularly downregulated in taf1 mutant zebrafish embryos. Variants in chromatin remodeling genes46,47, and histone genes in particular48,49, as well as GABA genes50 have repeatedly been linked to ID. In line with the PANTHER results, GSEA result of all differentially expressed genes, visualized using enrichment map, highlighted chromatin and DNA assembly pathways as well as ion transport pathways as two major themes. A recent study by Aneichyk et al. established XDP’s association with an intronic variant of TAF18 causing reduced TAF1 expression in stem cell-derived neuronal cells and, likely, XDP12,13,14. Transcriptomics on XDP patient-derived neuronal cells implied disturbed regulation of neuronal processes and axon guidance by analysis on a subset of differentially expressed genes. Hurst et al. recently investigated expression of 86 neuronal ion-channel genes in TAF1 deficient neuronal cells of which eight genes showed differential expression11. Four of these genes orthologs (ccnd1, cacna1g, kcnj14 and asic2) were also differently expressed in zebrafish mutants. Additionally, ATP-sensitive inward rectifier potassium channel 12 (kcnj12), belonging to the same gene family as kcnj14, was the most underexpressed gene in our data (log2 −6.1). Data provided by Aneichyk et al. displayed a moderate upregulation of kcnj12. Hurst et al. highlighted reduced expression of Cyclin D1 (CCND1) that is associated with proliferation and is important for cerebellar development and highlighted as dysregulated in patients with TAF1 variants by Ballouz et al. (https://www.bio-rxiv.org/content/10.1101/128439v2). We observed a slightly reduced expression of ccnd1 in our dataset (log2 −0.49). Apart from ccnd1, our dataset revealed striking upregulation of ccna1 (log2 3.6) and to a lesser extent dysregulation of other cyclins, including a2, b1, b3, c, e2, g1, l1 and t2. Up to this point, no key pathways or genes have been highlighted from the combined results of previous RNA studies. Compared to Aneichyk and Hurst et al., our analysis has the advantage of investigating all differentially expressed genes of a germline knockout during embryonic development. The result highlights chromatin processes and ion transport processes as particularly dysregulated, both processes have previously been linked to neurodevelopment. Combined, these results advocate that germline variants in TAF1 could cause XLID by dysregulation of genes essential for neurodevelopment. However, even though it is not within the scope of this study, further assessments are needed to clarify TAF1’s relation to synaptic transmission, chromatin assembly and ion transport in humans to better understand the mechanism causing syndromic XLID.
In conclusion, we present a five-generation family with six males affected by syndromic XLID associated with TAF1 c.3568C>T, p.(Arg1190Cys) hemizygosity. Five carrier females were asymptomatic and showed 100% skewed XCI. We investigated the role of taf1 during embryonic development by creating the first germline taf1 knockout model, where the lethal outcome in zebrafish embryos illuminated the importance of functional taf1 during embryonic development. Subsequent transcriptome analysis highlighted several genes important for neuronal processes to be associated with taf1 function, proposing that human TAF1 may be a key regulator of neurogenesis. Further investigation of TAF1 missense variants’ association to disturbed neurodevelopmental processes are important as they may help to understand the full etiology of syndromic XLID associated with TAF1.
Materials and Methods
Ethical consent
Prior to the initiation of the study, the local ethics committee for human research in Uppsala, Sweden, approved this study (Dnr 2012/321). Informed consent for the study was obtained from the participants and legal guardians of the participants below 18 years of age. Consent to publish identifying images in an online, open access journal has been obtained from participants and legal guardians of participants below 18 years of age. All clinical investigations and genetic analyzes were conducted in accordance with the guidelines of the Declaration of Helsinki. All animal experimental procedures were approved by the local ethics committee for animal research in Uppsala, Sweden (permit number C161/4).
Clinical assessment
The five-generation family consisted of 32 members with 6 out of 20 males diagnosed with a syndromic form of XLID. Comprehensive and continuous clinical examinations were performed since early childhood for patient V:4 and since young adulthood for patient IV:5. Patients III:2, III:5, III:8, and IV:1 were clinically assessed based on photographs and family history. Phenotype data has been submitted to ClinVar.
Polymerase chain reaction and Sanger sequencing
For genetic analysis, peripheral blood samples were collected from 17 family members. DNA and RNA was extracted according to standard protocols.
Polymerase chain reaction (PCR) and Sanger sequencing were performed on genomic DNA and reverse-transcribed cDNA. Approximately 500 ng of RNA was used for cDNA synthesis with oligo(dT) according to Maxima H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA). Taq PCR was performed on 1 μl of cDNA or 50 ng of DNA at 95 °C for 5 min, 20 cycles (95 °C 20 sec, 65–55 °C 30 sec, 72 °C 1 min) and 25 cycles (95 °C 20 sec, 55 °C 30 sec, 72 °C 1 min) following a standard protocol (Applied Biosystems, Waltham, MA). Sanger sequencing was performed on a 3130XL ABI Genetic Analyzer using the ABI PRISM BigDye Primer v3.0 Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Waltham, MA). Primer sequences are stated in Supplementary Table S2. Variant data has been submitted to ClinVar.
Assays for X-chromosome inactivation assessment
One μg of genomic DNA isolated from peripheral blood was digested with FastDigest HpaII in a 20 µl reaction volume using 1 µl of restriction enzyme according to standard protocol (Thermo Fisher Scientific, Waltham, MA). Amplification of the androgen receptor (AR) microsatellites was carried out in a 20 µl reaction containing 0.1 mM of dNTP, 250 nM of each primer, 1.25 mM of MgCl2, 2 µl of buffer, 1 U of Taq polymerase, and 50 ng of DNA or 2 µl of digested DNA, with primers and cycling conditions as previously described25. Amplification of the retinitis pigmentosa 2 (RP2) gene microsatellites was performed as previously described26 with an input of 50 ng of DNA or 2 µl of digestion product. 5′ primers were marked with (6)-Carboxyfluorescein protein (/56-FAM; Supplementary Table S2). Genotyping was performed through a fragment length analysis (FLA), as described in the Supplemental Data 1, and analyzed with the GeneMapper™ software (Thermo Fisher Scientific, Waltham, MA). Primer sequences are stated in Supplementary Table S2.
CRISPR/Cas9 target design
Two sgRNAs targeting the single zebrafish taf1 gene with no predicted off-target effects were designed using the online software CHOPCHOP51: one targeting exon 7 (5′-GGG CTA AGA AAA AAT CAG GGT GG-3′) and the other targeting exon 8 (5′-GGT GTC CCG GAG GAT GGC AGC GG-3′). The sgRNAs were prepared as previously described52, creating a fragment consisting of the T7 promotor, the targeted gene specific sequence, and the guide core sequence. The sgRNAs were synthesized by in vitro transcription using the HiScribe T7 High Yield RNA Synthesis Kit (New England Biolabs, Ipswich, MA). Cas9 mRNA was prepared by in vitro transcription with the mMESSAGE mMACHINE T3 Transcription Kit (Life Technologies, Carlsbad, CA) using 500 ng of linearized plasmid that was retrieved from 5 μg of p-T3TS-nCas9n plasmid (plasmid #46757; Addgene, Cambridge, MA) digested with XbaI (New England Biolabs, Ipswich, MA). The products were purified, and their integrity were assessed using a denaturation gel.
Animals
Fertilized zebrafish (Danio rerio) eggs (AB strain) were obtained by natural spawning. Embryos were injected at the one-cell stage with 150 pg of Cas9 mRNA and 50 pg of each sgRNA in RNase-free H2O as previously described52 and maintained at 28.5 °C in E3 medium53. The efficiency of the targets was estimated by the CRISPR-Somatic Tissue Activity Test (STAT) methodology in eight embryos at two days post-injection, as previously described54. The injected founder zebrafish (F0) were raised and incrossed. For genotyping the F1 zebrafish, DNA was extracted from a 1–3 mm amputation of the adult zebrafish caudal fin by lysing the tissue in 30 μl of 50 mM NaOH for 20 min at 95 °C, adding 60 μl of 0.1 mM Tris and diluting the obtained material (1:10). For the initial genotyping step, FLA analysis was used. Two μl of DNA (50–200 ng) was added to Platinum Taq DNA Polymerase55. The PCR mix was incubated at 94 °C for 12 min followed by 35 cycles (94 °C 30 sec, 57 °C 30 sec, 72 °C 30 sec) and 72 °C for 10 min. Size determination was carried out on a 3130XL ABI Genetic Analyzer (Applied Biosystems, Waltham, MA) and the data was analyzed using the Peak Scanner Software (Thermo Fisher Scientific, Waltham, MA). For the fish that screened positive for the variant, the FLA results were confirmed by Sanger sequencing as described above.
Phenotype assessment
Two strains with alleles containing frameshift deletions resulting in premature stop codons (taf1uu1931 and taf1uu1941) were selected for further experiments. The identified founders were crossed with wt zebrafish (AB strain), and their adult offspring were genotyped. Heterozygous carriers from both mutant lines were individually crossed and the offspring was observed. Phenotypic observation and quantification of eye, ear and length were performed on 43 and 45 embryos from the same clutch of taf1uu1941 and taf1uu1931, respectively. Twenty-nine and 42 embryos from the same clutch of taf1uu1941 and taf1uu1931 respectively, were raised under normal conditions with the addition of 0.003% 1-phenyl 2-thiourea (PTU) at 6 hours post fertilization to reduce pigmentation, which allowed measurement of head and tectum size as demonstrated before56. Eye, ear, head and tectum area as well as length was measured with ImageJ on images generated with a Leica microscope or the VAST bioImager™. Heart rate was measured in 44 and 45 embryos from the same clutch of taf1uu1941 and taf1uu1931, respectively by an in-house script quantifying the mean heart beats per minute from a ten second movie generated by Vertebrate Automated Screening Technology (VAST) bioImager™ platform. Twenty-four taf1uu1941 were kept until 5 dpf to assess the lethal phenotype. Embryos were sacrificed and genotyped by FLA and/or Sanger sequencing as described above (primer sequences are given in Supplementary Table S2). Data was visualized in boxplots generated by ggplot in R environment. Significance was established using Student’s t-test adjusted with Bonferroni correction. Specific genotype distributions of clutches are available in Supplementary Table S3.
RNA extraction from zebrafish embryos
Zebrafish embryos (3 dpf) from crossing three pairs of taf1uu1941/+ heterozygous adults were sedated, washed in PBS, sorted based on their phenotype (normal or mutant), and collected in 10 μl RNAlater /embryo (Ambion, Thermo Fisher Scientific, Waltham, MA). Mutant (taf1uu1941/uu1941) and sibling (taf1+/+ and taf1uu1941/+) zebrafish embryos were pooled as follows: n = 10 for pair 1 and n = 20 for pairs 2 and 3. Samples were held on ice and then incubated at 4 °C for 24 hours. The embryos were stored at −20 °C until the day of extraction. For RNA extraction, samples were thawed at room temperature and RNAlater was removed by pipetting. Samples were homogenized and lysed in 0.5 ml of TRIzol according to the manufacturer’s protocol (Ambion, Waltham, MA) using a BioVortexer mixer. RNA cleaning was carried out using a Qiagen RNeasy Micro Kit according to the manufacturer’s protocol (Appendix C). RNA quality was assessed using the Agilent 2100 Bioanalyzer and RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA).
RNA library preparation
Libraries were prepared using 190 ng of poly(A)-selected RNA with the TruSeq Stranded mRNA sample preparation kit according to the manufacturer’s protocol (Illumina Inc., San Diego, CA). The quality of the libraries was evaluated using the TapeStation (D1000 ScreenTape, Agilent Technologies), and the adapter-ligated fragments were quantified by qPCR using the Library Quantification Kits from Illumina (KAPA Biosystems, Boston, MA) on a StepOnePlus instrument (Applied Biosystems, Waltham, MA) prior to cluster generation and sequencing.
Transcriptome sequencing and analysis
RNA sequencing was carried out on Illumina’s HiSeq2500 with a PE125 read length (HCS v2.2.58/RTA v1.18.64) according to the manufacturer’s instructions (Illumina, San Diego, CA). Demultiplexing and conversion to FASTQ format was performed using the bcl2fastq2 (v2.19.1.403) software, provided by Illumina. Additional statistics on sequencing quality were compiled with an in-house script from the FASTQ, RTA, and bcl2fastq2 output files.
RNA sequencing data quality was assessed with FastQC (v0.11.5) and the RSeQC script package (v0.1.0). TrimGalore (v0.4.1) was used for trimming adapter contamination, followed by alignment to the reference sequence (GRCz10/danRer10) using Star (v2.5.1). FeatureCounts (v1.5.1) was used to receive transcript counts, and DESeq2 (v1.19.13) was used for paired differential gene expression analysis. An adjusted p-value < 0.01, corrected by the Benjamini-Hochberg procedure, was used to select significantly differentially expressed transcripts. PANTHER overrepresentation test (v13.1)29,30 corrected for multiple testing using Bonferroni correction was used to investigate enrichment of biological functions. GSEA was performed on all differentially expressed genes (adjusted p-value < 0.01) with 1109 zebrafish pathways retrieved from GO2MSIG57 filtered for minimum 15 genes, and maximum 200 genes, per pathway. The GSEA result was visualized with enrichment map using yFiles Organic layout with a Q-value < 0.01 and edge cut-off <0.0375, according to protocol58.
Data Availability
All data is available upon request.
References
Bieniossek, C. et al. The architecture of human general transcription factor TFIID core complex. Nature 493, 699–702, https://doi.org/10.1038/nature11791 (2013).
Goodrich, J. A. & Tjian, R. Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation. Nat Rev Genet 11, 549–558, https://doi.org/10.1038/nrg2847 (2010).
Warfield, L. et al. Transcription of Nearly All Yeast RNA Polymerase II-Transcribed Genes Is Dependent on Transcription Factor TFIID. Mol Cell 68, 118–129 e115, https://doi.org/10.1016/j.molcel.2017.08.014 (2017).
Rooms, L. et al. TBP as a candidate gene for mental retardation in patients with subtelomeric 6q deletions. Eur J Hum Genet 14, 1090–1096, https://doi.org/10.1038/sj.ejhg.5201674 (2006).
Hellman-Aharony, S. et al. Microcephaly thin corpus callosum intellectual disability syndrome caused by mutated TAF2. Pediatr Neurol 49, 411–416 e411, https://doi.org/10.1016/j.pediatrneurol.2013.07.017 (2013).
Alazami, A. M. et al. Accelerating novel candidate gene discovery in neurogenetic disorders via whole-exome sequencing of prescreened multiplex consanguineous families. Cell Rep 10, 148–161, https://doi.org/10.1016/j.celrep.2014.12.015 (2015).
Tawamie, H. et al. Hypomorphic Pathogenic Variants in TAF13 Are Associated with Autosomal-Recessive Intellectual Disability and Microcephaly. Am J Hum Genet 100, 555–561, https://doi.org/10.1016/j.ajhg.2017.01.032 (2017).
Aneichyk, T. et al. Dissecting the Causal Mechanism of X-Linked Dystonia-Parkinsonism by Integrating Genome and Transcriptome Assembly. Cell 172, 897–909 e821, https://doi.org/10.1016/j.cell.2018.02.011 (2018).
Hu, H. et al. X-exome sequencing of 405 unresolved families identifies seven novel intellectual disability genes. Mol Psychiatry 21, 133–148, https://doi.org/10.1038/mp.2014.193 (2016).
O’Rawe, J. A. et al. TAF1 Variants Are Associated with Dysmorphic Features, Intellectual Disability, and Neurological Manifestations. Am J Hum Genet 97, 922–932, https://doi.org/10.1016/j.ajhg.2015.11.005 (2015).
Hurst, S. E. L.-B. et al. A novel variant in TAF1 affects gene expression and is associated with X-linked TAF1 intellectual disability syndrome. Neuronal Signaling 2, https://doi.org/10.1042/NS20180141 (2018).
Makino, S. et al. Reduced neuron-specific expression of the TAF1 gene is associated with X-linked dystonia-parkinsonism. Am J Hum Genet 80, 393–406, https://doi.org/10.1086/512129 (2007).
Bragg, D. C. et al. Disease onset in X-linked dystonia-parkinsonism correlates with expansion of a hexameric repeat within an SVA retrotransposon in TAF1. Proc Natl Acad Sci USA 114, E11020–E11028, https://doi.org/10.1073/pnas.1712526114 (2017).
Herzfeld, T. et al. X-linked dystonia parkinsonism syndrome (XDP, lubag): disease-specific sequence change DSC3 in TAF1/DYT3 affects genes in vesicular transport and dopamine metabolism. Hum Mol Genet 22, 941–951, https://doi.org/10.1093/hmg/dds499 (2013).
Palmer, E. H. S. et al. De Novo Variants Disrupting the HX Repeat Motif of ATN1 Cause a Recognizable Non-Progressive Neurocognitive Syndrome. Am J Hum Genet, https://doi.org/10.1016/j.ajhg.2019.01.013 (2019).
Ng, S. B. et al. Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat Genet 42, 790–793, https://doi.org/10.1038/ng.646 (2010).
Wang, Y. et al. Integrative analysis revealed the molecular mechanism underlying RBM10-mediated splicing regulation. EMBO Mol Med 5, 1431–1442, https://doi.org/10.1002/emmm.201302663 (2013).
Jambaldorj, J., Makino, S., Munkhbat, B. & Tamiya, G. Sustained expression of a neuron-specific isoform of the Taf1 gene in development stages and aging in mice. Biochem Biophys Res Commun 425, 273–277, https://doi.org/10.1016/j.bbrc.2012.07.081 (2012).
Schwarz, J. M., Cooper, D. N., Schuelke, M. & Seelow, D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 11, 361–362, https://doi.org/10.1038/nmeth.2890 (2014).
Kumar, P., Henikoff, S. & Ng, P. C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4, 1073–1081, https://doi.org/10.1038/nprot.2009.86 (2009).
Pollard, K. S., Hubisz, M. J., Rosenbloom, K. R. & Siepel, A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res 20, 110–121, https://doi.org/10.1101/gr.097857.109 (2010).
Cooper, G. M. et al. Distribution and intensity of constraint in mammalian genomic sequence. Genome Res 15, 901–913, https://doi.org/10.1101/gr.3577405 (2005).
Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291, https://doi.org/10.1038/nature19057 (2016).
Ameur, A. et al. SweGen: a whole-genome data resource of genetic variability in a cross-section of the Swedish population. Eur J Hum Genet 25, 1253–1260, https://doi.org/10.1038/ejhg.2017.130 (2017).
Allen, R. C., Zoghbi, H. Y., Moseley, A. B., Rosenblatt, H. M. & Belmont, J. W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 51, 1229–1239 (1992).
Machado, F. B. et al. 5meCpG epigenetic marks neighboring a primate-conserved core promoter short tandem repeat indicate X-chromosome inactivation. PLoS One 9, e103714, https://doi.org/10.1371/journal.pone.0103714 (2014).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev Dyn 203, 253–310, https://doi.org/10.1002/aja.1002030302 (1995).
Robinson, J. T. et al. Integrative genomics viewer. Nat Biotechnol 29, 24–26, https://doi.org/10.1038/nbt.1754 (2011).
Mi, H., Muruganujan, A. & Thomas, P. D. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res 41, D377–386, https://doi.org/10.1093/nar/gks1118 (2013).
Thomas, P. D. et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 13, 2129–2141, https://doi.org/10.1101/gr.772403 (2003).
Neri, G., Schwartz, C. E., Lubs, H. A. & Stevenson, R. E. X-linked intellectual disability update 2017. Am J Med Genet A 176, 1375–1388, https://doi.org/10.1002/ajmg.a.38710 (2018).
Niranjan, T. S. et al. Affected kindred analysis of human X chromosome exomes to identify novel X-linked intellectual disability genes. PLoS One 10, e0116454, https://doi.org/10.1371/journal.pone.0116454 (2015).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17, 405–424, https://doi.org/10.1038/gim.2015.30 (2015).
Wang, H., Curran, E. C., Hinds, T. R., Wang, E. H. & Zheng, N. Crystal structure of a TAF1-TAF7 complex in human transcription factor IID reveals a promoter binding module. Cell Res 24, 1433–1444, https://doi.org/10.1038/cr.2014.148 (2014).
Firth, H. V. et al. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am J Hum Genet 84, 524–533, https://doi.org/10.1016/j.ajhg.2009.03.010 (2009).
Li, H. H., Li, A. G., Sheppard, H. M. & Liu, X. Phosphorylation on Thr-55 by TAF1 mediates degradation of p53: a role for TAF1 in cell G1 progression. Mol Cell 13, 867–878 (2004).
Severson, P. L., Vrba, L., Stampfer, M. R. & Futscher, B. W. Exome-wide mutation profile in benzo[a]pyrene-derived post-stasis and immortal human mammary epithelial cells. Mutat Res Genet Toxicol Environ Mutagen 775-776, 48–54, https://doi.org/10.1016/j.mrgentox.2014.10.011 (2014).
Oh, H. R., An, C. H., Yoo, N. J. & Lee, S. H. Frameshift Mutations in the Mononucleotide Repeats of TAF1 and TAF1L Genes in Gastric and Colorectal Cancers with Regional Heterogeneity. Pathol Oncol Res 23, 125–130, https://doi.org/10.1007/s12253-016-0107-0 (2017).
Le Gallo, M. et al. Somatic mutation profiles of clear cell endometrial tumors revealed by whole exome and targeted gene sequencing. Cancer 123, 3261–3268, https://doi.org/10.1002/cncr.30745 (2017).
Forbes, S. A. et al. COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res 45, D777–D783, https://doi.org/10.1093/nar/gkw1121 (2017).
Borrie, S. C., Brems, H., Legius, E. & Bagni, C. Cognitive Dysfunctions in Intellectual Disabilities: The Contributions of the Ras-MAPK and PI3K-AKT-mTOR Pathways. Annu Rev Genomics Hum Genet 18, 115–142, https://doi.org/10.1146/annurev-genom-091416-035332 (2017).
Jansen, S. et al. De Novo Truncating Mutations in the Last and Penultimate Exons of PPM1D Cause an Intellectual Disability Syndrome. Am J Hum Genet 100, 650–658, https://doi.org/10.1016/j.ajhg.2017.02.005 (2017).
Kumar, R. et al. Increased STAG2 dosage defines a novel cohesinopathy with intellectual disability and behavioral problems. Hum Mol Genet 24, 7171–7181, https://doi.org/10.1093/hmg/ddv414 (2015).
Fieremans, N. et al. Identification of Intellectual Disability Genes in Female Patients with a Skewed X-Inactivation Pattern. Hum Mutat 37, 804–811, https://doi.org/10.1002/humu.23012 (2016).
Plenge, R. M., Stevenson, R. A., Lubs, H. A., Schwartz, C. E. & Willard, H. F. Skewed X-chromosome inactivation is a common feature of X-linked mental retardation disorders. Am J Hum Genet 71, 168–173, https://doi.org/10.1086/341123 (2002).
Iwase, S. & Martin, D. M. Chromatin in nervous system development and disease. Mol Cell Neurosci 87, 1–3, https://doi.org/10.1016/j.mcn.2017.12.006 (2018).
Zaghlool, A. et al. A Role for the Chromatin-Remodeling Factor BAZ1A in Neurodevelopment. Hum Mutat 37, 964–975, https://doi.org/10.1002/humu.23034 (2016).
Zamurrad, S., Hatch, H. A. M., Drelon, C., Belalcazar, H. M. & Secombe, J. A Drosophila Model of Intellectual Disability Caused by Mutations in the Histone Demethylase KDM5. Cell Rep 22, 2359–2369, https://doi.org/10.1016/j.celrep.2018.02.018 (2018).
Martens, M. B. et al. Euchromatin histone methyltransferase 1 regulates cortical neuronal network development. Sci Rep 6, 35756, https://doi.org/10.1038/srep35756 (2016).
Milenkovic, I. et al. GABAA receptor subunit deregulation in the hippocampus of human foetuses with Down syndrome. Brain Struct Funct 223, 1501–1518, https://doi.org/10.1007/s00429-017-1563-3 (2018).
Labun, K., Montague, T. G., Gagnon, J. A., Thyme, S. B. & Valen, E. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res 44, W272–276, https://doi.org/10.1093/nar/gkw398 (2016).
Varshney, G. K. et al. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res 25, 1030–1042, https://doi.org/10.1101/gr.186379.114 (2015).
Westerfield, M. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). (Univ. of Oregon Press, 2000).
Carrington, B., Varshney, G. K., Burgess, S. M. & Sood, R. CRISPR-STAT: an easy and reliable PCR-based method to evaluate target-specific sgRNA activity. Nucleic Acids Res 43, e157, https://doi.org/10.1093/nar/gkv802 (2015).
Sood, R. et al. Efficient methods for targeted mutagenesis in zebrafish using zinc-finger nucleases: data from targeting of nine genes using CompoZr or CoDA ZFNs. PLoS One 8, e57239, https://doi.org/10.1371/journal.pone.0057239 (2013).
Chen, H. L., Yuh, C. H. & Wu, K. K. Nestin is essential for zebrafish brain and eye development through control of progenitor cell apoptosis. PLoS One 5, e9318, https://doi.org/10.1371/journal.pone.0009318 (2010).
Powell, J. A. GO2MSIG, an automated GO based multi-species gene set generator for gene set enrichment analysis. BMC Bioinformatics 15, 146, https://doi.org/10.1186/1471-2105-15-146 (2014).
Merico, D., Isserlin, R., Stueker, O., Emili, A. & Bader, G. D. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One 5, e13984, https://doi.org/10.1371/journal.pone.0013984 (2010).
Jacobson, R. H., Ladurner, A. G., King, D. S. & Tjian, R. Structure and function of a human TAFII250 double bromodomain module. Science 288, 1422–1425 (2000).
Acknowledgements
We would like to express our gratitude towards the family for their long-term participation in this study. We want to thank Professor Gary Bader and his team for valuable input on pathway analysis. Transcriptome sequencing and bioinformatics analysis were performed in collaboration with the SNP&SEQ Technology Platform at Science for Life Laboratory, Uppsala, Sweden, and the National Bioinformatics Infrastructure Sweden (NBIS). Zebrafish experiments were performed in collaboration with the Genome Engineering Zebrafish (GEZ) Facility at Science for Life Laboratory, Uppsala, Sweden. This work was supported by grants from Uppsala University, Faculty of Medicine, for psychiatric and neurological research, the Swedish Society of Medicine to S.G., grants from The Swedish Society of Medicine to M.W., grants from Uppsala University Hospital to M.-L.B. and the EU FP7 project GENCODYS [grant number 241995] to V.M.K., M.W. was supported by grants from the Swedish Society for Medical Research (SSMF). S.G. and J.J. was supported by grants from the Sävstaholm Foundation.
Author information
Authors and Affiliations
Contributions
S.G., V.M.K., J.L. and M.-L.B. designed the experiments and analyzed the data. S.G., M.W., B.F.-G., A.-M.M., S.E., J.J. and A.A. performed experiments. H.G. and G.A. performed clinical investigations. V.M.K., J.L. M.-L.B. supervised research. S.G. prepared the figures and the manuscript with input and approval from all authors.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gudmundsson, S., Wilbe, M., Filipek-Górniok, B. et al. TAF1, associated with intellectual disability in humans, is essential for embryogenesis and regulates neurodevelopmental processes in zebrafish. Sci Rep 9, 10730 (2019). https://doi.org/10.1038/s41598-019-46632-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46632-8
This article is cited by
-
Hierarchical TAF1-dependent co-translational assembly of the basal transcription factor TFIID
Nature Structural & Molecular Biology (2023)
-
A novel quantitative targeted analysis of X-chromosome inactivation (XCI) using nanopore sequencing
Scientific Reports (2023)
-
Network Biology Approaches to Uncover Therapeutic Targets Associated with Molecular Signaling Pathways from circRNA in Postoperative Cognitive Dysfunction Pathogenesis
Journal of Molecular Neuroscience (2022)
-
Understanding the Landscape of X-linked Variants Causing Intellectual Disability in Females Through Extreme X Chromosome Inactivation Skewing
Molecular Neurobiology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.