Abstract
The robot hand illusion (RoHI) is the perception of self-ownership and self-agency of a virtual (robot) hand that moves consistently with one’s own. The phenomenon shows that self-attribution can be established via temporal integration of visual and movement information. Our previous study showed that participants felt significantly greater RoHI (sense of self-ownership and sense of self-agency) when visuomotor temporal discrepancies were less than 200 ms. A weaker RoHI effect (sense of self-agency only) was observed when temporal discrepancies were between 300 and 500 ms. Here, we used functional near-infrared spectroscopy (fNIRS) to investigate brain activity associated with the RoHI under different visual feedback delays (100 ms, 400 ms, 700 ms). We found that the angular and supramarginal gyri exhibited significant activation in the 100-ms feedback condition. ANOVA indicated a significant difference between the 100-ms condition and the other conditions (p < 0.01). These results demonstrate that activity in the posterior parietal cortex was modulated by the delay between the motor command and the visual feedback of the virtual hand movements. Thus, we propose that the inferior parietal cortex is essential for integrating motor and visual information to distinguish one’s own body from others.
Similar content being viewed by others
Introduction
Most people have sense of strong control over their voluntary actions and can also distinguish between their own body and those of others. This implies that self-attribution comprises two sensory components: sense of agency (SoA) and sense of ownership (SoO)1,2. The SoA is the sense of authorship for a given action, which is a subjective feeling of control over one’s actions and their outcomes. The SoO is the conscious awareness that one’s body belongs to oneself.
In the rubber hand illusion (RHI), observers feel the SoO toward a fake hand (rubber hand) when their hand and the rubber hand are stroked simultaneously, while the observer does not feel that the rubber hand is their own hand when they receive visual and tactile information from the rubber hand asynchronously3,4,5,6. In addition, the result showed a significant difference in proprioceptive drift toward the rubber hand in synchronous condition in congruent posture. The proprioceptive drift is defined as the drift of the perceived location of one’s own hand before and after the stimulation. Usually, the drift occurs toward the rubber hand when the RHI takes place. The participants felt more strongly that the rubber hand is their own when the stimuli were applied on their hand and the rubber hand synchronously compared to the asynchronous stimulation5. Studies have addressed the time window for this self-attribution in RHI, showing that recognition was attenuated when visual information was delayed longer than 200–300 ms7,8.
While the integration of visual and tactile information, and thus the SoO, have been investigated in RHI studies, RHI have been expanded to investigate not only the SoO but also the SoA using a ‘moving rubber hand’ paradigm9,10. Kalckert & Ehrsson modified the RHI experimental paradigm to address the active movement, passive movement and visuotactile stimulation on the rubber hand. The participant’s index finger was connected to the index finger of a fake hand. The participant was instructed to lifts their index finger, so that they can feel the SoA of that finger movement of the fake hand. In the synchronous active condition, the participant felt both the SoO and SoA of the fake hand stronger than in other conditions. In addition, the participants also felt greater SoA when they moved their own hand (active condition) compared to when the experimenter moved the participant’s hand (passive condition). In addition, the virtual hand illusion using the same paradigm with the RHI also showed that the participants felt both the SoO and the SoA toward the virtual hand that is generated by computer graphics (CG) as their own hand in the synchronous condition compared to the asynchronous condition11,12.
In this study, we focused on the integration of visual and motor information on the fake hand, which is called as the robot hand illusion (RoHI)13,14,15. The RoHI is an illusion in which a virtual (robot) hand is perceived as one’s own hand when it moves consistently with one’s hand motions. Our previous study showed that attenuation of self-attribution toward a virtual hand occurred when visual feedback was delayed longer than 200 ms13. The present study aimed to further investigate the neural mechanism of the SoO and the SoA when experiencing the RoHI.
The neural mechanism that underlies self-attribution is still unclear. Several neuroimaging studies have used the RHI to show that the premotor cortex is related with the SoO16,17,18,19. Ehrsson et al. also suggested that activity in the intraparietal cortex preceding the onset of the RHI, which likely plays a role in the continued multisensory integration of stimuli with respect to the hand16. Furthermore, studies have found that the intraparietal region is related to the integration of visual and tactile information that is associated with the RHI17,18,20,21,22. The neural correlates of SoA have been also suggested to be related to activity in the inferior parietal lobule (IPL)23,24,25,26.
The above-mentioned studies have investigated the multisensory integration for the self-body senses, namely the SoO and the SoA, and the neural networks correlated with the self-attribution. The present study systematically investigated self-attribution in the RoHI under delayed visual feedback. We focused on the investigation of the temporal binding between visual and motor information and conducted a neuroimaging experiment by using functional near-infrared spectroscopy (fNIRS). We predicted that the temporal window needed for visual and motor integration in the RoHI is less than 200–300 ms like in the RHI, and that the IPL is essential to establish both the SoO and the SoA.
Results
Questionnaires
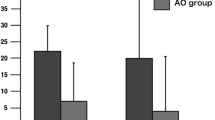
The average scores for questionnaire items related to the SoO (items 1–4) and those related to the SoA (items 9–12) are shown in Fig. 1 for all participants. Participant ratings were higher in both measures for the 100-ms delayed visual feedback condition than for the other conditions. The Kolmogorov-Smirnov test indicates that the data follow the normal distribution (SoO, D(64) = 0.144, p > 0.05; SoA, D(64) = 0.120, p > 0.05; ownership sense control, D(64) = 0.066, p > 0.05; agency sense control, D(64) = 0.91, p > 0.05).
The subjective ratings are shown in more detail in Figs 2 and 3. We performed a 4 × 2 two-way ANOVA with delay (100 ms, 400 ms, 700 ms, and control 1; see Methods) and sense (ownership and agency) as factors. The results showed a significant main effect of delay (F(3, 120) = 17.248, p < 0.01, η2 = 0.657), but no significant main effect of sense (F(1,120) = 1.860, p > 0.1, η2 = 0.125) or any interaction between the two (F(3,120) = 0.341, p > 0.1, η2 = 0.092). For both the SoO and the SoA, subsequent analyses (Tukey’s honestly significant difference, HSD) showed that the questionnaire scores for the 100-ms condition were significantly greater than those for the 700-ms and control conditions (p < 0.01), and those for the 400-ms condition were significantly greater than those for the control-1 condition (p < 0.01). Additionally, the SoO was significantly greater in the 100-ms condition than in the 400-ms condition (p < 0.05), and the SoA was greater in the 400-ms condition than in the 700-ms condition (p < 0.05). The post-hoc power analysis showed that we have enough power (96.1%) for this analysis (16 samples for each condition).
A one-way (delay) ANOVA was applied separately to the ownership-sense control and the agency-sense control items. For both items, the results showed no significant main effect of delay (ownership sense control, F(3, 60) = 0.525, p > 0.1, η2 = 0.162; agency sense control, F(3, 60) = 0.432, p > 0.1, η2 = 0.147; Fig. 1).
fNIRS results
Using correlated component analysis (CCA, see Methods), we analysed the differential effects among correlated components to determine which experimental conditions affect neural activity. Each component of the correlated activity has different spatial distributions on the scalp. Figure 4 shows the top three components (first component, C1; second component, C2; third component, C3). The inter-subject correlation (ISC, see Methods) was calculated for each component and condition, which then submitted to one-way ANOVA (100–700 ms delay, control 1 and 2) to determine the conditional effect.
The first CCA component was pronounced over the angular gyrus and the supramarginal gyrus in both hemispheres, the visual association cortex in the left hemisphere, and the somatosensory association cortex in the right hemisphere. ANOVA for this component showed a significant main effect of delay (F(4,75) = 3.466, p < 0.05, η2 = 0.430). Subsequent analyses (Tukey HSD) revealed that ISC for the 100-ms condition was significantly greater than those for the other conditions (p < 0.01). Similarly, ISC for the 700-ms condition was significantly greater than those for the control conditions (p < 0.01).
The second component was strong over the somatosensory cortex and the supramarginal gyrus in both hemispheres and the superior middle temporal gyrus in the right hemisphere. The result showed no significant main effect of delay (F(4,75) = 1.086, p > 0.1, η2 = 0.240).
The third component was strong over the visual association cortex in both hemispheres, the somatosensory association cortex and the angular gyrus in the left hemisphere. ANOVA showed a significant main effect of delay (F(4,75) = 2.519, p < 0.05, η2 = 0.367). Subsequent analyses revealed that ISC for the 700-ms condition was significantly weaker than those for the other conditions (p < 0.01).
Discussion
The questionnaire results in this experiment showed that the participants felt the sense of ownership only when visual feedback occurred within 100 ms of their movement, and the sense of agency when it occurred within 400 ms (see Figs 2 and 3). However, the participants rated the 700 ms condition with no significant difference compared to 0 (neutral) for both the SoO and the SoA questionnaires. In addition, the angular gyrus and the supramarginal gyrus showed significant activation in the 100-ms delayed visual feedback condition, which was significantly different from the other conditions (p < 0.01). This result was consistent with our previous findings that greater SoO and SoA occurred when the visual feedback was delivered within less than 200 ms delay and that weak SoA occurred with delays between 300 ms and 500 ms13. It was also shown that there was no significant SoO or SoA when visual feedback was delayed more than 500 ms. Several studies have shown that the time window for visual and tactile integration is 200–300 ms in the case of RHI7,8. Further, other studies of auditory and visual integration have also indicated that the time window for integrating these senses was less than 200 ms27,28,29. However, according to Costantini et al., the temporal limit on the RHI depends on individual’s perceptual temporal resolution30. The participants need to judge the simultaneity between visual and the tactile stimuli. In their study, the results showed that the average of the temporal binding window was 211 ± 59.9 ms, which are less than 300 ms. Then, the participant underwent the RHI stimulation and felt a significantly greater SoO in the synchronous condition compared to the asynchronous condition where the individual size of temporal binding window was used as the visual feedback delay in the asynchronous condition. This study is consistent with our finding where multisensory integration for self-attribution in RoHI occurs only when the two sensations occur within 300 ms of each other.
In this experiment, we focused on the RoHI to investigate brain activity related to the SoO and SoA. The feeling of body ownership has been suggested to be related to activity in the posterior parietal cortex and the intraparietal sulcus (PPC/IPS) as well as in the premotor cortex31,32,33. According to Makin et al. the PPC seems to integrate multisensory information with respect to the rubber hand31. Moreover, Ehrsson et al. identified activity within the anterior part of the IPS preceding the onset of the RHI, which likely plays a role on the continued multisensory integration with respect to the hand32. Linear relationship between proprioceptive drift and brain activity in the supramarginal gyrus is also reported18. This finding indicates that the SoO has been associated with activity in the supramarginal gyrus. These findings are consistent with our results, in which the first CCA component showed that the angular gyrus and the supramarginal gyrus activated in the 100 ms condition compared to the other conditions.
Additionally, the SoA has also been associated with activity in the inferior parietal lobule (IPL)25,34,35. According to Renes et al., the IPL showed more activation when the outcome matched with the participant’s goal and the participant felt greater SoA than when not36. This finding is consistent with our results that the first and third CCA components, which both include the IPL regions, showed more activation in the 100 ms delay condition compared to the other, especially to the 700 ms, delay conditions. The participants also rated the SoA score lower in the 700 ms condition than the other conditions. These indicate that the activation in the IPL is associated with a feeling of agency toward a robot hand. To sum, our result showed that the IPL was activated when participants felt both SoO and SoA in the 100-ms delayed visual feedback condition. We postulate that the IPL is an essential area for multisensory integration regarding the self-body.
Methods
Participants
Sixteen healthy students (all male; aged 22.5 ± 1.0 years) who were naive to the purpose of the study were recruited for the experiment. Thirteen participants were right-handed and three were left-handed and all had normal or corrected-to-normal vision. All participants provided written informed consent. The experiment was approved by the ethics committee of the School of Science and Technology, Meiji University (No. 08562), and was conducted according to the principles and guidelines of the Declaration of Helsinki.
Procedure
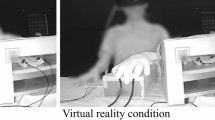
The participants were asked to sit at a table with a mirror and put their right palm facing down. The participants were not able to see their own right hand directly (see Fig. 5). Using the Cyberglove system (Cyberglove, CyberGlove Systems LLC, San Jose, California), the participants were instructed to move their hand to record their hand movement. An image of a virtual hand, which was generated using recorded data, was presented on a liquid-crystal display monitor (LMD-232W, SONY, Tokyo, Japan) through the reflection of the mirror. Visual feedback delay was constructed using a hardware device (EDS3305, Eletex, Osaka, Japan). Three delay conditions (100, 400 and 700 ms) and two control conditions (control 1, the subject observed the video of the virtual hand moved without moving their own hand; control 2, the subject moved their hand without observing the virtual hand) for hand-opening and hand-closing movements was tested for each participant. Each trial lasted 30 s (5 s for pre-rest, 10 s for task, and 15 s for post-rest), which was repeated 6 times. We recorded fNIRS data for each delay conditions. The order of the delay conditions was pseudo-random and counterbalanced across participants.
After completing each condition, participants completed a 16-item questionnaire identical to that used in previous studies except in control 2 condition because participants do not observe any image of hand9,13, in either the original English or in Japanese. They reported their subjective experience on a 7-point Likert-like scale ranging from −3 (totally disagree) to +3 (totally agree), with 0 indicating neither agreement nor disagreement (uncertain). Four statements referred to the feeling of ownership (e.g., “I felt as if I was looking at my own hand”), and four statements described sensations related to agency (e.g., “I felt as if I was causing the movement I saw”). The remaining eight statements were control statements which is describe the fake question of the senses; four describing ownership and the other four describing agency (e.g., “I felt as if I had more than one right hand” and “It seemed as if the hand image had a will of its own”).
fNIRS data acquisition
The fNIRS data were recorded using a multichannel fNIRS unit operating at 780-nm, 805-nm, and 830-nm wavelengths (OMM-3000, Shimadzu, Japan) at a sampling frequency of 10 Hz. Oxy-haemoglobin (oxyHb), deoxy-haemoglobin (deoxyHb), and total haemoglobin (totalHb) concentrations were recorded in the temporal, parietal, and occipital lobes. The optode locations were recorded using a 3D magnetic space digitizer (Fastrak, Polhemus, USA) to estimate the anatomical brain region beneath the fNIRS channels. To determine the correspondence between the measured position data and the fNIRS channels, we used the probabilistic spatial registration method37 to generate a probabilistic mapping between each fNIRS channel and its corresponding anatomical brain region. This was then used for interpreting fNIRS-activation data.
fNIRS data analysis
The change in oxyHb is considered the main parameter that changes with regional cerebral blood flow. A 2-Hz low-pass filter and a 0.2-Hz high-pass filter were applied to the fNIRS data. CCA was applied to the fNIRS signal for each condition to extract the common signal that was contained in all conditions38,39,40 and to analyze up to the third component. In CCA, we find weight vector for the data to maximize the correlation coefficient between experiment conditions. The signals commonly contained in different conditions for each channel was extracted through CCA. Additionally, the brain region involved in the component was determined using a forward model, A = RW(WTRW) − 141,42, using weight (W) that produced from CCA and data covariance matrix (R). Each component has been normalized so that the maximum becomes 1. In order to compare the degree to which cortical activity was correlated among the conditions, we took the sum of the averaged correlations for every pair of participants for each component (known as ISC)40. It extracts the common brain activity among the subjects performing the same task. We used general linear model (GLM) which is an analysis method for statistically examining how much the observed signal (of one participant) can be fitted with a model called design matrix (another participant’s signal). Comparisons across conditions were performed using analysis of variance (ANOVA).
References
Gallagher, S. Philosophical conceptions of the self: Implications for cognitive science. Trends Cogn. Sci. 4(1), 14–21 (2000).
Tsakiris, M., Schutz-Bosbach, S. & Gallagher, S. On agency and body-ownership: Phenomenological and neurocognitive reflections. Conscious Cogn. 16(3), 645–660 (2007).
Botvinick, M. & Cohen, J. Rubber hands ‘feel’ touch that eyes see. Nature. 391, 756 (1998).
Armel, K. C. & Ramachandran, V. S. Projecting sensations to external objects: Evidence from skin conduc- tance response. Proc. Biol Sci. 270(1533), 1499–1506 (2003).
Tsakiris., M. & Haggard, P. The rubber hand illusion revisited: Visuotactile integration and self-attribution. J. Exp. Psychol. Hum. Percept. Perform. 31(1), 80–91 (2005).
Longo, M. R., Schuur, F., Kammers, M. P., Tsakiris, M. & Haggard, P. What is embodiment? A psychometric approach. Cognition. 107(3), 978–998 (2008).
Shimada, S., Fukuda, K. & Hiraki, K. Rubber hand illusion under delayed visual feedback. PLoS ONE. 4(7), e6185, https://doi.org/10.1371/journal.pone.0006185 (2009).
Shimada, S., Suzuki, T., Yoda, N. & Hayashi, T. Relationship between sensitivity to visuotactile temporal discrepancy and the rubber hand illusion. Neuroscience Research. 85, 33–38 (2014).
Kalckert, A. & Ehrsson, H. H. Moving a rubber hand that feels like your own: A dissociation of ownership and agency. Frontiers in Human Neuroscience. 6, 40, https://doi.org/10.3389/fnhum.2012.00040 (2012).
Kalckert, A. & Ehrsson, H. H. The moving rubber hand illusion revisited: comparing movements and visuotactile stimulation to induce illusory ownership. Conscious Cogn. 26, 117–32 (2014).
Slater, M., Perez-Marcos, D., Ehrsson, H. H. & Sanchez-Vives, M. V. Towards a digital body: the virtual arm illusion. Frontiers in Human Neuroscience. 2, 6, https://doi.org/10.3389/neuro.09.006.2008 (2008).
Ma, K., Lippelt, D. P. & Hommel, B. Creating Virtual-hand and Virtual-face Illusions to Investigate Self-representation. J. Vis. Exp. 121, e54784, https://doi.org/10.3791/54784 (2017).
Ismail, M. A. F. & Shimada, S. Robot Hand Illusion under Delayed Visual Feedback: Relationship between the Senses of Ownership and Agency. PLoS ONE. 11(7), e0159619, https://doi.org/10.1371/journal.pone.0159619 (2016).
Romano, D., Caffa, E., Hernandez-Arieta, A., Brugger, P. & Maravita, A. The robot hand illusion: inducing proprioceptive drift through visuo-motor congruency. Neuropsychologia. 70, 414–420 (2015).
Caspar, E. A. et al. New frontiers in the rubber hand experiment: when a robotic hand becomes one’s own. Behav. Res. Methods. 47(3), 744–755 (2015).
Ehrsson, H. H., Spence, C. & Passingham, R. E. That’s my hand! activity in premotor cortex reflects feeling of ownership of a limb. Science. 305, 875–877 (2004).
Petkova, V. I. et al. From part- to whole-body ownership in the multisensory brain. Curr. Biol. 21, 1118–1122 (2011).
Brozzoli, C., Gentile, G. & Ehrsson, H. H. That’s near my hand! Parietal and premotor coding of hand-centered space contributes to localization and self- attribution of the hand. J. Neurosci. 32, 14573–14582 (2012).
Bekrater-Bodmann, R. et al. The importance of synchrony and temporal order of visual and tactile input for illusory limb ownership experiences - an FMRI study applying virtual reality. PLoS ONE. 9(1), e87013, https://doi.org/10.1371/journal.pone.0087013 (2014).
Guterstam, A. et al. Decoding illusory self-location from activity in the human hippocampus. Front. Hum. Neurosci. 9, 412, https://doi.org/10.3389/fnhum.2015.00412 (2015).
Guterstam, A., Gentile, G. & Ehrsson, H. H. The invisible hand illusion: multisensory integration leads to the embodiment of a discrete volume of empty space. J. Cogn. Neurosci. 25(7), 1078–1099 (2013).
Limanowski, J. & Blankenburg, F. Network activity underlying the illusory self-attribution of a dummy arm. Hum. Brain Mapp. 36(6), 2284–2304 (2015).
Blakemore, S. J., Rees, G. & Frith, C. D. How do we predict the consequences of our actions? A functional imaging study. Neuropsychologia. 36(6), 521–529 (1998).
Chaminade, T. & Decety, J. Leader or follower? Involvement of the inferior parietal lobule in agency. Neuroreport. 13(15), 1975–1978 (2002).
Farrer, C. & Frith, C. D. Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency. Neuroimage. 15(3), 596–603 (2002).
Farrer, C. et al. Modulating the experience of agency: a positron emission tomography study. Neuroimage. 18(2), 324–33 (2003).
van Wassenhove, V., Grant, K. W. & Poeppel, D. Temporal window of integration in auditory-visual speech perception. Neuropsychologia. 45(3), 598–607 (2007).
Stevenson, R. A. & Wallace, M. T. Multisensory temporal integration: task and stimulus dependencies. Exp Brain Res. 227(2), 249–261 (2013).
Wallace, M. T. & Stevenson, R. A. The construct of the multisensory temporal binding window and its dysregulation in developmental disabilities. Neuropsychologia. 64, 105–123 (2014).
Costantini, M. et al. Temporal limits on rubber hand illusion reflect individuals’ temporal resolution in multisensory perception. Cognition. 157, 39–48 (2016).
Makin, T. R., Holmes, N. P. & Ehrsson, H. H. On the other hand: Dummy hands and peripersonal space. Behavioral Brain Research. 191(1), 1–10 (2008).
Ehrsson, H. H. et al. Upper limb amputees can be induced to experience a rubber hand as their own. Brain. 131, 3443–3452 (2008).
Shimada, S., Hiraki, K. & Oda, I. The parietal role in the sense of self-ownership with temporal discrepancy between visual and proprioceptive feedbacks. Neuroimage. 24(4), 1225–1232 (2005).
Farrer, C. et al. The angular gyrus computes action awareness representations. Cerebral Cortex. 18, 254–261 (2008).
Schnell, K. et al. An fMRI approach to particularize the frontoparietal network for visuomotor action monitoring: Detection of incongruence between test subjects’ actions and resulting perceptions. Neuroimage. 34(1), 332–341 (2007).
Renes, R. A., van Haren, N. E., Aarts, H. & Vink, M. An exploratory fMRI study into inferences of self-agency. Social cognitive and affective neuroscience. 10(5), 708–712 (2015).
Singh, A. K., Okamoto, M., Dan, H., Jurcak, V. & Dan, I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage. 27(4), 842–851 (2005).
Dmochowski, J. P., Sajda, P., Dias, J. & Parra, L. C. Correlated components of ongoing EEG point to emotionally laden attention - a possible marker of engagement? Front Hum. Neurosci. 6, 112, https://doi.org/10.3389/fnhum.2012.00112 (2012).
Dmochowski, J. P., Ki, J. J., DeGuzman, P., Sajda, P. & Parra, L. C. Extracting multidimensional stimulus-response correlations using hybrid encoding-decoding of neural activity. Neuroimage. 180(Pt A), 134–146 (2018).
Ki, J. J., Kelly, S. P. & Parra, L. C. Attention Strongly Modulates Reliability of Neural Responses to Naturalistic Narrative Stimuli. J. Neurosci. 36(10), 3092–3101 (2016).
Parra, L. C., Spence, C. D., Gerson, A. D. & Sajda, P. Recipes for the linear analysis of EEG. NeuroImage 28(2), 326–341 (2005).
Haufe, S. et al. On the interpretation of weight vectors of linear models in multivariate neuroimaging. Neuroimage. 87, 96–110 (2014).
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (16H02839 and 17H05915) from the Japan Society for the Promotion of Science (https://www.jsps.go.jp/english/e-grants/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
I.M.A.F. and S.S. designed the experiment, I.M.A.F. conducted the experiment, I.M.A.F. analyzed the data, and I.M.A.F. and S.S. wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ismail, M.A.F.B., Shimada, S. Activity of the inferior parietal cortex is modulated by visual feedback delay in the robot hand illusion. Sci Rep 9, 10030 (2019). https://doi.org/10.1038/s41598-019-46527-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46527-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.