Abstract
One of the major consequences of the lack of a functional VHL protein in von Hippel-Lindau disease, a rare cancer, is the constitutive activation of the HIF pathway. This activation ends up in the generation of Central Nervous System (CNS) Hemangioblastomas among other tumours along the lifespan of the patient. Nowadays, only surgery has been proven efficient as therapy since the systemic attempts have failed. Propranolol, a non-specific β1-and β2-adrenergic receptor antagonist, was recently designated as the first therapeutic (orphan) drug for VHL disease. Nevertheless, its β1 affinity provokes the decrease in blood pressure, being not recommended for low or regular blood pressure VHL patients. In order to overcome the β1-drawback, the properties of a high specific β2-adrenergic receptor blocker named ICI-118,551 have been studied. ICI-118,551 was able to decrease Hemangioblastomas cell viability in a specific manner, by triggering apoptosis. Moreover, ICI-118,551 also impaired the nuclear internalization of HIF-1α in Hemangioblastomas and hypoxic primary endothelial cells, reducing significantly the activation of HIF-target genes and halting the tumour-related angiogenic processes. In this work, we demonstrate the therapeutical properties of ICI-118,551 in VHL-derived CNS-Hemangioblastoma primary cultures, becoming a promising drug for VHL disease and other HIF-related diseases.
Similar content being viewed by others
Introduction
Von Hippel-Lindau (VHL) disease is an autosomal dominant inherited genetic disorder with an incidence of 1 per 36,000 individuals in the general population and is considered as a rare disease or rare cancer1,2.
Patients with VHL disease harbour a single mutation allele in the tumour suppressor gene VHL (3p25–p26). VHL protein (pVHL) controls the cytoplasmic levels of the Hypoxia Inducible Factor (HIF) complex. In normoxic conditions, pVHL binds to the previously hydroxylated prolyl residues of HIF-1α and HIF-2α, being ubiquitinated and targeted to the proteasome for rapid degradation. When patients suffer a spontaneous inactivation or loss of the second wild-type VHL allele (loss of heterozygosity), either there is no expression of the pVHL or the mutated form is not functional3,4.
Therefore, in the absence of functional pVHL, HIF-1α and HIF-2α subunits accumulate within the cytoplasm and translocate to the nucleus, triggering the hypoxia program by targeting hypoxia responsive genes, which are normally silenced in normoxia5. HIF-1α and HIF-2α are involved in cell proliferation, angiogenesis, extracellular matrix degradation, vascular tone, and erythropoiesis, among other processes. Hence, cells from VHL tumours have a constitutively active HIF program (a pseudo-hypoxic state) due to the absence of functional pVHL6,7.
As a consequence of this pseudo-hypoxic state, the clinical manifestations of the disease include multiple benign and malignant tumours that appear throughout the lifespan of the patient: retinal hemangioblastoma, Central Nervous System (CNS) hemangioblastoma, clear cell renal cell carcinoma (ccRCC), pheochromocytoma, pancreatic islet tumour, endolymphatic sac tumours, and cysts in testes and broad ligament8,9,10,11.
Thus far, therapeutic options for VHL patients are only derived from surgery and, unfortunately, the preoperative neurological deficits do not resolve after surgery12. In addition, the systemic therapies, mainly antiangiogenic, used for metastatic cancers had shown limited response in VHL tumours13,14. Therefore, and due to the lack of effective therapies for diffuse or recurrent disease, there is an urgent demand for effective drugs for VHL patients, especially those medicines that might halt the progression of tumours and subsequently delay surgical treatment.
In 2008, Léauté-Labrèze and col. showed for the first time the therapeutical antiangiogenic properties of Propranolol, a non-specific β1-and β2-blocker with more than fifty years on the market. Propranolol is prescribed for the treatment of arrhythmia, hypertension, migraines, and other cardiac and neurological diseases, as well as infantile hemangioma15,16,17,18. In addition, our lab has demonstrated in vitro and in a clinical trial (EudraCT: 2014-003671-30), the therapeutical properties of Propranolol in VHL disease19,20. In brief, Propranolol-treated primary cultured hemangioblastoma (HB) cells decreased the RNA and protein expression levels of HIF-1α and HIF-2α, and therefore the pro-angiogenic and HIF-1α targets such us Endoglin, VEGF, EPO, and SOX. Moreover, VEGF and miR210 plasma levels from treated patients were reduced after treatment with Propranolol19,21.
Remarkably, the main property of Propranolol, its capacity of reduce the blood pressure, is the highest drawback for low or regular blood pressure VHL patients. Alongside the clinical trial, hypotension and bradycardia were the main side effects registered. These side effects of Propranolol are driven exclusively by its β1-adrenergic receptor blockade22, so it would be possible to overtake them and keep the benefits by using a high specific β2-adrenergic receptor antagonist.
In this line, β2-blockers have shown antiangiogenic properties in vitro and in vivo23,24,25, becoming a promising therapeutical molecule for VHL Hemangioblastomas26. Among them lays the selective antagonist of the β2-adrenergic receptor erythro-D,L-1(methylinden-4-yloxy)-3-isopropylaminobutan-2-ol, known as ICI-118,551 (ICI).
ICI was developed by Imperial Chemical Industries in the 80’s. Up to now, ICI has no known therapeutic application in humans so far, although it has been used widely in research to understand the action of the β2 adrenergic receptor due to its β2/β1 selectivity ratio of 123 folds and a Kd in nM range27,28,29,30,31,32,33. Several human clinical studies as well as murine/rodent models developed in the 80’s used ICI in comparative analysis of different β-antagonists. Supplementary Table 1 shows a compilation of the assays performed with ICI in humans, including purpose, results, dose, and length of the treatment. Nevertheless, little is known about ICI’s pharmacokinetics; it is known that ICI is able to cross the blood–brain barrier (BBB)34,35,36 (Supplementary Table 1). Based on the clinical trials and in vivo assays done, systemic doses have been used without toxicity in humans (up to 20 mg/kg), rodents (0.5 or 1 mg/kg) or rhesus monkeys (0.1 μg/kg). ICI, nowadays, is mainly used as control of β-blockers’s specificity in physiological processes such us control of heart and vascular tone and SNC and muscular signaling37,38,39,40. Finally, ICI has been also used in cancer research showing a role in cell proliferation and signalling regulation41,42.
Here we show a future therapeutic approach of ICI for VHL disease and a potential mechanism of action using different HB primary cultures. Blockage of the β2-receptor impairs the viability of HBs primary culture cells by triggering the apoptotic caspases 3 and 7 cascade. Moreover, ICI is able to halt or almost delay two main angiogenic processes such us cell migration and tube formation. Finally, and based on the idea that β-antagonists downregulate HIF signalling, we demonstrate that ICI prevents the nuclear internalization of HIF-1α in HBs and human endothelial primary cultures under hypoxic conditions.
Taken together the above mentioned effects on VHL hemangioblastomas plus the lack of side-off effects on healthy endothelial cells and blood pressure43, ICI arises as a promising new (and almost unique) molecule on the field of VHL therapies. Its properties suggest also a potential therapeutic role on other angiogenesis-related diseases like Hereditary Hemorrhagic Telangiectasia (HHT) or carcinomas.
Results
ICI-118,551 decreases viability in hemangioblastomas by inducing cell apoptosis
The selective β2-receptor blocker ICI mirrors the results previously obtained with the non-selective β1-and β2-antagonist Propranolol21. When testing viability of HB cells in a dose-response curve Propranolol (Fig. 1A) and ICI (Fig. 1B) decreased viability of primary cultures of VHL HB cells in a dose-dependent manner. Our lab had previously established 100 μM Propranolol as the effective dose; this dose is able to impair cell viability at least by 55–60% when compared to untreated cells, and ICI gives similar results. Dose-effect curves for ICI and Propranolol are also shown for HUVECs (Fig. 1C). Interestingly, the effect of the β-blockers seems to be rather specific for HB cells, since at 100 μM there is a significant difference of viability, while in HUVECs is of 80%, in HB drops to 40–45%.
Effect of β2-adrenergic receptor blockage on HB cells viability. HB (dark grey) and HUVEC (light grey) primary cultures were incubated for 48 hours with increasing doses [0–100/200 µM] Propranolol (A), ICI (B), and Atenolol. (C) Propranolol and ICI significantly impair cell viability only in HB cells, while Atenolol showed no changes in cell viability neither in HBs nor in HUVECs. Cell viability was measured as described in Material and Methods. (D) mRNA quantification of β1-and β2-receptors in HB cells versus HUVECs by RT-qPCR. Data show a higher expression of β1-and β2-receptors in HBs compared with HUVECs. (E) Representative pictures of 8 HB primary cell cultures treated with 50–100 µM Propranolol or ICI for 96 hours. Data denote mean ± S.D (n = 3). Scale bars represent 100 µm.
In order to determine the β2-receptor specificity, Atenolol, a specific β1-receptor antagonist, was used to test for the effect on viability at 50 and 100 μM (Fig. 1C). Atenolol, in comparison with ICI (Fig. 1B) and Propranolol (Fig. 1A), hardly provokes any effect on HB and HUVECs.
As the difference in viability between HUVECs and HB cells could be explained by the number of β-receptors expressed, we then measured the gene expression levels by RT-qPCR of β1 and β2 receptors. As it is shown in Fig. 1D, the amount of both β-receptors and more specific the levels of β2 receptors were higher in HB than HUVECs.
Figure 1E shows the cells remaining in HB cultures from 2 different patients after 72 hours of treatment with 100 μM Propranolol and 50 and 100 μM ICI compared with untreated cells. A significant visual reduction in the number of cells with respect to the untreated ones is observed.
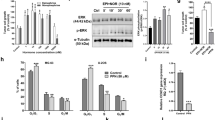
The decrease in viability of HB following ICI treatment is due to apoptosis as it happened for Propranolol21. This fact has been investigated by quantifying the expression of mRNA of two pro-apoptotic genes, BAX and the executor protease Caspase 9. Figure 2A shows how the expression of both genes is significantly upregulated (between 1.3–2 fold) after treatment with ICI in HBs, from different patients. On the other hand, apoptosis was also quantified measuring Caspase 3 and 7 activity by luminometry (Fig. 2B) following ICI treatment. A significant increment of caspase enzymatic activity of 1.7 fold was detected in treated versus untreated cells.
ICI-118,551 treatment induces cell apoptosis by stimulating pro-apoptotic genes Bax and Caspase 9. HB 11 (light grey) and HB26 (dark grey) primary cultures were incubated with 100 μM ICI for 72 h incubation. Relative mRNA expression levels of the apoptosis-related genes BAX and CASP9, measured by RT-qPCR. (A) Caspases 3&7 activity was quantified using the luminometric kit Caspase-Glo® 3/7 Assay. (B) HB18 cells were cultivated on coverslip in P-24 well plates, and treated with either 100 μM ICI or Propranolol for 24 or 48 h. Cells were fixed with PFA 4% for 30 min at 4 °C and washed with PBS. Coverslips were mounted in a drop of Pro-long DAPI mounting reagent (Life) and observed by fluorescence microscopy to determine nuclear integrity. (C) Representative pictures of 3 HB primary cell cultures. Scale bars represent 10 µm.
In order to obtain a visual confirmation of the apoptotic process on HB cells after Porpranolol or ICI treatment, nuclear integrity was assessed by DAPI staining. As shown in Fig. 2C, incubation for 48 hours with 100 μM Propranolol and ICI trigger the nuclear disassembly, supporting the genetic and molecular results shown in Fig. 2A,B.
ICI-118,551 inhibits hemangiosphere formation from VHL tumour cells
Tumour cells, when cultured avoiding adherence, form organized and clearly defined border spheres around a core of cancer stem cells (CSC)44,45. Anti-tumoral agents show its properties by inhibiting or disrupting the formation of the spheres by disaggregation of cells. Figure 3 shows hemangiosphere cultures inhibition from 5 different HBs which were treated or not with 100 μM Propranolol or ICI for 7 days. As shown in Fig. 3, hemangiospheres are disrupted by β-blockers being especially conspicuous after ICI treatment on HB4 and HB23 cells.
Propranolol and ICI-118,551 inhibit hemangiosphere formation from VHL patient cells. Six different HB primary cell cultures were treated for 7 days with either 100 μM Propranolol or ICI. Round sphere-like structures with a well-defined border appear as aggregates from VHL cell tumours. However, after the treatment, the spheres appear disaggregated and the observed groups are irregular with few cells. Pictures were taken the last day of treatment. Scale bars represent 100 µm.
In vitro antiangiogenic properties of ICI-118,551
Angiogenesis is the property of formation of new vessels from pre-existing ones. Basically, the angiogenic process is based on the activation and further migration of the endothelial cells and tubulogenesis or formation of new vessels. These processes can be studied in vitro by the test of wound healing (Fig. 4A) and Matrigel tubulogenesis assay (Fig. 4B).
Propranolol and ICI-118,551 inhibit the angiogenic process (wound healing and tube formation assays) in vitro. (A). Shows representative images of scratch wound healing assay of HUVEC cells treated with either 100 μM ICI or Propranolol (P). Area covered after 6 hours was pictures and quantified showing that β-receptor blockage significantly impairs endothelial cell migration. (B) Shows representative images of tube formation assay of HUVEC cells treated with either 100 μM ICI (I) or Propranolol (P) for 6 hours. The number of closed cells (density network) was quantified as described in Material and Methods and averaged from 5 different fields in each condition. Density network quantification shows that Propranolol and ICI significantly inhibit the tube formation process. Data denote mean ± S.D. Scale bars represent 100 µm.
Confluent monolayers of HUVECs were disrupted by scratching their surface. This wound healing test was followed in time to monitor the migration of cells. Figure 4A shows how treatment with either 100 μM ICI or Propranolol delay the closure of the wound compared to control cells interfering with the migration process, as shown in the graph after quantification.
Figure 4B shows the characteristic tube network formed by endothelial cells on Matrigel after 6 hours, imitating the in vivo vessels network. However, if cells are pre-treated either with 100 μM ICI or Propranolol, the number of closed structures is dramatically decreased. The average quantification of closed tubules in five different optic fields is shown in Fig. 4B. Notably, Propranolol and ICI are acting as inhibitors of angiogenesis, migration, and of tubulogenesis processes.
ICI-118,551 is an antiangiogenic agent acting by inhibiting HIF-target genes
Propranolol and ICI are both able to block β2-adrenergic receptors. To test the hypothesis that by blocking the adrenergic β2-receptors, HIF transcriptional activity is inhibited, a system of luciferase reporter cell, HeLa 9xHRE-Luc, was used. To induce hypoxic conditions, HeLa 9xHRE-Luc cells were cultured with 100 μM Deferoxamine (DFO) and then treated with 100 μM Propranolol, ICI, or Atenolol for 48 hours. The luciferase activity was measured by luminometry. Figure 5A shows how luciferase expression is triggered under hypoxic conditions, and how this upregulation is dampened by Propranolol and ICI. Interestingly, there is some effect of Atenolol on the luciferase activity but this is negligible compared to the β2-antagonists (Propranolol and ICI). This result suggests that the decrease of HIF responsive transcription is mainly mediated through the β2-receptor blockade.
(A) Effect of β2-adrenergic receptor blockage on HIF targets. HeLa 9xHRE-Luc cells under normoxia (white bars) or hypoxia induced with 100 µM DFO (grey bars) were incubated with either 100 µM Atenolol, or ICI, or Propranolol for 24 hours. Then, luciferase-HIF dependent activity was measured. The bar histograms show the relative luciferase activity measured. Treatment with the β2-blockers Propranolol or ICI significantly inhibited the hypoxia stimulation induced by DFO. Data denote mean ± S.D (n = 3). (B) Propranolol and ICI, decrease the expression of the HIF target AQP-1. Different HB cells cultures (HB19, HB23, HB28) were treated with both β-blockers during 48 h and the expression of AQP-1 mRNA were measured by Real Time PCR. Propranolol and ICI significantly decrease the AQP-1 expression.
To test the impact of Propranolol and ICI on HIF-1α driven target genes, Aquaporin 1 (AQP-1) expression was studied by real time PCR. This target is of special interest in HBs, since AQP-1 codes for a transmembrane protein water channel. AQP-1 expression is induced by HIF-1α46,47,48. This enhanced expression may increase liquid flow across the cell membrane leading to cystic growth, common in VHL tumours. As shown in Fig. 5B AQP-1 expression was significantly decreased in different HB cells cultures (HB19, HB23, HB28) treated with Propranolol or ICI.
HIF-1α nuclear translocation is altered by the β-adrenergic receptor blockers
To unravel the possible molecular mechanism of action of Propranolol and ICI and to understand its effect on HIF target downregulation targets, HIF-1α cellular distribution was addressed by an immunofluorescence confocal assay. Blocking HIF-1α nuclear translocation should impair HIF targets, as described in cancer cells treated with Vorinostat49.
Firstly, as a proof of principle HUVEC cells under an hypoxic controlled condition with 100 µM DFO for 24 h, were treated with 100 µM of Atenolol, or Propranolol, or ICI, or vehicle. In this assay, HIF-1α was visualized and its nuclear or cytoplasmic localization (Fig. 6A) was measured in a quantitative manner (Fig. 6B). Figure 6A shows HIF-1α labelled with Alexa 568 (in red), nucleus with DAPI (in blue) and merged image, as well as cellular distribution histograms plus a normalized HIF-1α density distribution. In control or Atenolol treated HUVECs, HIF-1α is preferentially allocated in nuclei. However, treatment with Propranolol or ICI changed significantly its cellular distribution. The translocation of HIF-1α to the nucleus is somehow precluded since HIF-1α now is also found over the cytoplasm, as shown in Fig. 6A,B.
Effect of β2-adrenergic receptor blockage on HIF-1α subcellular distribution in endothelial cells. HUVEC cells under 100 µM DFO were incubated with either 100 µM Atenolol, or Propranolol, or ICI for 48 hours. Immunofluorescence detection and confocal microscopy processes are described in Material and Methods. (A) Shows representative images of the mouse anti-human HIF-1α antibody stains (red), DAPI nuclear staining (blue), and merged (middle column). Furthermore, the analysis of the distribution of HIF-1α alongside the cell is shown in two manners: i) as intensity histograms; were nuclei appear in blue (DAPI) and HIF-1α in red and ii) as a normalized distribution of HIF-1α intensity. (B) Shows the percent distribution of HIF-1α in the nuclei (blue) or cytoplasm (red). Treatment with the β2-antagonists Propranolol or ICI significantly inhibited the internalization of HIF-1α, being accumulated into the cytoplasm. Data denote mean ± S.D (n = 8–10). Scale bars represent 50 µm.
Next, the same procedure was performed in HBs cultures. In untreated (Control) and Atenolol-treated HB cells, HIF-1α is translocated and therefore, is mainly localized inside the nucleus (Fig. 7A), although some staining is also visible in cytoplasm, due to the constitutively hypoxic physiology of HBs. The situation turns out completely different when Propranolol and ICI treatments are added. In these cases, HIF-1α is not as localized in nuclei as before. This situation can be clearly observed comparing the histograms shown in Fig. 7B, where the staining distribution was quantified by the ImageJ-FIJI. Notably, there is no significant difference between untreated and Atenolol treated-HB cells, and neither between Propranolol and ICI, indicating that the effect is mainly via β2-receptors.
Effect of β2-adrenergic receptor blockage on HIF-1α subcellular distribution in HB primary culture cells. HB primary culture cells were incubated with either 100 µM Atenolol, or Propranolol, or ICI for 48 hours. Immunofluorescence detection and confocal microscopy processes are described in Material and Methods. (A) Shows representative images of the mouse anti-human HIF-1α antibody stains (red), DAPI nuclear staining (blue), and merged (middle column). Furthermore, the analysis of the distribution of HIF-1α alongside the cell is shown in two manners: i) as intensity histograms; were nuclei appear in blue (DAPI) and HIF-1α in red and ii) as a normalized distribution of HIF-1α intensity. (B) Shows the percent distribution of HIF-1α in the nuclei (blue) or cytoplasm (red). Treatment with the β2-blockers Propranolol or ICI significantly inhibited the internalization of HIF-1α, being accumulated into the cytoplasm. Data denote mean ± S.D (n = 8–10). Scale bars represent 50 µm.
In summary, the results of Figs 6 and 7 demonstrate that Propranolol and ICI, both β2-blockers are preventing in some way the translocation of HIF-1α to nuclei which may explain the decrease of gene expression of HIF-targets shown in Fig. 5 and was previously shown in Albiñana et al., 2015/201719,21.
Discussion
Most of rare diseases are not of primary interest for the industry due to their low incidence and the lack of benefits for the industry. The case of VHL disease, an hereditary rare cancer, maybe an exception due to its close connection with a bigger pathology like cancer.
VHL patients need several different CNS and retinal surgeries along their lifespan. Unluckily, there is an accumulative morbidity in neurologic and retinal functions after each surgery. After CNS HB surgical resection, only 20% of the patients maintain or improve their preoperative symptoms50.
Furthermore, the systemic therapy used for VHL Hemangioblastomas in CNS has been unsuccessful so far. Even the most recent clinical trial reported only 42% of response in renal and pancreatic tumours but no response in CNS tumours14,51.
In this scenario, where surgery and pharmacological therapies show limited recovery and response for CNS VHL disease, an urgent requirement for new and more effective drugs with reduced side effects for these patients are demanded.
Some previous studies from Shepard et al. and our group have demonstrated that Propranolol, a non-specific β1-and β2-blocker, was efficient on in vitro treatment of CNS Hemangioblastomas primary cultures19,21,52. Basically, Propranolol was acting in two ways: i) decreasing cell viability by triggering apoptosis and ii) inhibiting the expression of HIF-dependent targets in VHL HB cells, where HIF-1α degradation is impaired21. Moreover, a phase 3 clinical trial, conducted by the Spanish VHL Alliance, addressed the therapeutical benefits of Propranolol in VHL patients suffering from retina HBs (a different subpopulation of the CNS Hemangioblastomas), Over the time of the trial, retinal tumours did not grow and HB-derived exudates present in 2 different patients, disappeared completely between 3 and 6 months of treatment19,20.
Importantly, Propranolol side effects of hypotension and bradycardia were registered and make difficult to keep a sustained treatment, in particular in patients with tendency to hypotension. These cardio-specific side effects are due to Propranolol binding to β1-specific cardio-receptors. Therefore, the elucidation of whether the therapeutical effects from Propranolol are derived from the blockage of β1, β2 or both receptors was critical.
The lack of effect of Atenolol (a specific β1-receptor antagonist) on cell viability and HIF regulation on HBs cultures (Fig. 1B) or HeLa 9xHRE-Luc reporter cells (Fig. 5) gave us the clue to look for an almost specific β2-blocker. By finding this, we should avoid the undesired cardio specific effects while keeping the therapeutical effects related to β2-blockade.
The present work demonstrates that the selective β2-adrenergic receptor blocker ICI, has similar effects that Propranolol20 and in some tests shows improved results. In particular, as shown in results, ICI decreases viability, triggers apoptosis, inhibits hemangiosphere formation, cell migration, and angiogenesis processes, as well as decreases HIF levels and HIF-1 α nuclear translocation. Moreover, we have also assessed that ICI has a similar antitumoral activity as Propranolol by lowering cAMP levels22,48, as shown in Supplementary Fig. 1. Altogether, ICI is partially counteracting the HIF response, constitutively activated in VHL tumour cells.
It is particularly interesting the fact shown in Fig. 1 that ICI and Propranolol are specifically decreasing the viability of HB cells derived from VHL tumours, while at the same doses healthy endothelial primary cultures are much less affected. This is due to the fact that HB cells express more β2-receptors than HUVECs in this work (Fig. 1D). Thus, the effect of the β2-blockers is preferentially exerted on HB tumour cells, this becoming a very important issue for the specificity and safety of the treatment. On the other hand, the lack of effect of Atenolol on the viability of either HB or HUVECs proves that the decrease in viability is exerted through the two β2-antagonists, Propranolol and ICI (Fig. 1C).
An effect reported for the first time, to the best of our knowledge, is the ability of HB primary cultures, to form hemangiospheres. These spheres, observed currently in cancer cell lines from breast (MCF-7)53 or glioblastoma (U-87)54 are organized structures around at least one CSC in the core of the sphere. This proves that, although HBs are considered “non malignant tumours” they also contain some CSC, able to organize hemangiospheres. However, there may be less abundant CSC compared to other tumours, since hemangiospheres are smaller and show size variability in vitro among different HB primary cultures. One of the “standard” in vitro tests, for antitumoral therapeutic agents is the capacity to disrupt, the formation of these structures. Interestingly, as shown in Fig. 3, Propranolol and ICI inhibit hemangiosphere formation in HB cells derived from VHL patients, showing its potential therapeutical capabilities.
Moreover, as VHL is a pseudohypoxic syndrome, the inhibition by Propranolol and ICI of in vitro angiogenesis is highly relevant. As shown in Fig. 4, Propranolol and ICI inhibit the processes of cell migration and tubulogenesis both are necessary processes for angiogenesis. Angiogenesis is dependent on the activation of HIF program, constitutively active in VHL hemangioblastomas. We demonstrated in 2015 that Propranolol was able to inhibit the hypoxia inducible activity in HeLa 9xHRE-Luc cells. In this work we also show that ICI is acting as Propranolol, by precluding the hypoxia activation of HIF targets21. More important, since we are postulating that this effect is also dependent on the β2-blockade, Fig. 5 supports our hypothesis since the decrease in the HIF reporter activity is minimal after Atenolol treatment when compared to the effect of the β2-blockers Propranolol and ICI.
One important HIF target in VHL disease is AQP-1. Its expression was found to be involved not only in water transport but also in tumorigenesis, HBs become clinically manifest through the development of huge associated cysts46,47. As we can see in different HBs, the expression of AQP1 decreased significantly after Propranolol and ICI treatment.
Previously, we hypothesized a mechanism of action for Propranolol, where the HIF dependent HRE targets, VEGF, Sox2, Oct-4, Epo, and miR210, were clearly downregulated, also showing how Propranolol decreased the amount of HIF19,21. Trying to deepen into the mechanistic pathway, Figs 6 and 7 beautifully show how ICI treated cells have decreased amounts of HIF-1α in the nuclei.
In 2017, Propranolol was designated as orphan drug for the treatment of VHL by the European Medicines Agency. We propose ICI as a new therapeutic step forward, following the line of the β2-antagonists, but avoiding the cardio specific effects due to β1-binding as with Propranolol.
Taking into account Propranolol’s β1-2 antagonist activity and success in off-label clinical trials for rare cancer (melanoma, angiosarcoma, and metastasic paraganglioma), we think that ICI may show similar therapeutical properties for the same type of rare carcinomas, avoiding the β1 blocking vascular effect. In addition, tumours like clear cell renal carcinomas (ccRCC) where VHL is mutated arise as a potential target for β-blockers55,56,57,58.
Altogether, we conclude that ICI might be an interesting drug to have in the common pharmaceutical arsenal available to treat pseudohypoxic syndromes, in particular the VHL disease, without excluding other types of HIF-mediated diseases.
Methods
Cell culture and treatments
CNS HB primary cultures from excess of resected surgery pieces of VHL patients were obtained after the patients had provided written informed consent. HB primary cultures were purified as previously described21 and cultured in RPMI supplemented with 20% Fetal Bovine Serum (FBS), 2 mM L-glutamine, and 100 U/ml penicillin/streptomycin (all from GIBCO, Grand Island, NY, USA).
Primary human umbilical vein endothelial cells (HUVEC), were cultured in EGM-2 (Lonza, Walkersville, MD, USA) supplemented with 10% FBS, 2 mM L-glutamine, and 100 U/ml penicillin/streptomycin (GIBCO).
The human cervix adenocarcinoma HeLa cells (ATCC, CCL-2), stably transfected with a hypoxia reporter formed by nine copies in tandem of the hypoxia responsive element plus the luciferase gene (HeLa 9xHRE-Luc), were cultured in DMEM supplemented with 10% FBS; 2 mM L-glutamine, and 100 U/ml penicillin/streptomycin (GIBCO).
To induce hypoxic conditions, HUVEC and HeLa 9xHRE-Luc cells were treated with 100 μM desferrioxamine (DFO) (Sigma-Aldrich, San Luis, MO, US).
CNS Hemangioblastomas, HeLa 9xHRE-Luc, and HUVEC cells were incubated with different doses of β-adrenergic receptor blockers for the time and dose indicated on each experiment. Atenolol (Sigma), was dissolved in DMSO (Merck, Darmstadt, Germany), while Propranolol and ICI (Sigma) were dissolved in distilled water.
All the cellular assays were performed at 37 °C, 5% CO2 and humidity conditions.
Cell viability assay
The viability of HB and HUVEC cells was measured by the Luminescent Cell Viability Assay (Promega, Madison, WI, USA). This is a homogeneous quantitative method to determine the number of viable cells in culture based on quantitation of the ATP presence, which indicates metabolically active cells. Briefly, 5 × 103 cells were seeded in 96-well/plates and cultured in 100 μl with increasing doses [0–200 μM] of Atenolol, or Propranolol, or ICI for the time indicated for each experiment in results. Then, 100 μl/well of Cell Titer-Glo reagent (Lysis buffer, Ultra-Glo Recombinant Luciferase, Luciferine, and Mg2+) was added and gently mixed for 15 minutes at room temperature (RT). Next, luminescence was measured using a Glomax Multidetection System (Promega).
Caspase activation assay
The Caspase-Glo® 3/7 Assay (Promega) is a luminescent assay that measures caspase-3 and caspase-7 activities. Luminescence is proportional to the amount of caspase activity presence. Briefly, 5 × 103 cells were seeded in 96 well/plates and cultured in 100 μl with increasing doses of [0–200 μM] Atenolol, or Propranolol, or ICI for the time indicated for each experiment in results. Then, 100 μl/well of Caspase Glo® 3/7 Reagent (Lysis buffer, Ultra-Glo Recombinant Luciferase, DEVD-aminoluciferine, and Mg2+) was added and gently mixed for 1 hour at RT. Next, the luminescence was measured in a Glomax Multidetection System (Promega).
Hemangiospheres formation and culture
To induce hemangiospheres formation, HB cells were grown in suspension in phenol red free DMEM:F-12 medium (GIBCO) supplemented with GlutaMAX (GIBCO), B27 Supplement 50X (GIBCO), 20 ng/ml EGF (Lonza, Basel, Switzerland), 20 ng/ml b FGF (Lonza), and 1% penicillin/streptomycin (GIBCO). 5 × 104 cells/ml were plated at a density of on ultra-low attachment 75 cm2 cell culture flasks (Corning, Corning, NY, US). Cultures were treated or not with 100 μM Propranolol or ICI for 7 days.
Wound healing and tube formation in vitro assays
Cell motility was assessed by wound healing recovery in vitro assay, in which a confluent monolayer of HUVECs in a 24-well plate was scratched by T-200 tip to make wounded gaps. Then, cell debris was washed out with PBS and fresh complete medium supplemented with PBS (as control), 100 µM Propranolol, or ICI for 6 hours. Endothelial cell migration into the denuded area was monitored at 0 and 6 hours post-wounding. Images were taken with an Olympus digital camera and ImageJ program (NIH, Bethesda, MD, US) was used to quantify the wound healing process.
For tube formation assays, 24 well-plates were previously coated with 100 μl standard Matrigel (BD Biosciences, Bedford, MA, USA) diluted 1:2 in serum-free DMEM plain medium (GIBCO). Then, 1 × 105 HUVECs were seeded and cultured in fresh complete medium supplemented with PBS (as control), or with 100 µM Propranolol or ICI for 6 hours. Images were taken with an Olympus digital camera and quantification of connected tubules was performed using Adobe Photoshop CS3 software (San José, CA, US).
Luciferase reporter assay
1 × 104 HeLa 9xHRE-Luc cells were cultured in a 24 well-plate. On the next day, cells under normoxia (as control) or under hypoxia with 100 µM DFO were treated or not with 100 µM Atenolol, Propranolol, or ICI for 48 h. After the incubation, cell lysate luciferase activity was analyzed using the Luciferase reporter assay system (Promega) in a GloMax®-Multi Detection System (Promega), following the manufacturer’s instructions.
Real-time RT-PCR
Total RNA was extracted from HB cells using Nucleo Spin RNA kit (Macherey-Nagel, Düren, Germany). One microgram of total RNA was reverse-transcribed in a final volume of 20 μl with the First Strand cDNA Synthesis Kit (Roche, Mannheim, Germany) using random primers. SYBR Green PCR system (BioRad, Hercules, CA, US) was used to carry out real-time PCR with an iQ5 system. Primers used for qPCR are: 18S Fwd: 5′-CTCAACACGGGAAACCTCAC-3′, 18S Rv: 5′-CGCTCCACCAACTAAGAACG-3′; BAX Fwd: 5′-CACTCCCGCCACAAAGAT-3′, BAX Rv: 5′-CAAGACCAGGGTGGTTGG-3′, CASP9 Fwd: 5′-CCCAAGCTCTTTTTCATCCA-3′, CASP9 Rv: 5′-TTACTGCCAGGGGACTCGT-3′, AQP1 Fwd: 5′-CCTCCCTGACTGGGAACTC-3′, AQP1 Rv: 5′-GGAGGGTCCCGATGATCT-3′.
cAMP quantitative determination
The cAMP levels in HB were determined using the cAMP-Glo Assay (Promega). 5 × 103 HB cells were seeded in 96 well/plates and cultured in the induction buffer: serum-free RPMI basal medium, 100 μM Ro 20-1724 (Sigma-Aldrich) and 500 μM IBMX (Sigma-Aldrich). The cells were then incubated with ICI or Propranolol for 30 minutes at 37 °C. Then, lysis buffer, cAMP reaction buffer, and cAMP detection solution were added according to the manufacturer’s specification. Finally, Kinase-Glo luminescent reagent was added and luminescence (cAMP activity) was measured in a Glomax luminometer (Promega).
DAPI staining and fluorescence microscopy
The DAPI (4′,6-diamidino-2-phenylindole) staining method was used to detect the visual symptoms of apoptosis generated by ICI or Propranolol in HB cells.
Briefly, 5 × 103 HB cells were seeded on sterile, collagen-coated coverslips (13 mm diameter, VWR international, Radnor, PA, US) placed at the bottom of a 24 well-plate. On the next day, HB cells were treated with 100 µM Propranolol, or ICI for 24 and 48 h. Then, cells were washed with PBS and fixed with 3% PFA for 10 minutes at RT. After two PBS washing steps, samples were incubated mounted on glass slides using Prolong + DAPI mounting media (Molecular Probes, Eugene, OR, US). 40x confocal images were taken using the fluorescence confocal microscope Sp5 (DMI6000 CS Leica Microsystems, Wetzlar, Germany). FIJI-Image J software tool (NIH, MD, US) was used to identify and analyze the apoptotic nuclei.
Immunofluorescence microscopy
Immunofluorescence analyses were performed to evaluate the effect of Atenolol, Propranolol, and ICI on translocation of HIF-1α from cytoplasm to the nucleus. 5 × 103 HUVEC and HB cells were seeded on sterile, collagen-coated coverslips (VWR international) placed at the bottom of a 24 well-plate. On the next day, HB cells which have a constitutive hypoxic condition and HUVECs cells under 100 µM DFO were both treated with 100 µM Atenolol, or Propranolol, or ICI for 48 h.
Then, cells were washed with PBS and fixed with 3% PFA for 10 minutes at RT. After two PBS washing steps, samples were incubated with blocking solution (1% Goat Serum and 1% BSA in PBS) for 1 h at RT. Then, cells were incubated overnight at 4 °C with mouse anti-human HIF-1α antibody (1:100) (BD, Franklin Lakes, NJ, US.). Following this, cells were washed thoroughly four times with PBS and incubated for 1 h at RT with goat anti- Mouse IgG (H + L)-Alexa fluor 568 conjugate antibody (1:200) (Thermo Fisher Scientific, Waltham, MA, US). Finally, cells were washed with PBS and coverslips were mounted on glass slides using Prolong + DAPI mounting media (Molecular Probes). 40x confocal images were taken using the fluorescence confocal microscope Sp5 (Leica Microsystems). Red and blue channels represent HIF-1α and DAPI stains, respectively. FIJI-Image J software tool (NIH) and LAS AF Lite program from Leica, were used to identify and quantify the nuclear or cellular intensity of HIF-1α alongside the longer diameter of the selected cells.
Statistical analysis
Results are presented as mean ± SEM. Statistical analyses were performed using the Student’s t-test. Statistical significance was defined when P < 0.05 (*P < 0.05; **P < 0.01, ***P < 0.001).
Ethical issues
All methods described in the present manuscript were carried out in accordance with all the international relevant guidelines and regulations of obliged fulfilment in the National Research Council of Spain (CSIC), and in agreement with worldwide regulations for Molecular Research. The Ethical committee of CSIC approved the protocols and procedures of the projects SAF 2014 52374-R and SAF2017-83351R, framework and funding for the experiments presented.
Informed consent was obtained from all subjects or, if subjects are under 18, from a parent and/or legal guardian.
References
Maher, E. R., Neumann, H. P. & Richard, S. Von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 19, 617–623 (2011).
Richard, S., Gardie, B., Couvé, S. & Gad, S. Von Hippel-Lindau: how a rare disease illuminates cancer biology. Semin Cancer Biol. 23, 26–37 (2013).
Gläsker, S. et al. Reconsideration of biallelic inactivation of the VHL tumour suppressor gene in hemangioblastomas of the central nervous system. J Neurol Neurosurg Psychiatry. 70, 644–648 (2001).
Gossage, L., Eisen, T. & Maher, E. R. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 15, 55–64 (2015).
Bader, H. L., Hsu, T. & Systemic, V. H. L. gene functions and the VHL disease. FEBS Lett. 586, 1562–1569 (2012).
Haddad, N. M., Cavallerano, J. D. & Silva, P. S. Von hippel-lindau disease: a genetic and clinical review. Semin Ophthalmol. 28, 377–378 (2013).
Kassardjian, C. D., Macdonald, R. L. & Munoz, D. G. Hemangioblastomas in the elderly: epidemiology and clinical characteristics. J Clin Neurosci. 21, 1205–1208 (2014).
Kaelin, W. G. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2, 673–682 (2002).
Kaelin, W. G. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 8, 865–873 (2008).
Bausch, B. et al. Renal cancer in von Hippel-Lindau disease and related syndromes. Nat Rev Nephrol. 9, 529–538 (2013).
Vortmeyer, A. O., Falke, E. A., Gläske, S., Li, J. & Oldfield, E. H. Nervous system involvement in von Hippel-Lindau disease: pathology and mechanisms. Acta Neuropathol 125, 333–350 (2013).
Van Velthoven, V., Reinacher, P. C., Klisch, J., Neumann, H. P. & Gläsker, S. Treatment of intramedullary hemangioblastomas, with special attention to von Hippel-Lindau disease. Neurosurgery. 53, 1306–1313 (2003).
Capitanio, J. F., Mazza, E., Motta, M., Mortini, P. & Reni, M. Mechanisms, indications and results of salvage systemic therapy for sporadic and von Hippel-Lindau related hemangioblastomas of the central nervous system. Crit Rev Oncol Hematol. 86, 69–84 (2013).
Jonasch, E. et al. Pazopanib in patients with von Hippel-Lindau disease: a single-arm, single-centre, phase 2 trial. Lancet Oncol. 19, 1351–1359 (2018).
Leaute-Labreze, C. et al. Propranolol for severe hemangiomas of infancy. N Engl J Med 358, 2649–2651 (2008).
Storch, C. H. & Hoeger, P. H. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol 163, 269–274 (2010).
Sanchez-Carpintero, I., Ruiz-Rodriguez, R. & Lopez-Gutierrez, J. C. Propranolol in the treatment of infantile hemangioma: clinical effectiveness, risks, and recommendations. Actas Dermosifiliogr. 102, 766–779 (2011).
Léauté-Labrèze, C. et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 372, 735–746 (2015).
Albiñana, V. et al. Repurposing propranolol as a drug for the treatment of retinal haemangioblastomas in von Hippel-Lindau disease. Orphanet J Rare Dis 12, 122 (2017).
González-Rodríguez, B. et al. Evaluation of the safety and effectiveness of oral propranolol in patients with von Hippel-Lindau disease and retinal hemangioblastomas: phase III clinical trial. BMJ Open. Ophthalmology 4, e000203, https://doi.org/10.1136/bmjophth-2018-000203 (2019).
Albiñana, V. et al. Propranolol reduces viability and induces apoptosis in hemangioblastoma cells from von Hippel-Lindau patients. Orphanet J Rare Dis. 10, 118 (2015).
Munabi, N. C. et al. Propranolol Targets Hemangioma Stem Cells via cAMP and Mitogen-Activated Protein Kinase Regulation. Stem Cells Transl Med. 5, 45–55 (2016).
Wong, H. P. et al. Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation. Toxicol Sci. 97, 279–287 (2007).
Martini, D. et al. Antiangiogenic effects of β2 -adrenergic receptor blockade in a mouse model of oxygen-induced retinopathy. J Neurochem. 119, 1317–29 (2011).
Sharifpanah, F., Saliu, F., Bekhite, M. M., Wartenberg, M. & Sauer, H. β-Adrenergic receptor antagonists inhibit vasculogenesis of embryonic stem cells by downregulation of nitric oxide generation and interference with VEGF signalling. Cell Tissue Res. 358, 443–452 (2014).
Perron, L., Bairati, I., Harel, F. & Meyer, F. Antihypertensive drug use and the risk of prostate cancer (Canada). Cancer Causes Control. 15, 535–541 (2004).
Nathanson, J. A. Beta-adrenergic-sensitive adenylate cyclase in secretory cells of choroid plexus. Science. 204, 843–844 (1979).
Nathanson, J. A. Cerebral microvessels contain a beta 2-adrenergic receptor. Life Sci. 26, 1793–1799 (1980).
Minneman, K. P., Hegstrand, L. R. & Molinoff, P. B. The pharmacological specificity of beta-1 and beta-2 adrenergic receptors in rat heart and lung in vitro. Mol Pharmacol. 16, 21–33 (1979).
Minneman, K. P., Hedberg, A. & Molinoff, P. B. Comparison of beta adrenergic receptor subtypes in mammalian tissues. J Pharmacol Exp Ther. 211, 502–508 (1979).
O’Donnell, S. R. & Wanstall, J. C. Evidence that ICI 118, 551 is a potent, highly Beta 2-selective adrenoceptor antagonist and can be used to characterize Beta-adrenoceptor populations in tissues. Life Sci. 27, 671–677 (1980).
Bilski, A. J., Halliday, S. E., Fitzgerald, J. D. & Wale, J. L. The pharmacology of a beta 2-selective adrenoceptor antagonist (ICI 118,551). J Cardiovasc Pharmacol. 5, 430–437 (1983).
Ruffolo, R. R. Jr. ed. Adrenoceptors: Structure, Function and Pharmacology. (Harwood Academic Publisher, Luxembourg, 1995).
Hare, G. M. et al. Beta2 adrenergic antagonist inhibits cerebral cortical oxygen delivery after severe haemodilution in rats. Br J Anaesth. 97(5), 617–23 (2006).
Uchida, S., Kawashima, K. & Lee, T. J. Nicotine-induced NO-mediated increase in cortical cerebral blood flow is blocked by beta2-adrenoceptor antagonists in the anesthetized rats. Auton Neurosci. 96(2), 126–30 (2002).
Moresco, R. M. et al. Synthesis and in vivo evaluation of [11C]ICI 118551 as a putative subtype selective beta2-adrenergic radioligand. Int J Pharm 25(204(1–2)), 101–109 (2000).
Ozkan, M. H. & Uma, S. β-adrenergic Receptor Blocker ICI 118,551 Selectively Increases Intermediate-Conductance Calcium-Activated Potassium Channel (IK(Ca))-Mediated Relaxations in Rat Main Mesenteric Artery. Basic Clin Pharmacol Toxicol. 122(6), 570–576 (2018).
Gong, H., Li, Y., Wang, L., Lv, Q. & Wang, X. Short-term effects of β2-AR blocker ICI 118,551 on sarcoplasmic reticulum SERCA2a and cardiac function of rats with heart failure. Exp Ther Med. Sep 12(3), 1355–1362 (2016).
Propping, S., Newe, M., Lorenz, K., Wirth, M. P. & Ravens, U. β-Adrenoceptor-Mediated Relaxation of Carbachol-Pre-Contracted Mouse Detrusor. Urol Int. 95(1), 92–98 (2015).
Sonekatsu, M., Gu, S. L., Kanda, H. & Gu, J. G. Effects of norepinephrine and β2 receptor antagonist ICI 118,551 on whisker hair follicle mechanoreceptors dissatisfy Merkel discs being adrenergic synapses. Mol Brain. 12(1), 31 (2019).
Coelho, M. et al. Antiproliferative effects of β-blockers on human colorectal cancer cells. Oncol Rep. 33(5), 2513–2520 (2015).
Chen, H. et al. β2-AR activation induces chemoresistance by modulating p53 acetylation through upregulating Sirt1 in cervical cancer cells. Cancer Sci. 108(7), 1310–1317 (2017).
McCaffrey, P. M., Riddell, J. G. & Shanks, R. G. Selectivity of xamoterol, prenalterol and salbutamol as assessed by their effects in the presence and absence of ICI 118,551. Eur Heart J. Apr 11(Suppl A), 54–5 (1990).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature. 414, 105–111 (2001).
Abbaszadegan, M. R. et al. Isolation, identification, and characterization of cancer stem cells: A review. J Cell Physiol. 232, 2008–2018 (2017).
Abreu-Rodríguez, I. et al. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1α. PLoS One. 6(12) (2011).
Yin, T. et al. Correlation between the expression of aquaporin 1 and hypoxia-inducible factor 1 in breast cancer tissues. J Huazhong Univ Sci Technolog Med Sci. 28(3), 346–348 (2008).
Baker, J. G., Hall, I. P. & Hill, S. J. Agonist and inverse agonist actions of beta-blockers at the human beta 2-adrenoceptor provide evidence for agonist-directed signaling. Mol. Pharmacol. 64, 1357–1369 (2003).
Zhang, C. et al. Vorinostat suppresses hypoxia signaling by modulating nuclear translocation of hypoxia inducible factor 1 alpha. Oncotarget 8 (2017).
Gläsker, V. & Klingler, J. H. RareConnect.org webinar: Hemangioblastomas of the central nervous system in patients with VHL, https://youtu.be/F0wSl9AQxrg (2018).
Giles, R. H. & Gläsker, S. The first prospective trial for von Hippel-Lindau disease: pazopanib. Lancet Oncol. 19, 1267–1269 (2018).
Shepard, M. J. et al. Repurposing propranolol as an antitumor agent in von Hippel-Lindau disease. J. Neurosurg. 1–9 (2018).
Rincón-Fernández, D. et al. In1-ghrelin splicing variant is associated with reduced disease-free survival of breast cancer patients and increases malignancy of breast cancer cells lines. Carcinogenesis. 39, 447–457 (2018).
Liu, Y. C., Lee, I. C. & Chen, P. Y. Biomimetic brain tumor niche regulates glioblastoma cells towards a cancer stem cell phenotype. J Neurooncol. 137, 511–522 (2018).
Shan, T. et al. β2-AR-HIF-1α: a novel regulatory axis for stress-induced pancreatic tumor growth and angiogenesis. Curr Mol Med. 13(6), 1023–1034 (2013).
Galván, D. C., Ayyappan, A. P. & Bryan, B. A. Regression of primary cardiac angiosarcoma and metastatic nodules following propranolol as a single agent treatment. Oncoscience. 5(9–10), 264–268 (2018).
De Giorgi, V. et al. Propranolol for Off-label Treatment of Patients With Melanoma: Results From a Cohort Study. JAMA Oncol. 4(2) (2018).
Díaz-Castellanos, M. A. et al. Case Report: Propranolol increases the therapeutic response to temozolomide in a patient with metastatic paraganglioma. F1000Res. 6, 2087 (2017).
Acknowledgements
Funding was provided by the projects SAF 2014-52374-R and SAF2017-83351-R from the Ministery of Economy and Competitivity to LMB. The Spanish Alliance of VHL patients was funding ACM through CIBER-rare diseases grant. LR-P is recipient of a contract from PIE-201820E073 (CSIC). EG-V was funded by SAF2013-43421-R.
Author information
Authors and Affiliations
Contributions
L.M.B. designed the complete study. L.M.B., A.C.M., V.A. and E.G.-V. wrote the main manuscript text and figures. All experiments were done equally by ACM (apoptosis, viability and PCR assays), V.A. (hemangiosphere, tubulogenesis and wound healing assays), E.G.-V. (luciferase and confocal assays) and I.R.-P. (cAMP assays) with technical support of L.R.-P. D.A. provided clinical samples. K.V.G.-H. provided clinical review of the manuscript. I.R.-P. contributed with the supplementary data and figures. All authors critically reviewed the manuscript and approved the final manuscript as submitted and agree to be accountable for all aspects of the work A.C.M., V.A., E.G.-V. and L.M.B. wrote the main manuscript text and prepared all figures. K.V.G.-H., D.A. and I.R.-P. assisted with improving the writing of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cuesta, A.M., Albiñana, V., Gallardo-Vara, E. et al. The β2-adrenergic receptor antagonist ICI-118,551 blocks the constitutively activated HIF signalling in hemangioblastomas from von Hippel-Lindau disease. Sci Rep 9, 10062 (2019). https://doi.org/10.1038/s41598-019-46448-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46448-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.