Abstract
Vanadium dioxide (VO2) is one of the most extensively studied materials in the strongly correlated electron family capable of sustaining an insulator-to-metal transition. Here we present our studies of high-quality thin films of epitaxially grown VO2 on c-Al2O3(0001) and TiO2(001) via reactive DC pulsed magnetron sputtering. We present the structural transition probed via Reflection High Energy Electron Diffraction (RHEED) for the first time and we correlate the surface microstructure measurements with simulations before, during, and after the thermally induced transition. We also study the photoelectric conversion of VO2 on TiO2(001) and c-Al2O3(0001) under 405 nm light and demonstrate up to a 2000% increase in quantum efficiency as the power of the light is varied for VO2 on TiO2(001).
Similar content being viewed by others
Introduction
A Mott transition is a fundamental concept that governs the emergence of various electronic phases and physical properties in correlated electron materials, represented for example by the insulator-metal transition (IMT) in vanadium dioxide (VO2). Vanadium dioxide is one of the most extensively studied materials in the correlated electron family where it is known to undergo a characteristic reversible first order transition from insulator (T < Tc) to metal (T > Tc) where Tc, ~68 °C in bulk, is the temperature at which the transition occurs1,2. This IMT is also accompanied by an associated structural transition from an insulating monoclinic phase to a metallic rutile phase2. Thus, it is subject of significant importance to understand the dynamics of electron and lattice systems across such transition in these highly correlated materials3,4,5. An interesting proposed aspect of VO2 in its monoclinic phase is its ability to undergo photoelectric conversion; where holes are formed in the valence band of the substrate and transported to the O2p band of VO2 then electrons from the VO2 d|| band move to fill the holes in the lower O2p band6. Therefore, a key approach to understand the occurrence of this photoelectric conversion is to investigate the correlations between surface microstructure and photoelectric conversion in thin films deposited on different substrates to explore the potential parameter space for possible applications.
Additionally, it has been argued that the binding between doubly occupied (doublon) and empty (holon) sites plays a key role in the Mott transition in strongly correlated Mott-Hubbard systems and could play a distinct role in photelectric conversion7. In this photo-electric conversion, the application of an electric field to the monoclinic VO2 phase drives carrier tunneling to create doublon-holon pairs via nonlinear excitation processes such as multiphoton absorption and quantum-tunneling8,9. This pair creation results in an instantaneous insulator to metal transition without direct interaction with the lattice8,9. Allowing for a purely electronic phase transition, however, the thin film surface microstructure and morphology will affect carrier scattering, and the dynamics will be reflected in the ultimate quantum-efficiency9,10,11.
Results
Crystal analysis and determination
All films are 30 nm thick and were epitaxially grown via reactive DC magnetron sputtering on c-Al2O3 and TiO2 as discussed in the experimental section. The initial microstructure characterization of the films was carried out using X-Ray Diffraction (XRD) symmetric scans as shown in (Fig. 1a). Upon examination of the 2θ scans we determined that the peak location was consistent with the bulk location for the VO2 grown on c-Al2O3, with a slight degree of strain toward the substrate peak as expected. We note that the main peak location for the VO2 grown on TiO2 is consistent with previous reports for growth on this substrate12. For VO2 on TiO2(001), the VO2 peak location indicated that the film was also strained toward the substrate peak by ~0.5° in comparison to existing data, suggesting that in this case the film contains a more strained monoclinic phase toward the rutile phase than previously reported12.
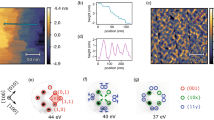
The experimental XRD, AFM, and simulated RHEED patterns of VO2 on c-Al2O3 and TiO2. (a) The XRD 2θ scan for VO2 on c-Al2O3(0001) and TiO2(001) where the intensity scale in arbitrary units is log scaled. (b) The simulated RHEED pattern of the 0° rotation for the monoclinic phase of VO2 where the streak intensity patterns are recorded on top and the simulated diagrams are reported on bottom with the in plane lattice planes are recorded for each. (c) The simulated RHEED patterns of the 0° rotation for the rutile phase of VO2 where the placement of the plots is the same as the previous monoclinic phase. (d) The AFM image of the surface microstructure of VO2 on c-Al2O3(0001) where the scale is 5.0 μm × 5.0 μm. (e) The AFM image of the of the surface microstructure of VO2 on TiO2(001) where the scale is 5.0 μm × 5.0 μm. (f) The orientations of the vanadium and oxygen atoms through the 0° and 90° rotations of one unit cell where the first two surface layers of atoms are shown in the c-direction where the red layer is displaced ~1.44 nm below the blue layer.
We also determined the mosaicity (the degree of crystallite misorientation) for each sample and found a nominal degree of mosaicity ~0.08° for the sample grown on c-Al2O3 and ~0.047° for the sample grown on TiO2.
RHEED structural analysis
Figure 1b,c shows the simulated Reflection High Energy Electron Diffraction (RHEED) patterns and streak diagrams expected for the bulk-like phases of VO2 through the IMT13,14. As evidenced by (Figs 1b,c,f and S1a–c), upon in-plane rotation of the sample with respect to the electron-beam, the diffraction pattern of the sample changes greatly due to the atomic positions of the vanadium and oxygen atoms on the lattice. The oxygen atoms play a distinct role in these diffraction patterns, as they appear to be the main contributors to streak intensity. Each in-plane rotation shows a large degree of variation in diffraction pattern, with a significant change expected for the monoclinic structure due to the canted angles exhibited by the oxygen atoms15.
The positions of the vanadium and oxygen atoms have been extensively studied both theoretically and experimentally for both phases across the IMT where space groups for the two phases of VO2 across the IMT are P42/mmm(136) for rutile and P21/c (14) for monoclinic and for our simulation atom positions were determined from these space groups as shown in Tables 1 and 215. The rutile structure possesses greater in-plane symmetry than the monoclinic does. Thus, through each 90° in-plane azimuthal rotation a repetition of patterns will occur with a clear differentiation in diffraction patterns expected along the 45° rotation directions, and with differing patterns between 45° and 135°, depending upon whether the oxygen atoms are in-line with the main axis or rotated 90° off axis.
This in-plane azimuthal dependency is illustrated in (Figs 1f and S2c) in which the first two layers of atoms account for the majority of the diffracted e–beam intensity in the patterns. For the present study, each of the chosen substrates ensures a good lattice match between the square lattice face of the substrates and the square face of the rutile structure for VO2 (a = 4.55 Å). In the case of c-Al2O3 the lattice mismatch is greater (a = 4.785 Å) than that of TiO2(001) (a = 4.584 Å) however both provide adequate lattice matching to facilitate epitaxial growth of VO2 as evidenced from the XRD in (Fig. 1a)15,16,17.
It is worth noting that the spotty nature of the simulated RHEED pattern (Figs 1b,c and S1a,b) is due to the fact that all surface atoms in the VO2 structure are not in the same plane, since all surface atoms in the same plane would lead to vertical streaks in the diffraction pattern. In the present case, this spotty pattern is compounded in the experimental RHEED data by the inherent surface roughness that is evidenced in the Atomic Force Microscopy (AFM) images of the actual samples (Fig. 1d,e). We also note that the surface of VO2 on c-Al2O3 has a larger degree of average roughness, ~30 nm, due to the larger spacing between terraces leading to a roughness on the order of the film thickness. In comparison, (Fig. 1e), corresponding to VO2 on TiO2(001), exhibits enhanced proximity between terraces, with each terrace sitting directly adjacent to its neighbor. This lack of separation between terraces accounts for a roughness ~13 nm thus yielding a smoother overall film in comparison to VO2 on c-Al2O3(0001). This larger degree of surface roughness in the VO2 on c-Al2O3(0001) may also be a contributor to the lesser photocurrent produced for the VO2 on c-Al2O3(0001) compared to the VO2 on TiO2(001) sample due to scattering effects discussed later.
We also present here a comparison study of bulk VO2 RHEED patterns predicted via simulation and the RHEED patterns recorded from VO2 grown on c-Al2O3. As shown in (Figs 2a and S2a) the experimentally determined RHEED patterns exhibit similar streak configurations and orientations as those determined in the simulated patterns. Figure S2a exhibits numerous cases of pattern matching as well as streak spacing suggesting that the simulations and the observed diffraction patterns for the monoclinic VO2 phase are in good agreement. We then proceeded to heat the samples up to 100 °C to allow for the VO2 to undergo the full IMT which for this sample occurred at ~55 °C. This shift in transition temperature from bulk is likely due to the thin film character of sample, where factors like lattice parameter mismatch between sample and substrate play a key role as previously reported for TiO212,18. Once the sample fully transitioned, the RHEED patterns in (Figs 2b and S2b) (right) were compared to the simulations shown in (Figs 2b and S2b) (left). The streak patterns from the rutile phase VO2 exhibit the same configurations as those predicted in the simulation for (Figs 2b and S2b), i.e. experimentally determined patterns are similar to the simulated ones, in combination with the AFM images and the XRD data, further assert that the patterns observed in both the simulations and experiments, are quite close to bulk-like crystalline VO2 in both the monoclinic and rutile states, and with low mosaicity.
The simulated and experimentally determined RHEED patterns for VO2 on c-Al2O3(0001) and VO2 on TiO2(001). For VO2 on c-Al2O3(0001): Simulated and experimental RHEED patterns of VO2 azimuthal rotations and for the two phases. (a) The left two images are simulations of the 0° rotation for the monoclinic phase of VO2; the top image denotes the 2-D lattice planes for the streak patterns while the bottom image has streaks highlighted. The right image is the experimentally determined RHEED pattern the highlighted streaks correspond to the simulated image. This plot orientation is maintained for (b) as well where the simulated images are shown at left and experimental images at right. (b) The 0°/90° rotation for the rutile phase of VO2. (c) The experimentally determined RHEED patterns for the 0° degree rotation through the thermal IMT where the image on the left is prior to transition and the image on the right is post transition. For VO2 on TiO2(001): (f) The top leftmost two images are simulations of the 0° rotation for the monoclinic phase (left) and rutile phase (right) of VO2. The bottom left image is a superimposed image of both simulated images. The right image is the experimentally determined RHEED pattern for VO2 on TiO2(001) where the highlighted streaks correspond to the simulated images. (e) The left top image is the simulation and right top and bottom images experimental patterns for VO2 grown on TiO2(001); the 0° rotation is right and 90° is bottom. (f) The experimentally determined RHEED patterns for the 0° degree rotation through the thermal IMT where the image on the left is prior to transition and the image on the right is post transition. (Note: All images have been rotated 45 degrees and the contrast has been increased for ease of streak identification).
We also examined the RHEED pattern through the transition to investigate the sample evolution in real time. To do so, we heated the sample in 5 °C increments from 25 °C to 100 °C recording the diffraction pattern after each increment. Upon ramping the temperature, we found a marked change in the RHEED pattern at the critical temperature. We observed the disappearance of several peripheral streaks exhibited in the monoclinic phase as well as a strengthening of the intensity of the main streaks that create a repeating “rectangular” pattern as designated by (Fig. 2c and Supplementary Video S1). We propose that this change in diffraction pattern is likely due to the change in the atomic positions of the oxygen atoms when transitioning from the monoclinic to the rutile phase. During this transition there is a lattice parameter change of ~ −1.2 Å from a = 5.75 Å in the monoclinic phase to a = 4.55 Å in the rutile phase15.
Finally, we sought to compare our bulk VO2 simulations to a VO2 sample grown on TiO2(001). Upon examination of the VO2 film prior transition we find in (Fig. 2d) a plausible superposition of the monoclinic and rutile phases with a greater emphasis on the streak locations of the rutile phase. Especially in the case of (Fig. 2d), the non-transitioned locations have a distinctly rutile like pattern that suggests structural strain toward the rutile phase. Thus for (Figs 2d and S2c), we have interlaid the monoclinic and the rutile simulations to make the microstructure more apparent enabling fruitful comparison with experiment. This combined with a 0° rotation asserts that there is microstructural strain toward the rutile phase in the non-transitioned VO2 grown on TiO2. We then heated the sample to 100 °C to allow full IMT, which for this sample is ~42 °C where film substrate strain effects are known to play a key role in lowering the transition temperature12,18.
Once we heated the sample, we compared the simulations with the experimentally determined RHEED patterns. Here we find that the patterns for the 0° and the 90° azimuth positions, observed in (Fig. 2e), have several streaks well matched to the simulations. In comparison to the monoclinic phase, the center streak is missing but the four streaks that make up the “rectangular” shape of the pattern still persist with a slightly larger streak spacing than that of VO2 on c-Al2O3. We also see this in (Fig. S2d) the 45° azimuth position for the diffraction pattern, along with similar streaks seen in the simulation. Also, this pattern now exhibits a slight slant to the locations of the streak that would occupy the same lattice plane. This is likely due to the structural strain on the atomic locations on the lattice, namely the oxygen locations. Slight displacements off the main axis of the lattice could also cause shifts to the pattern, which is the case for the 45° direction. This slight displacement is also visible in the 0° and the 90° azimuth in-plane directions, but the shifts are lesser here since these directions represent less sensitive directions to oxygen displacements.
We examined the RHEED pattern through the transition to see changes due to the IMT. In order to do this, we heated the sample in 5 °C increments from 25 °C to 100 °C recording the diffraction pattern at each increment in agreement with the previous VO2 on c-Al2O3 sample. Upon ramping through the temperature increments, we found a marked change that occurred at the critical temperature. We observed the disappearance of the center streak in the rutile state that was apparent in the non-transitioned phase as demonstrated by (Fig. 2f) and Supplementary Video S2. This change occurs abruptly upon the IMT. As previously described, due to the strong dependence of the streak patterns to the oxygen location and the larger degree of structural strain on the sample, this large pattern adjustment agrees with the expected strained monoclinic structure as previously discussed.
The structural characterization across the transition for these samples provides a suitable framework for subsequent characterization of the photo responsivity of the same samples described below.
Photocurrent analysis
As demonstrated by (Fig. 3a) the VO2/TiO2 band structure illustrates that such film substrate heterostructure as a promising candidate for an efficient near-UV to deep UV photo-sensor; which is of interest due to lack of efficient, low power photodetectors in this spectral range. Thus, we examined how the photocurrent produced in these samples under 405 nm illumination is affected through the IMT. Due to the close match between the energy of the incoming photon and the TiO2 substrate band gap energy, the electron carriers are excited from the O2p band into the conduction band of the TiO2 substrate. By exciting carriers in the TiO2 substrate, the holes left behind in the TiO2 O2p band are then transferred to the VO2 O2p band leading to carriers from the d|| band of VO2 moving to the O2p band thus inducing a photocurrent6. Worth noting is that strain effects on the structure of the VO2 could result in adjustments to the band structure shown. We anticipate that the d|| and d||* bands of the VO2 could exist closer together energetically resulting in a more metallic electronic structure as the lattice is strained toward the rutile phase.
The electronic structure of VO2 on TiO2 and c-Al2O3 as well as studies of the photoelectric properties of VO2 on TiO2. (a) Schematic band diagram of the hole transfer mechanism for VO2 on TiO2 and c-Al2O3 where the photon energy is demonstrated for the VO2 on TiO2 as being sufficient to excite carriers in the TiO2 layer and insufficient in the case of VO2 on c-Al2O3. Where the hole transfer is designated by the segmented line and the carrier movement is designated by the solid lines6,19. (b) Reflectivity measurements with a 405 nm diode laser illuminating VO2 deposited on TiO2(001) as the sample underwent a thermally induced IMT via heating and subsequent cooling where the dashed line indicated transition temperature. (c) The photocurrent switching of VO2 on TiO2(001) upon solely 405 nm illumination as the laser power was varied. The light was held on for 40 seconds post switching. (d) The same photocurrent switching measurement of VO2 on c-Al2O3(0001) where the 405 nm laser power was varied. The light was held on for 40 seconds post switching. (e) The photocurrent switching cycle for VO2 on TiO2(001) upon 405 nm illumination switching over 10 cycles. (f) External quantum efficiency measurement for VO2 on TiO2 as varied with laser power. (g) The average photocurrent and optical response of VO2 on TiO2(001) as the sample was thermally ramped through the optical transition where the 405 nm laser power was 1 mW through the thermal ramping where the dashed line indicates transition temperature.
This photocurrent is only possible in the monoclinic phase as once VO2 undergoes the phase transition to the rutile phase the band gap collapses and it takes on characteristics of a metal, therefore producing no photocurrent. To this end, we first demonstrated the optical response of VO2 on TiO2 as it undergoes the thermal IMT. This optical transition is demonstrated in (Fig. 3b) where a decrease in voltage is correlated to a decrease in resistivity due to the IMT. We then investigated if a photocurrent could be produced under solely illumination of 405 nm light, chosen because its energy (~3.06 eV) is close to the band gap of TiO2 is (~3.0 eV)20 thus providing optimal photon energy to properly excite carriers in the TiO2 substrate as demonstrated by (Fig. 3a). In (Fig. 3c) we show that the 405 nm laser produces a clear photocurrent, and its magnitude is correlated with the laser power. We also observed a reliable switching of the photocurrent switching through multiple on/off cycles, demonstrating its survivability as demonstrated by (Fig. 3e). We additionally examined the quantum efficiency of the TiO2 samples under various laser powers in (Fig. 3f). The external quantum efficiency was calculated via
Where ΔI is the photoexcited current, P is the total light power irradiated on the VO2, h is Planck’s constant, e is electron charge, and λ is the excitation wavelength. We find that as we decrease the laser power on the surface of the VO2 the quantum efficiency exhibits a 2000% increase through the laser power reduction as demonstrated by (Fig. 3f). This is potentially due to the carrier excitation in the substrate being inefficient at high powers due to the large influx of photons and the number of available carriers is less than the number of photons available to excite said carriers; as the laser power is decreased the photon to carrier excitation matching (and thus the overall carrier excitation per photon absorbed) is improved, in turn improving the overall quantum efficiency. We also attempted to measure the photocurrent of VO2 on c-Al2O3(0001) upon 405 nm illumination. Since the 405 nm light’s photon energy is insufficient to excite carriers in c-Al2O3(0001) across its ~8.8 eV21 band gap,, we see limited to no photocurrent in the VO2 film as shown in (Fig. 3d). We in fact found that the produced photocurrent was 4 orders of magnitude smaller and on the order of the dark current, the small current that exists in photosensitive devices when in absence of photons, leading to the lack of defined features in the corresponding graph as opposed to VO2 on TiO2(001).
Finally, we examined the photocurrent produced upon heating the sample and correlated the transition with the photocurrent. As demonstrated in (Fig. 3g), we observe that as the sample is heated, a marked change in the photocurrent produced is noted, approaching zero until it eventually reaches zero. This reduction in photocurrent is due to the IMT where the collapse of the band gap results in VO2 becoming metallic preventing the production of a photocurrent as previously described. Here our reflected optical signal tracks the phase change optically through the transition, where a reduction in optical response is correlated with the phase change from monoclinic to rutile. However, we note that the photocurrent reduces to zero before the optical transition is complete. This finding would suggest that the migration of carriers in VO2 is greatly reduced before the band gap is fully collapsed. One possible explanation follows from earlier descriptions of the nucleation of the metallic phase upon heating where localized puddles of the metallic phase in the VO2 sample become so prevalent such that hole migration from the substrate to the film is substantially impeded preventing the production of photocurrent before the film is fully transitioned to the metallic phase1,4,22.
Thus, local “puddle” formation results in loss of effective carrier movement before the sample has fully transitioned such that a global surface current is no longer possible reducing the photocurrent to zero although the surface of the sample is not fully phase transitioned. This “puddling” across the thermally induced IMT in VO2 has been extensively studied and reported1,4,22.
Discussion
We have shown that epitaxially grown VO2 on both c-Al2O3 and TiO2(001) by pulsed DC sputtering exhibit good crystal character and a low degree of mosaicity. They also exhibit a characteristic surface morphology with terracing plateaus due to strain. We have been able to accurately compare simulations of VO2 RHEED patterns consistent with our experimental data on the films that we have studied. We have also presented a first of its kind RHEED temperature transition analysis for VO2 in which we were able to analyze and conceptualize the structural phase transition of VO2 on both c-Al2O3 and TiO2(001), evidencing the dynamical changes that VO2 undergoes through its critical transition temperature.
We also determined the photocurrent switching of VO2 on TiO2(001) and c-Al2O3(0001) at 405 nm and saw a stark reduction in photocurrent for VO2 grown on c-Al2O3(0001). This reduction in photocurrent is likely a product of the high resistivity and large substrate band gap as well as VO2 films with much rougher surface, where roughness also contributes to scattering effects resulting in lower carrier efficiency thus reducing the photocurrent. We have also demonstrated a marked increase in the quantum efficiency of the VO2 on TiO2 with decreasing laser power seeing as large as a 2000% difference in quantum efficiency from 4.6 mW to 250 nW laser power. Finally, we have demonstrated how the IMT influences the produced photocurrent under illumination determining that as the VO2 transitions through the IMT to the metallic state there is a significant reduction of produced photocurrent, reaching zero value once the global state of the surface is metallic hindering production of photocurrent for VO2.
Methods
Sample growth
Epitaxial VO2 films were sputtered-deposited on one side polished (1-sp) c-Al2O3(0001) and 1-sp TiO2(001) substrates in an ultrahigh vacuum (UHV) deposition system with a base pressure ~3.0 × 10−7 Torr range. The films were deposited via reactive D.C. pulsed magnetron sputtering with a vanadium target of 99.95% purity in a 90% Ar and 10% O2 environment at 550 °C. Prior to deposition the substrates were annealed in-situ for 30 minutes at 600 °C to de-gas and recrystallize the top-most surface layers on each substrate. The target voltage was periodically pulsed to a positive charge to eject the excess insulating oxide layers that usually poison the surface of the target during sputter deposition. Growth rates were determined via X-Ray Reflectivity (XRR) in calibration samples. The films were evaluated via Reflection High-Energy Electron Diffraction (RHEED), Atomic Force Microscopy (AFM), X-Ray Diffraction (XRD), and Van der Pauw 4-point probe (VDP) measurement.
Rheed, AFM, XRD, and photocurrent characterization
RHEED was used to determine the crystallographic structure of the film surface. This technique utilizes glancing incident angle geometry for an electron beam on the probed surface allowing the beam to sample mainly the top-most layers of the film13,14. Thus, RHEED enables qualitative and quantitative descriptions of the in-plane surface microstructure. The space groups for the two phases of VO2 across the IMT are P42/mmm(136) for rutile with lattice spacing a = 4.55 Å, c = 2.88 Å, and P21/c (14) for monoclinic with lattice spacing a = 5.75 Å, b = 5.42 Å, c = 5.38 Å (we note that the drastic change in c lattice spacing is due to a doubling of the unit cell along the c direction)15. This information allows comparisons of various experimental diffraction patterns to predicted models for both phases. This technique also allows us to map the crystallographic changes through the thermally induced IMT.
The surface morphology of the samples was characterized via ex-situ AFM. The AFM images were collected using a Nanotec Cervantes AFM instrument. The AFM was operated in non-contact mode with an AppNano ACTA AFM tip with nominal tip diameter ~10 nm. The software WSxM from Nanotec was used for analysis of the AFM images23. All images were flattened in the WSxM program and the images were processed via the root mean squared (RMS) analysis tool in the program. The RMS roughness gives an average estimate of the surface roughness of the film, and this quantitative description can be paired to the qualitative description obtained from RHEED.
The microstructure of the films throughout their full thickness and out of plane crystallographic information was determined via XRD. The instrument used was a four-circle diffractometer with a quasi-monochromatic CuKalpha (λ = 1.5406 Å) beam. This technique was used to determine the out of plane lattice parameter, grain size24, and mosaicity of the films.
The electrical and quantum efficiency information was determined with an MMR Technology Variable Temperature Microprobe System (VTMP) and a 405 nm diode laser for the VO2 grown on TiO2 as well as a 1520 nm laser for the VO2 grown on c-Al2O3. The sheet resistance was determined via the Van der Pauw method25. The samples were illuminated with a 405 nm laser and the produced photocurrent was measured. The samples were then thermally ramped through the IMT and both the photocurrent, using the VTMP, and the optical response, using a 405 nm photodetector, were recorded simultaneously for each sample.
RHEED simulations
The simulations for this experiment were carried out using the RHEEDsim MATLAB program26. This software utilizes the atomic positions of the atoms in VO2 films as well as TiO2 and c-Al2O3 substrates determined from the space groups of the respective molecules. The program utilizes a kinematic approach for single scattering events, and then approximates the Ewald sphere to a planar surface to compute streak intensities. Additionally, intensity modulation extends to within the z direction26. The form factors for atomic scattering were determined via NIST standards for X-ray form factor, attenuation, and scattering tables27. This simulations yield realistic representations for comparison to experimentally determined RHEED diffraction patterns.
References
Wang, L. et al. Distinct Length Scales in the VO2 Metal-Insulator Transition Revealed by Bi-chromatic Optical Probing. Adv. Opt. Mater. 2, 30–33 (2014).
Morin, F. J. Oxides which show a metal-to-insulator transition at the neel temperature. Phys. Rev. Lett. 3, 34–36 (1959).
Zylbersztejn, A. & Mott, N. F. Metal-insulator transition in vanadium dioxide. Phys. Rev. B 11, 4383–4395 (1975).
Qazilbash, M. M. et al. Electrostatic modification of infrared response in gated structures based on VO2. Appl. Phys. Lett. 92, 241906 (2008).
Chae, B. G., Kim, H. T., Youn, D. H. & Kang, K. Y. Abrupt metal-insulator transition observed in VO2 thin films induced by a switching voltage pulse. Phys. B Condens. Matter 369, 76–80 (2005).
Muraoka, Y., Yamauchi, T., Ueda, Y. & Hiroi, Z. Efficient photocarrier injection in a transition metal oxide heterostructure. J. Phys. Condens. Matter 14, L757–L763 (2002).
Zhou, S., Wang, Y. & Wang, Z. Doublon-holon binding, Mott transition, and fractionalized antiferromagnet in the Hubbard model. https://doi.org/10.1103/PhysRevB.89.195119 (2013).
Oka, T. & Aoki, H. Ground-state decay rate for the Zener breakdown in band and mott insulators. Phys. Rev. Lett. 95, 137601 (2005).
Oka, T. Nonlinear doublon production in a Mott insulator: Landau-Dykhne method applied to an integrable model. Phys. Rev. B - Condens. Matter Mater. Phys. 86, 075148 (2012).
Tellier, C. R. & Tosser, A. J. Thermoelectric power of metallic films in the Mayadas-Shatzkes model. Thin Solid Films 41, 161–166 (1977).
Tellier, C. R. A theoretical description of grain boundary electron scattering by an effective mean free path. Thin Solid Films 51, 311–317 (1978).
Muraoka, Y. & Hiroi, Z. Metal–insulator transition of VO2 thin films grown on TiO2 (001) and (110) substrates. Appl. Phys. Lett. 80, 583–585 (2002).
Mahan, J. E., Geib, K. M., Robinson, G. Y. & Long, R. G. A review of the geometrical fundamentals of reflection high‐energy electron diffraction with application to silicon surfaces. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 8, 3692–3700 (1990).
Droopad, R., Williams, R. L. & Parker, S. D. RHEED intensity oscillations observed during the MBE growth of InSb (100). Semicond. Sci. Technol. 4, 111–113 (1989).
Leroux, C., Nihoul, G. & Van Tendeloo, G. From VO2(B) to VO2(R): Theoretical structures of VO2 polymorphs and in situ electron microscopy. Phys. Rev. B 57, 5111–5121 (1998).
Gražulis, S. et al. Crystallography Open Database (COD): an open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 40, D420–D427 (2012).
Gražulis, S. et al. Crystallography Open Database - An open-access collection of crystal structures. J. Appl. Crystallogr. 42, 726–729 (2009).
Radue, E. et al. Substrate-induced microstructure effects on the dynamics of the photo-induced metal–insulator transition in VO 2 thin films. J. Opt. 17, 025503 (2015).
Shin, S. et al. Vacuum-ultraviolet reflectance and photoemission study of the metal-insulator phase transitions in VO2, V6O13, and V2O3. Phys. Rev. B 41, 4993–5009 (1990).
Pascual, J., Camassel, J. & Mathieu, H. Resolved quadrupolar transition in TiO2. Phys. Rev. Lett. 39, 1490–1493 (1977).
Robertson, J. Band offsets of wide-band-gap oxides and implications for future electronic devices. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 18, 1785 (2000).
Qazilbash, M. M. et al. Mott Transition in VO2 Revealed by Infrared Spectroscopy and Nano-Imaging. Science (80-.). 318, 1750–1753 (2007).
Horcas, I. et al. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 78, 013705 (2007).
Patterson, A. L. The scherrer formula for X-ray particle size determination. Phys. Rev. 56, 978–982 (1939).
van der Pauw, L. J. A Method of Measuring The Resistivity And Hall Coefficient On Lamellae Of Arbitrary Shape. Phillips Tech. Rev. 20, 220–224 (1958).
Wang, K. & Smith, A. R. Efficient kinematical simulation of reflection high-energy electron diffraction streak patterns for crystal surfaces. 1–9 (2011).
Chantler, C. T. Detailed tabulation of atomic form factors, photoelectric absorption and scattering cross section, and mass attenuation coefficients in the vicinity of absorption edges in the soft X-ray (Z = 30–36, Z = 60–89, E = 0.1–10 keV) – addressing convergence iss. J. Synchrotron Radiat. 8, 1124–1124 (2001).
Acknowledgements
The project depicted is sponsored by the Department of the Defense, Defense Threat Reduction Agency (HDTRA 1-16-1-0056). The content of the information does not necessarily reflect the position or the policy of the federal government, and no official endorsement should be inferred. We also acknowledge the William & Mary Applied Research Center for the usage of their instruments.
Author information
Authors and Affiliations
Contributions
J.A.C., S.E.M., D.B.B. and M.R.B. contributed to the experiments. J.A.C. analyzed the data. J.A.C. prepared the figures in the manuscript. J.A.C. wrote the main manuscript. I.N. and R.A.L. supervised the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41598_2019_45806_MOESM1_ESM.doc
Supplementary Information for Structural and Photoelectric Properties of Epitaxially Grown Vanadium Dioxide Thin Films on c-Plane Sapphire and Titanium Dioxide
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Creeden, J.A., Madaras, S.E., Beringer, D.B. et al. Structural and Photoelectric Properties of Epitaxially Grown Vanadium Dioxide Thin Films on c-Plane Sapphire and Titanium Dioxide. Sci Rep 9, 9362 (2019). https://doi.org/10.1038/s41598-019-45806-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45806-8
This article is cited by
-
Insulator-to-metal transition in ultrathin rutile VO2/TiO2(001)
npj Quantum Materials (2022)
-
Structural mapping of single-crystal VO2 microrods through metal-to-insulator phase transition
Journal of Materials Science (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.