Abstract
The bulge area of the hair follicle contains hair-follicle-associated pluripotent (HAP) stem cells. Here, we present effective cryopreservation procedures of the human hair follicle that preserve the differentiation potential of HAP stem cells. Whole hair follicles isolated from human scalp were cryopreserved by a slow-rate cooling medium and stored in liquid nitrogen. A careful thawing method was used to collect the upper parts of the human hair follicles which were cultured for four weeks in a Dulbecco’s Modified Eagle’s Medium with fetal bovine serum (FBS). Proliferating hair follicle cells were then shifted to DMEM/Ham’s Nutrient Mixture F-12 medium without FBS and allowed to grow for one week. These proliferating cells were able to produce HAP stem cell colonies with multilineage differentiation capacity. They produced keratinocytes, smooth muscle cells, cardiac muscle cells, neurons and glial cells. Interestingly, these cryopreserved hair follicles produced pluripotent HAP stem cell colonies similar to fresh follicles. These findings suggest that the cryopreserved whole human hair follicle preserves the ability to produce HAP stem cells, which will enable any individual to preserve a bank of these stem cells for personalized regenerative medicine.
Similar content being viewed by others
Introduction
Hair-follicle bulge stem cells were originally shown to have the capacity to form hair-follicle cells, sebaceous-gland basal cells, and epidermis1,2,3,4,5. In addition to normal tissues homeostasis, these stem cells are involved in the regeneration of hair follicles and acute epithelial wound repair6,7. Subsequently, we discovered nestin-expressing stem cells in the bulge area of the hair follicle8,9. We found that nestin-expressing stem cells from both mouse and human have multilineage differentiation capacity that could produce neurons and other cell types10,11,12,13. We termed these nestin-expressing stem cells hair-follicle-associated pluripotent (HAP) stem cells. Previously, we demonstrated that isoproterenol and hypoxia can enhance the HAP stem cells to form cardiac muscle cells13,14. Further, it was shown that HAP stem cells from both mouse and human can fully repair the severed sciatic nerve and spinal cord of mice15,16,17 and showed homing capacity in experimental cortical dysplasia18.
Using a slow-rate cooling method, we previously demonstrated that cryopreserved whole-mouse hair follicles were able to maintain the pluripotency of HAP stem cells19. In the present study, we established effective cryopreservation procedures of the whole human hair follicle by slow-rate cooling and storage in liquid nitrogen, which preserved the multilineage-differentiation capacity of human HAP stem cells.
Materials and Methods
Isolation of human scalp skin samples
The human HAP stem cells were isolated from specimens obtained with surgery of normal human scalp from five patients20. The age of five patients ranged from 32 to 72 years. All patients had given informed consent to Kitasato University, School of Medicine to perform this study. This study was done with the approval of the Kitasato University Medical Ethics Organization. Experiments in the present study were performed per the Declaration of Helsinki guidelines and in agreement with national regulations for the experimental use of human material20.
Cryopreservation of human whole hair follicle
Cryopreservation of human whole hair follicles was done as previously reported19,20. Five whole hair follicles were extracted from the scalp and placed to cryovials19,20, followed by the addition of TC-Protector medium (DS Pharma Biomedical, Osaka, Japan). Cryovials were then kept overnight in a −80 °C freezer. After that they were placed in a liquid nitrogen tank. Stored cryovials containing whole hair follicles were thawed in a 37 °C water bath by gentle shaking. These thawed cells were divided in three fractions (upper, middle and lower). For inducing differentiation, the upper parts of hair follicles were used. The upper parts of hair follicles were dispersed in medium containing fresh DMEM (Sigma-Aldrich) with 10% FBS, 2 mM L-glutamine (Gibco), 10 mM Hepes (MP Biomedicals) and 50 µg/ml gentamycin (Gibco) in 6-well Corning Flat Bottom Cell Culture plates20 (Fig. 1).
Immunofluorescence staining and flow cytometry analysis of the differentiated cells
Immunofluorescence staining was done as previously reported19,20. Immunostaining with primary antibodies for identification of differentiated cells and HAP stem cell colonies, for stem cell-markers are presented in Table 1. The following secondary antibodies were used: goat anti-mouse IgG and anti-mouse IgM, goat anti-rabbit IgG, goat anti-rat IgM, and donkey anti-goat IgG, conjugated to Alexa Fluor® 568 or Alexa Fluor® 594 (1:400; Molecular Probes). 4′, 6-diamino-2-phenylindole, dihydrochloride (DAPI) (Molecular Probes) was used to stain DNA.
For flow cytometry analysis, the following secondary antibodies were used: goat anti-mouse IgG H&L phycoerythrin (1:500; Abcam), goat anti-chicken IgY biotinylated (1:500; R&D) antibodies, and Brilliant Violet 421TM streptavidin (1:500; BioLegend).
Real-time RT-PCR of stem cell marker genes
RT-PCR protocols to detect stem cell marker genes were performed as previously described19. Using RNeasy Plus Mini kit (QIAGEN), total RNA was extracted from 100 HAP stem cell colonies cultured from fresh and cryopreserved hair follicles. A high capacity RNA to cDNA kit (ABI) was used to synthesize c-DNA. CFX96 (Bio-Rad) with TaqMan Gene Expression Assays (ABI) was used to perform real-time PCR. The following TaqMan Probes were utilized: nestin: Hs00707120_s1, Oct3/4: Hs00999632_gl, Nanog: Hs02387400_g1, and r18s: Hs99999901_s1. Reference gene (r18s)19 was used to normalize the mRNA levels19.
Western blotting
Western blotting was done as previously described19. Total proteins (30 μg/well) were extracted from HAP stem cell colonies cultured from fresh and cryopreserved hair follicles. Sodium dodecylsulfate polyacrylamide (4~20%) was used to perform electrophoresis. A immobilon-P membrane (Millipore Corporation) was used to transfer proteins. Specific protein on Immobilon-P were detected by rabbit polyclonal anti-nestin antibody (1:100), rabbit polyclonal anti-Oct4 antibody (1:500, Abcam), and rabbit polyclonal anti-GAPDH antibody (1:4000, BioLegend). The secondary antibody was peroxidase-conjugated protein A/G (Pierce). An enhanced chemiluminescence (ECL), together with a Western Blotting Detection System (Amersham Biosciences), were used to detect protein. ECL was measured by a Light-Capture CCD Camera System (ATTO, Tokyo, Japan).
Statistical analysis
The experimental data represent the mean (±standard deviation, SD). Statistical analysis was conducted using an unpaired two-tailed students t-test. A p-value of <0.05 is considered statistically significant.
Results
Cryopreserved human hair follicles maintain the differentiation capacity of HAP stem cells
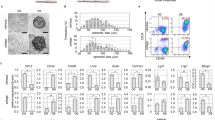
To test the effect of cryopreservation on human hair follicles, we thawed and cultured them for four weeks in DMEM containing 10% FBS. Then the upper parts of the hair follicles were transferred to cell-culture dishes. The attachment rate of the hair follicles and numbers of proliferating cells were quantified. Fresh hair follicles (non-frozen control) showed an 82.2 ± 12.2% attachment rate and 5.0 ± 1.7 × 104 growing cells/human hair follicle. Cryopreserved human hair follicles showed an 83.1 ± 20.3% attachment rate and 3.9 ± 2.0 × 104 growing cells/ hair follicle. These results demonstrated that cryopreserved human hair follicles have the same outgrowth ability as fresh human hair follicles (Fig. 2a,b).
After culturing the upper part of human hair follicles in DMEM containing 10% FBS for four weeks, human HAP stem cells proliferated and differentiated to nestin+ and βIII-tubulin+ neurons, GFAP+ glial cells, K15+ keratinocytes, SMA+ smooth-muscle cells, and cTnT+ cardiac muscle cells (Fig. 3). The following are the quantitative analysis of the differentiation ability of HAP stem cells: βIII-tubulin+ neurons, fresh 60.4 ± 16.6%, cryopreserved 55.1 ± 15.0%; SMA+ smooth muscle cells, fresh 18.3 ± 11.4%, cryopreserved 19.4 ± 11.0%; K15+ keratinocytes, fresh 5.2 ± 1.3%, cryopreserved 5.1 ± 3.1%; Troponin T+ cardiac muscle cells, fresh 2.2 ± 1.6%, cryopreserved 1.4 ± 1.5%; GFAP+ glial cells, fresh 7.3 ± 9.2%, cryopreserved 8.1 ± 9.8% (Table 2). These results show that HAP stem cells grown from cultured cryopreserved human hair follicles have a similar proliferation and pluripotent differentiation capacity as fresh human hair follicles.
Cell types differentiated from the cultured upper part of cryopreserved hair follicles. Nestin- and βIII-tubulin-positive neurons, GFAP-positive glial cells, K15-positive keratinocytes, smooth muscle actin (SMA)-positive smooth muscle cells, and troponin (cTnT)-positive cardiac muscle cells. Staining for nestin, βIII-tubulin, GFAP, K15, SMA, and cTnT is shown in red color in corresponding panels. DAPI is shown in blue color in each panel. Scale bar = 100 µm.
Cultured cryopreserved human hair follicles produce HAP stem cell colonies
Outgrowing cells from the upper part of the cryopreserved hair follicle were also cultured for a week in DMEM/F12 medium without FBS containing B-27, and every two days were supplemented with basic fibroblast growth factor (bFGF). These cultured cells were able to produce similar number of HAP stem cell colonies as the upper part of fresh human hair follicles. HAP stem-cell colonies formed were: 49.2 ± 51.5 from fresh human hair follicles and 48.6 ± 30.9 from cryopreserved human hair follicles. These results show that cryopreserved human hair follicles have the same colony-forming ability as fresh human hair follicles (Fig. 2c).
Immunofluorescence staining for stem cell marker expression in HAP stem cell colonies from cryopreserved hair follicles was performed on day 35 of culture, which showed SSEA1-negative (SSEA-1−), SSEA3-positive (SSEA-3+), SSEA4-positive (SSEA-4+), Nanog-positive, Oct3/4-positive, and nestin-positive (Fig. 4), which is similar to the HAP stem-cell colonies obtained from freshly-cultured human hair follicles20.
Stem-cell marker expression in HAP stem cell colonies produced from cultured cryopreserved human hair follicles. SSEA1-negative, SSEA3-positive, SSEA4-positive, Nanog-positive, Oct3/4-positive, and nestin-positive. Staining for SSEA1, SSEA3, SSEA4, Nanog, Oct3/4 and nestin is shown in red color in corresponding panels. DAPI is shown in blue color in each panel. Scale bar = 100 µm.
The HAP stem cell colonies that were obtained from the upper part of cryopreserved human hair follicle were cultured again back in DMEM with 10% FBS. After the switch into 10% FBS-containing DMEM for 2 weeks, the differentiating cells moved away from nestin-expressing HAP stem cell colonies and formed 4.0 ± 2.6 × 103 differentiated cells/HAP-stem-cell colony. The HAP stem cell colonies that were obtained from the upper part of cultured cryopreserved human hair follicles formed 1.6 ± 1.2 × 103 differentiated cells/HAP stem cell colony (Fig. 2d).
The cultured HAP stem cell colonies differentiated to nestin+ and βIII-tubulin+ neurons, GFAP+ glial cells, K15+ keratinocytes, SMA+ smooth muscle cells, and troponin+ cardiac muscle cells (Fig. 5). Immunofluorescence staining was performed on day 49. We then compared HAP stem cell colonies, produced from both the upper part of fresh human hair follicles and from the upper part of cryopreserved human hair follicles, for pluripotent differentiation capability. Both fresh and cryopreserved follicles had similar HAP stem-cell pluripotency and differentiating capacity as they produced multiple cell types: βIII-tubulin+ neurons, fresh 76.3 ± 3.0%, cryopreserved 67.8 ± 6.1%; SMA+ smooth muscle cells, fresh 12.4 ± 5.2%, cryopreserved 15.9 ± 3.7%; K15+ keratinocytes, fresh 5.0 ± 3.7%, cryopreserved 2.2 ± 1.5%; Troponin T+ cardiac muscle cells, fresh 0.4 ± 0.4%, cryopreserved 0.4 ± 0.5%; GFAP+ glial cells, fresh 9.2 ± 11.3%, cryopreserved 6.3 ± 5.4% (Table 2).
Cell differentiation from human HAP stem-cell colonies produced from cultured cryopreserved hair follicles. HAP stem cell colonies differentiated to nestin and βIII-tubulin-positive neurons, GFAP-positive glial cells, K15-positive keratinocytes, SMA-positive smooth muscle cells, and cTnT-positive cardiac muscle cells. Staining for nestin, βIII-tubulin, GFAP, K15, SMA, and cTnT is shown in red color in corresponding panels. DAPI is shown in blue color in each panel. Scale bar = 100 µm.
Gene expression analysis in HAP stem cell colonies produced from cultured cryopreserved hair follicles compared to fresh hair follicles
RT-PCR analysis (on day 35) showed the relative mRNA level of the HAP stem cell colonies formed from cultured cryopreserved hair follicles: for nestin 4.09 ± 0.90; for Oct3/4 0.71 ± 0.4; for Nanog 0.38 ± 0.31. The values are normalized to the expression of these genes for fresh fair follicles (Fig. 6, Supplementary Information). These results show that the mRNA levels of stem-cell marker genes such as nestin, Oct3/4, and Nanog were preserved in the HAP stem-cell colonies formed from cryopreserved human hair follicles.
The expression of nestin and Oct4 was compared by western blot analysis, between HAP stem cell colonies and cryopreserved hair follicles to fresh hair follicles. In comparison to HAP stem cell colonies from fresh hair follicles, the relative protein levels of HAP stem cell colonies formed from cryopreserved hair follicles were nestin 1.02 ± 0.14 and cultured Oct4 1.06 ± 0.25 (Fig. 6). These results show that HAP stem-cell colonies formed from cultured cryopreserved human hair follicles maintained normal protein levels.
Discussion
The human hair follicle cryopreservation method presented in this study is suitable to produce large amount of HAP stem cells for future stem-cell-related regeneration medicine in the clinic. With this method, each individual will be able to obtain and bank their own HAP stem cells, which will be ideal because they will be free from immunological rejection as well as ethical or oncogenic issues compared to human oocytes, embryonic stem (ES) cells or induced pluripotent stem (iPS) cells.
The unique capacity of stem cells to produce various cell types is vital for regenerative medicine, drug screening, and tissue engineering. For efficient industrial-scale and clinical use of stem cells, vigorous and functional cryopreservation methods are required. Cryopreservation methods have been reported using ES cells, mesenchymal stem cells (MSCs), iPS and several somatic stem cells21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40. ES cells and iPS cells are sensitive to cryopreservation, which means they required specialized methods to maintain optimal cell viability and recovery30. Cryopreservation is also important to minimize metabolic activity, preserve viability, and differentiation potential of stem cells31,32. Zhao et al.24 reported that fetal human liver hematopoietic progenitor cells (LHSCs) can be cryopreserved for approximately two years with fully functional differentiation ability. Human dental pulp stem cells were shown to maintain proliferation and differentiation potential even after being stored in liquid nitrogen for two years33. Marquez-Curtis et al.34 demonstrated the ability of placental mesenchymal stem cells (MSCs) to differentiate after cryopreservation. In a study using bone marrow-MSCs (BM-MSCs), Shen et al.35 have shown that BM-MSCs retain multilineage differentiation ability even after 20 years of cryopreservation. Successful differentiation potential and viability was also found in cryopreserved adipose-derived stem cells (ADSCs)36. Hill et al.37 reported that cryopreservation does not affect the multipotency and differentiation capacity of human skin derived precursor cells (SDPCs). Lavoie et al.38 found no difference in growth potential of skin epithelial stem cells before and after cryopreservation. Previously, we showed that mouse whisker hair follicles when cryopreserved retained HAP stem cells and growth capacity after transplantation as well as viability of the HAP stem cells39. Gho et al.40 reported that cryopreserved human hair follicle bulge-derived stem cells that grew out from fresh follicles were able to maintain multilineage differentiation ability. Recently, several reliable long-term cryopreservation methods were developed for various kinds of stem cells with full preservation of multipotency41,42,43,44,45,46.
Here, we showed that the whole human hair follicle when cryopreserved by slow-rate cooling procedure maintains the pluripotency of their stem cells. The thawed follicles can be transplanted directly or cultured to produce HAP stem cell colonies for differentiation to nerve, glial, smooth muscle, cardiac, keratinocyte, and other cells. The cryopreservation method presented here will enable each individual to keep a bank of pluripotent stem cells from the cryopreserved hair follicles for future clinical application. The entire follicle can be cryopreserved without culture or other manipulation which makes this method potentially widely used.
References
Cotsarelis, G., Sun, T. T. & Lavker, R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61(7), 1329–37 (1990).
Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T.-T. & Lavker, R. M. Involvement of Follicular stem cells in forming not only the follicle but also the epidermis. Cell 102, 451–61 (2000).
Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104, 233–45 (2001).
Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L. & Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–48 (2004).
Morris, R. J. et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22, 411–17 (2004).
Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11(12), 1351–4 (2005).
Brownell, I., Guevara, E., Bai, C. B., Loomis, C. A. & Joyner, A. L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8(5), 552–65 (2011).
Li, L. et al. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci USA 100(17), 9958–61 (2003).
Uchugonova, A., Duong, J., Zhang, N., König, K. & Hoffman, R. M. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem 112(8), 2046–50 (2011).
Amoh, Y. et al. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA 102, 5530–4 (2005).
Yashiro, M. et al. From hair to heart: nestin expressing hair follicle associated pluripotent (HAP) stem cells differentiate to beating cardiac muscle cells. Cell Cycle 14, 2362–6 (2015).
Amoh, Y. et al. Multipotent nestin-expressing stem cells capable of forming neurons are located in the upper, middle, and lower part of the vibrissa hair follicle. Cell Cycle 11, 3513–17 (2012).
Yamazaki, A. et al. Isoproterenol directs hair follicle-associated pluripotent (HAP) stem cells to differentiate in vitro to cardiac muscle cells which can be induced to form beating heart muscle tissue sheets. Cell Cycle 15, 760–65 (2016).
Shirai, K. et al. Hypoxia Enhances Differentiation of Hair Follicle-Associated-Pluripotent (HAP) Stem Cells to Cardiac-Muscle Cells. J Cell Biochem 118(3), 554–558 (2017).
Yamazaki, A. et al. Implanted hair-follicle-associated pluripotent (HAP) stem cells encapsulated in polyvinylidene fluoride membrane cylinders promote effective recovery of peripheral nerve injury. Cell Cycle 16, 1927–32 (2017).
Obara, K. et al. Hair-Follicle-Associated Pluripotent (HAP) Stem Cells Encapsulated on Polyvinylidene Fluoride Membranes (PFM) Promote Functional Recovery from Spinal Cord Injury. Stem Cell Rev Reports 15(1), 59–66 (2019).
Liu, F. et al. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle 10(5), 830–9 (2011).
Omidi, A. et al. Homing of allogeneic nestin-positive hair follicle-associated pluripotent stem cells after maternal transplantation in experimental model of cortical dysplasia. Biochem Cell Biol 93(6), 619–25 (2015).
Kajiura, S. et al. Cryopreservation of the hair follicle maintains pluripotency of nestin-expressing stem cells. Tissue Eng Part C Methods 21, 825–31 (2015).
Tohgi, N. et al. Human hair-follicle associated pluripotent (hHAP) stem cells differentiate to cardiac-muscle cells. Cell Cycle 16, 95–9 (2017).
Berz, D., McCormack, E. M., Winer, E. S., Colvin, G. A. & Quesenberry, P. J. Cryopreservation of hematopoietic stem cells. Am J Hematol 82(6), 463–72 (2007).
Cohen, R. I., Thompson M. L., Schryver B. & Ehrhardt R. O. Standardized cryopreservation of pluripotent stem cells. Curr Protoc Stem Cell Biol 28, Unit 1C. 14, https://doi.org/10.1002/9780470151808.sc01c14s28 (2014).
Takebe, Y. et al. Cryopreservation Method for the Effective Collection of Dental Pulp Stem Cells. Tissue Eng Part C Methods 23(5), 251–261 (2017).
Zhao, J., Hao, H. N., Thomas, R. L. & Lyman, W. D. An efficient method for the cryopreservation of fetal human liver hematopoeitic progenitor cells. Stem Cells 19, 212–218 (2001).
Nishigaki, T. et al. Highly efficient cryopreservation of human induced pluripotent stem cells using a dimethyl sulfoxide-free solution. Int J Dev Biol 55, 305–311 (2011).
Milani, P. et al. Cell freezing protocol suitable for ATAC-Seq on motor neurons derived from human induced pluripotent stem cells. Sci Rep 6, 25474 (2016).
Somal, A. et al. Impact of Cryopreservation on Caprine Fetal Adnexa Derived Stem Cells and Its Evaluation for Growth Kinetics, Phenotypic Characterization, and Wound Healing Potential in Xenogenic Rat Model. J Cell Physiol 232(8), 2186–2200 (2017).
Vakhshori, V. et al. Cryopreservation of Human Adipose-Derived Stem Cells for Use in ex vivo Regional Gene Therapy for Bone Repair. Hum Gene Ther Methods 29, 269–277 (2018).
Moraveji, S. F. et al. Optimizing methods for human testicular tissue cryopreservation and spermatogonial stem cell isolation. J Cell Biochem 120(1), 613–621 (2019).
Thompson, M. L., Kunkel E. J. & Ehrhardt R. O. Standardized Cryopreservation of Stem Cells. In: Srivastava A., Snyder E., Teng Y. (eds) Stem Cell Technologies in Neuroscience. Neuromethods, vol 126. Humana Press, New York, NY (2017).
Woods, E. J., Benson, J. D., Agca, Y. & Critser, J. K. Fundamental cryobiology of reproductive cells and tissues. Cryobiology 48, 146–156 (2004).
Baust, J. G., Gao, D. & Baust, J. M. Cryopreservation: An emerging paradigm change. Organogenesis 5, 90–96 (2009).
Zhang, W., Walboomers, X. F., Shi, S., Fan, M. & Jansen, J. A. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng 12(10), 2813–23 (2006).
Marquez-Curtis, L. A., Janowska-Wieczorek, A., McGann, L. E. & Elliott, J. A. Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology 71(2), 181–97 (2015).
Shen, J. L. et al. Effectiveness of human mesenchymal stem cells derived from bone marrow cryopreserved for 23–25 years. Cryobiology 64(3), 167–75 (2012).
Goh, B. C., Thirumala, S., Kilroy, G., Devireddy, R. V. & Gimble, J. M. Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med 1(4), 322–4 (2007).
Hill, R. P. et al. Generation and characterization of multipotent stem cells from established dermal cultures. PLoS One 7(11), e50742 (2012).
Lavoie, A. et al. Human epithelial stem cells persist within tissue-engineered skin produced by the self-assembly approach. Tissue Eng Part A 19(7–8), 1023–38 (2013).
Cao, W. et al. Extensive hair shaft growth after mouse whisker follicle isolation, Cryopreservation and transplantation in nude mice. PLoS One 10(12), e0145997 (2015).
Gho, C. G. et al. Isolation, expansion and neural differentiation of stem cells from human plucked hair: a further step towards autologous nerve recovery. Cytotechnology 68(5), 1849–58 (2016).
Yong, K. W. et al. Phenotypic and functional characterization of long-term cryopreserved human adipose-derived stem cells. Sci Rep 5, 9596 (2015).
Yuan, Y. et al. Efficient long-term cryopreservation of pluripotent stem cells at −80 °C. Sci Rep 6, 34476 (2016).
Sultani, A. B., Marquez-Curtis L. A., Elliott J. A. & McGann L. E. Improved cryopreservation of human umbilical vein endothelial cells: a systematic approach. Sci Rep 6, 34393 (2016).
Bissoyi, A., Bit, A., Singh, B. K., Singh, A. K. & Patra, P. K. Enhanced cryopreservation of MSCs in microfluidic bioreactor by regulated shear flow. Sci Rep 6, 35416 (2016).
Nevi, L. et al. Cryopreservation protocol for human biliary tree stem/progenitors, hepatic and pancreatic precursors. Sci Rep 7(1), 6080 (2017).
Zhang, X., Simmons, C. A. & Santerre, J. P. Alterations of MEK1/2-ERK1/2, IFNγ and Smad2/3 associated Signalling pathways during cryopreservation of ASCs affect their differentiation towards VSMC-like cells. Stem Cell Res 32, 115–125 (2018).
Acknowledgements
This study was partly funded by Grant-in-Aid for Scientific Research (C) 16K10173 from the Ministry of Education, Science, Sports, and Culture of Japan, a grant from the Ministry of Education, Culture, Sports, Science, and Technology of the Japan Government (Assistance for Strategic Creation of Research Basis, 2014–2016), and the Terumo Life Science Foundation (to Y. Amoh).
Author information
Authors and Affiliations
Contributions
K.O., Y.A. and R.M.H. were involved in study conception and design. K.O., Y.A., N.T., S.M., Y.H., N.A. and R.A. involved in acquisition of data. K.O., Y.A., N.T., S.M., Y.H., N.A., R.A. S.R.S. and R.M.H. analyzed and interpreted the data. Y.A. and R.M.H. prepared the manuscript. S.R.S. was involved in critical revision of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obara, K., Tohgi, N., Mii, S. et al. Hair-follicle-associated pluripotent stem cells derived from cryopreserved intact human hair follicles sustain multilineage differentiation potential. Sci Rep 9, 9326 (2019). https://doi.org/10.1038/s41598-019-45740-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45740-9
This article is cited by
-
Hair follicle associated pluripotent (HAP) stem cells jump from transplanted whiskers to pelage follicles and stimulate hair growth
Scientific Reports (2022)
-
Live slow-frozen human tumor tissues viable for 2D, 3D, ex vivo cultures and single-cell RNAseq
Communications Biology (2022)
-
Hair-follicle-associated pluripotent (HAP) stem cells differentiate into mature beating cardiomyocyte sheets on flexible substrates in vitro
Medical Molecular Morphology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.