Abstract
Recently we introduced a modified Mini Nutritional Assessment (MNA) Short Form (MNA-SF) and Long Form (MNA-SF) with operationalization of the ‘mobility’ and ‘neuropsychological problems’ items of the MNA using scores on Barthel Index mobility item and Mini Mental State Examination and Geriatric Depression Scale scores. We have now evaluated the abilities of this modified MNA-SF and MNA-LF to predict mortality in comparison with the standard MNA-SF and MNA-LF and the Nutritional Risk Screening 2002 (NRS 2002) and the Malnutrition Universal Screening Tool (MUST). A prospective analysis was performed in 240 hospitalised geriatric patients aged ≥ 65 years. Malnutrition and/or malnutrition risk were assessed using the modified MNA-SF and MNA-LF, the standard MNA-SF and MNA-LF, and the NRS 2002 and MUST. The modified MNA-SF and MNA-LF and the standard MNA-SF and MNA-LF assessments (all p < 0.05), but not NRS 2002 or MUST (all p ≥ 0.05), predicted six-month and/or one-year mortality. Prediction of six-month and/or one-year mortality by the modified MNA-SF was comparable with predictions by the standard MNA-SF and MNA-LF (all p ≥ 0.05). The modified MNA-LF showed better prediction of six-month and one-year mortality than the standard MNA-SF and MNA-LF (all p < 0.05). The modified MNA-LF (all adjusted p < 0.05), but none of the other instruments (all adjusted p ≥ 0.05), predicted six-month and one-year mortality independently of age, sex, frailty, comorbidity and ADL disability burden. The modified MNA-SF and MNA-LF emerged as potentially valuable tools for predicting mortality in patients hospitalised on geriatric wards.
Similar content being viewed by others
Introduction
Malnutrition is an acute, subacute or chronic state of overnutrition or undernutrition which can include an inflammatory component and impacts body composition and function1,2. Malnutrition is often found in individuals with impairments to body functions such as dysphagia3, immobility4, depression5, limited perception of hunger and thirst, polypharmacy6,7, and acute and chronic diseases7,8. Patients on geriatric wards frequently show a high prevalence of malnutrition or are at high risk of malnutrition7,9,10,11. Malnourished patients have higher risks of sarcopenia12,13, frailty14,15, morbidity8,16 and mortality8,16,17,18,19,20. Previous analysis has shown that malnourished geriatric patients experience clinical endpoints, including mortality, more often or, strictly speaking, earlier than well-nourished people of the same age8,16,17. There are therefore advantages in detecting malnutrition and the risk of malnutrition early to drive timely dietary interventions in patients on geriatric wards.
Several different screening tools for malnutrition and/or the risk of malnutrition have been introduced. These include the Mini Nutritional Assessment (MNA) Short Form (MNA-SF)21,22 and Long Form (full MNA or MNA-LF)23, the Nutritional Risk Screening 2002 (NRS 2002)24,25, and the Malnutrition Universal Screening Tool (MUST)26, among others. Of these, the MNA21,22,23 and in particular the MNA-SF21,22 may be the instruments of choice in all geriatric healthcare settings, including patients on geriatric wards27. However, one drawback is that some items on the MNA-SF and MNA-LF, particularly ‘mobility’ and ‘neuropsychological problems’, depend on subjective interpretation by the examiner28. This suggests that from the clinical point of view a more standardized and objective implementation of these two MNA items would be advantageous28.
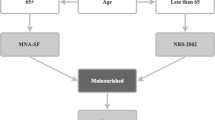
In Germany, patients hospitalised for acute care on geriatric wards are routinely evaluated in relation to 5 dimensions of a comprehensive geriatric assessment (CGA29) by the multidisciplinary geriatric team30. These five dimensions are cognition, emotion, mobility, activities of daily living (ADL), and social situation29. The Mini Mental State Examination (MMSE), Geriatric Depression Scale (GDS), the Timed Up and Go Test (TUG), Barthel Index, and a 5-item questionnaire addressing the patient’s social situation are frequently applied as assessment instruments29. As the prevalence or risk of malnutrition in patients hospitalised on geriatric wards is high, ever more geriatricians in Germany prefer to include evaluation of nutritional status in their assessments. With the aim of providing an objective and efficient approach to evaluating the nutritional status of older in-patients on geriatric wards, there may be value in using results from the mobility item of the Barthel Index and MMSE and GDS scores to operationalize the ‘mobility’ and ‘neuropsychological problems’ items in parallel with the other standard items of the MNA-SF and MNA-LF28.
We have recently introduced a modified MNA in which the ‘mobility’ and ‘neuropsychological problems’ items of the MNA are operationalized on the basis of results from the mobility item of the Barthel Index and MMSE and GDS scores28. We have reported evaluation of the completion rate, prevalence, and agreement with respect to categorisation of nutritional status determined using the modified MNA-SF and MNA-LF in comparison with the NRS 2002 instrument in geriatric inpatients in a cross-sectional analysis28. However, there are no data on the predictive ability of the modified MNA-SF and MNA-LF for adverse clinical outcomes such as mortality. Of note, the standard MNA-SF and MNA-LF have repeatedly been found to have predictive power for mortality31,32,33,34,35,36. The ability to predict mortality can therefore be regarded as a characteristic feature of the MNA-SF and MNA-LF. We therefore believe there is value in determining whether or not the modified MNA-SF and MNA-LF also have predictive power for mortality and whether or not the hypothesised predictive power for mortality of the modified MNA-SF and MNA-LF is comparable to that of the standard MNA-SF and MNA-LF.
In the study presented here, we now aimed to analyse the abilities of the modified MNA-SF and MNA-LF28 to predict six-month and one-year mortality in hospitalised geriatric patients. Moreover, we aimed to compare the predictive abilities of the modified MNA-SF and MNA-LF28 for mortality in comparison with those of the standard MNA-SF and MNA-LF21,22,23, NRS 200224,25 and MUST26 in this group of patients.
Materials and Methods
Study design and population
The study was a prospective longitudinal analysis in patients hospitalised on the geriatric wards of the Department of Internal Medicine III (Medicine of Ageing), Malteser Waldkrankenhaus St. Marien, Erlangen, Germany. The study population consisted of 240 patients. Patients were enrolled in the study programme between October 2015 and March 2016. Inclusion criteria were age ≥ 65 years, willingness and provision of consent for all relevant medical data to be held after baseline examination, even should death occur, and provision of consent to contact relatives, legal guardians, general practitioners, and the local town authority for residents/inhabitants to obtain information on current place of residence and living status (alive/deceased). Patients were evaluated in relation to malnutrition and risk of malnutrition by a single trained investigator (LB) during hospital stays on geriatric wards (baseline examination). Comprehensive geriatric assessment was performed in all 240 study participants by the geriatric team (including physical therapists, psychologists, occupational therapists, speech therapists nurses, and others) by applying routine comprehensive geriatric assessment instruments (Mini Mental State Examination37, Geriatric Depression Scale38, Timed Up and Go Test39, Barthel Index40, social situation assessment using a 5-item questionnaire41, frailty phenotype42, Cumulative Illness Rating Scale for Geriatrics43, and others). Patients were followed up six months and one year after baseline examination. Patients, patients’ relatives or legal guardians, and general practitioners were contacted by telephone six months and one year after baseline examination to obtain pertinent follow-up data relating to medical endpoints such as death due to any cause (including exact date of death). The study was approved by the Ethics Committee of the Friedrich-Alexander University, Erlangen-Nuremberg, Germany and complied with currently applicable laws. Informed written consent by the participant or a legal guardian was obtained before inclusion of patients into the study.
Malnutrition screening tools
Standard mini nutritional assessment short form (MNA-SF) and mini nutritional assessment long form (MNA-LF)
In the early 1990s Guigoz et al. developed and introduced the 18-item MNA (full MNA, or MNA-LF) as a malnutrition screening instrument23. In 1996, Rubenstein et al. developed and validated a short form (MNA-SF) including only six of the 18 items of the MNA-LF21. In 2009, Kaiser et al. showed that the ‘Body Mass Index (BMI)’ item of the MNA-SF and MNA-LF could be replaced by calf circumference when BMI could not be determined22. The six items of the standard MNA-SF were: ‘decline of food intake over the past three months’ (item A), ‘weight loss during the last three months’ (item B), ‘mobility’ (item C), ‘psychological stress or acute disease during the last three months’ (item D), ‘neuropsychological problems’ (item E), and ‘Body Mass Index’ (item F1), replacing BMI with ‘calf circumference (CC) in cm’ when BMI could not be determined (item F2)21,22. The items in the standard MNA-LF included the six items of the MNA-SF, along with twelve additional items: ‘independent living situation (not living in nursing home)’ (item G), ‘intake of more than 3 prescription drugs per day’ (item H), ‘pressure sores or skin ulcers’ (item I), ‘number of full daily meals’ (item J), ‘consumption markers for protein intake’ (item K), ‘consumption of two or more portions of fruit or vegetables per day’ (item L), ‘fluid intake’ (item M), ‘mode of feeding’ (item N), ‘self-assessment of nutritional status’ (item O), ‘self-assessment of health status in comparison with people of the same age’ (item P), ‘mid- arm circumference (MAC) in cm’ (item Q), and ‘calf circumference (CC) in cm’ (item R)23. Patients’ nutritional status in the standard MNA-SF and MNA-LF was classified as ‘normal nutritional status’, ‘at risk of malnutrition’, and ‘malnutrition’ on the basis of criteria described in detail elsewhere21,22,23.
Modified mini nutritional assessment short form (MNA-SF) and mini nutritional assessment long form (MNA-LF)
In the modified MNA-SF and MNA-LF, item C ‘mobility’ of the standard MNA-SF and MNA-LF was operationalized on the basis of the score on the mobility item of the Barthel Index. In brief, the categories “bed or chair bound”, “able to move in the ward” and “able to leave the ward” of item C, ‘mobility’, of the standard MNA-SF and MNA-LF were operationalized in the modified MNA-SF and MNA-LF using scores of 0, 5 to 10, and 15 points on the mobility item of the Barthel Index respectively28. Item E, ‘neuropsychological problems’, of the modified MNA-SF and MNA-LF was operationalized on the basis of Mini Mental State Examination (MMSE) and Geriatric Depression Scale (GDS) scores28. Thus, the categories “severe dementia or depression”, “mild dementia”, and “no psychological problems” of item E, ‘neuropsychological problems’, of the standard MNA-SF and MNA-LF were operationalized in the modified MNA-SF and MNA-LF based on an MMSE score of <20 or a GDS score of 10–15 points, an MMSE score of 20–26 points or a GDS score of 6–9 points, and an MMSE score of 27–30 points or a GDS score < 6 points respectively28. In addition, the modified MNA-SF and/or MNA-LF also included A, B, D, and F-R of the standard MNA-SF and/or MNA-LF, using the standard implementation and scoring of these items of the standard MNA-SF and MNA-LF21,22,23,28. Like the standard MNA-SF and MNA-LF, the modified MNA-SF and MNA-LF classified patients’ nutritional status into ‘normal nutritional status, ‘at risk of malnutrition’, and ‘malnutrition’21,22,23,28.
Nutritional risk screening 2002 (NRS 2002)
The NRS 2002 consists of a prescreen and a final screen. The prescreen includes four questions: I) ‘is BMI < 20.5 kg/m²?’, II) ‘has the patient lost weight within the last three months?’; III) ‘has the patient had a reduced dietary intake in the last week?’; and IV) ‘is the patient severely ill (i.e., in intensive therapy)?’, which have yes/no answers24,25. If the prescreen is positive, i.e., at least one of the four prescreening questions generates a ‘yes’ answer, a final and more comprehensive screening is performed and includes three dimensions: I) ‘nutritional, anthropometric and clinical status’; II) ‘disease severity; and III) ‘age of 70 years or older’24,25. Patients are identified as ‘no risk for malnutrition’ or ‘at risk of malnutrition’ on the NRS 2002, as described in detail elsewhere24,25.
Malnutrition universal screening tool (MUST)
The MUST addresses three items: ‘body mass index’, ‘undesired weight loss between the last three to six-months’, and ‘acute disease causing fasting for more than five days’26,44. Patients are identified as at ‘low risk’, ‘medium risk’, or ‘high risk of malnutrition’ on the MUST, as described in detail elsewhere26,44.
Statistical analysis
Data were analysed in SPSS version 24 (IBM SPSS Statistics, Armonk, NY, USA). Results are presented in the text and/or tables as mean ± standard deviation and/or percentages. Groups of patients, i.e., patients who had died/survived to follow-up at six months and one year, were compared using the Mann-Whitney U-test or the chi-squared test as appropriate. The six-month and one-year mortality rates for the different categories defined by the malnutrition screening instruments were analysed by Kaplan Meier analysis. The log-rank test was used to differentiate the predictive power of the different categories defined by the malnutrition screening instruments in relation to six-month and one-year mortality. Cox proportional hazard models were run to analyse the hazard ratios for six-month and one-year mortality of each increment in category on the various malnutrition screening instruments. Hazard ratios (HRs) for six-month and one-year mortality of the various malnutrition screening instruments were adjusted for potential confounding factors (i.e., sex, age, frailty status, Cumulative Illness Rating Scale – Geriatrics (CIRS-G), and Barthel-Index score) and were considered both separately and together. Receivers operating characteristic (ROC) curves were used to estimate the area under the curves (AUC) for the various malnutrition screening tools, analysed as categorical variables, in relation to six-month and one-year mortality. The abilities of the various malnutrition screening tools to predict six-month and one-year mortality were compared by comparing the AUC of the malnutrition screening tools for six-month and one-year mortality using the Hanley and McNeil method45. Statistically significant differences were identified at p < 0.05.
Results
Clinical characteristics of the study cohort
The study cohort consisted of 240 patients hospitalised on geriatric wards (159 female and 81 male). The clinical characteristics of the overall study cohort and individuals stratified by death/survival at six months and one year are shown in Table 1. Follow-up data at six months were not available for one person. Of the remaining 239 for whom follow-up data were available at six month follow-up, 28 individuals (11.7%) had died. Among these 239 patients, 67.4% were female. Mean age was 82.5 ± 6.1 years, mean BMI was 24.8 ± 8.1 kg/m2, mean weight was 66.6 ± 22.3 kg, and mean height was 158.0 ± 30.7 cm. A total of 51.9% of these patients described weight loss of >4.5 kg in the last year, 76.2% had had falls, 36.0% had heart failure, 10.5% had myocardial infarcts, 21.8% had stroke, 17.6% had cancer, 34.3% had diabetes mellitus, 25.9% had pulmonary disease, 41.8% had kidney disease, 11.3% had constipation, 61.9% had urinary incontinence or bladder catheter, 16.3% had bowel incontinence, and 96.2% received more than 5 medications. Mean MMSE was 25.6 ± 3.9 points, GDS was 4.4 ± 2.7 points, Barthel Index was 68.8 ± 19.6 points, CIRS-G was 18.4 ± 5.4, 77.4% were frail on the Frailty Phenotype, and 65.3% had TUG > 19 sec or were unable to perform the TUG. Follow-up data at one year were not available for four persons, including the individual from whom follow-up data were not available at six months. Of the remaining 236 patients for whom follow-up data were available at one-year, 49 individuals (20.8%) had died. These 236 patients included 67.8% females, had an average age of 82.5 ± 6.1 years, a mean BMI of 24.9 ± 8.0 kg/m², a mean weight of 66.7 ± 22 kg, and a mean height of 158.0 ± 30.8 cm. A total of 51.3% of these 236 patients described weight loss >4.5 kg in the last year, 76.3% had had falls, 35.6% had heart failure, 10.2% had myocardial infarcts, 21.6% had stroke, 17.4% had cancer, 34.7% had diabetes mellitus, 25.8% had pulmonary disease, 41.9% had kidney disease, 11.4% had constipation, 61.4% had urinary incontinence or were catheterised, 15.3% had bowel incontinence, and 96.2% took more than five medications. Mean MMSE was 25.6 ± 4.0 points, GDS was 4.4 ± 2.7 points, Barthel Index was 67.2 ± 19.4 points, CIRS-G was 18.4 ± 5.4, 77.1% were frail on the Frailty Phenotype, and 64.8% of the patients had a TUG > 19 sec or were unable to perform the TUG. A total of 16.1% of these 236 patients were institutionalised.
Assessment on both the modified MNA-SF and standard MNA-SF categorised 98.8% of the 240 study participants as “malnourished or at risk of malnutrition”. The proportions classified as malnourished and at risk for malnutrition differed: the modified MNA-SF identified 51.7% of individuals as “malnourished” and 47.1% as “at risk of malnutrition,” whereas the standard MNA-SF identified 56.7% of study participants as “malnourished” and 42.0% as “at risk of malnutrition.” The modified MNA-LF and standard MNA-LF categorised 97.5% and 97.9% patients as “malnourished or at risk of malnutrition”. The modified MNA-LF identified 37.5% as “malnourished” and 60.0% as “at risk of malnutrition”, while the standard MNA-LF classified 37.1% as “malnourished” and 60.8% as “at risk of malnutrition”.
Comparison of mortality rates at six-month and one-year follow-up stratified by nutritional status as determined with the various malnutrition screening instruments
Mortality rates at six-month and one-year follow-up stratified by nutritional status on the various malnutrition screening instruments are given in Table 2 and Figs 1 and 2. Patients with adverse malnutrition status on the modified MNA-SF and MNA-LF and the standard MNA-SF and MNA-LF had higher mortality rates than individuals with better nutritional status (all p < 0.05) (see Table 2). Mortality rates for six-month mortality showed no statistically significant differences between patients with different malnutrition status on the NRS 2002 (p ≥ 0.05), though one-year mortality was higher in patients with adverse malnutrition status than in individuals with better nutritional status (all p < 0.05). Mortality rates showed no statistically significant differences between patients with different malnutrition status on the MUST for either six-month and/or one-year mortality (all p ≥ 0.05) (see Table 2)
Prediction of six-month and one-year mortality
The abilities of the various malnutrition screening instruments to predict six-month and one-year mortality are shown and compared in Table 3. The modified MNA-SF and MNA-LF and the standard MNA-SF and MNA-LF, but not the NRS 2002 or the MUST, were able to predict six-month and one-year mortality (see Table 3). The ability of the modified MNA-SF to predict six-month mortality was better than that of the NRS 2002 but showed no differences compared with the modified MNA-LF, standard MNA-SF, MNA-LF and MUST. The ability of the modified MNA-SF to predict one-year mortality showed no difference compared with the standard MNA-SF, MNA-LF, NRS 2002, and MUST and was worse than that of the modified MNA-LF. With the exception of the modified MNA-SF, the modified MNA-LF yielded better predictions of six-month mortality than all other malnutrition screening instruments evaluated in this study. The modified MNA-LF gave better prediction of one-year mortality than all other malnutrition screening tools evaluated here.
Hazard Ratios and adjusted Hazard ratios for each increment in category on the various malnutrition screening tools in relation to six-month and one-year mortality
Unadjusted and adjusted HR for each increment in category on the different malnutrition screening instruments are given in Table 4. Each increment in category on the modified MNA-SF and MNA-LF and the standard MNA-SF and MNA-LF and the MUST, but not the NRS 2002, increased the risk for six-month and one-year mortality (see Table 4). In an adjusted model taking age and sex taking into account, each increment in category of the modified MNA-SF, the modified MNA-LF, the standard MNA-SF, the standard MNA-LF, and the MUST, but not NRS 2002, was associated with an increased risk for six-month and one-year mortality (see Table 4). In a further adjusted model taking several potential confounding factors into account (age, sex, frailty status according to the frailty phenotype, Cumulative Illness Rating Scale for Geriatrics (CIRS-G), and Barthel Index score), each increment in category on the modified MNA-LF, but not on the modified MNA-SF, standard MNA-SF, standard MNA-LF, NRS 2002, or MUST, was associated with an increased risk for six-month and one-year mortality (see Table 4).
Discussion
The major finding in the present study was that the modified MNA-SF and the standard MNA-SF did not differ in the ability to predict six-month and one-year mortality in our cohort of hospitalised geriatric patients. This indicates that the power of the modified MNA-SF to predict six-month and one-year mortality may be comparable with that of the standard MNA-SF in this group of patients. Thus, the modified MNA-SF emerges as a potentially valuable tool for predicting mortality in those hospitalised geriatric patients in whom the mobility item of the Barthel Index, MMSE and GDS is routinely applied during standard comprehensive geriatric assessments.
A further major finding of this study is that the modified MNA-LF displayed better prediction of six-month and one-year mortality than the standard MNA-LF. This indicates that the modification of the two items - ‘mobility’ and ‘neuropsychological problems’ - in the modified MNA-LF may improve the ability of this malnutrition screening instrument to predict mortality in hospitalised geriatric patients. Moreover, with the exception of the modified MNA-SF (the modified MNA-LF and modified MNA-SF did not differ in the ability to predict six-month mortality), the modified MNA-LF yielded better predictions of six-month and one-year mortality than all other malnutrition screening instruments evaluated here. Similarly, Kiesswetter et al. reported that the MNA-LF gave better prediction of one-year mortality than the MNA-SF in a cohort of 309 older adults aged ≥ 65 years receiving home care31. In a study of 246 institutionalised individuals aged 76.5 ± 11 years, the MNA-LF had higher predictive value for survival of well-nourished participants then the other malnutrition screening instruments (MNA-SF, NRS 2002 and MUST)32. In contrast, a Taiwanese prospective cohort study of 2872 participants aged ≥ 65 years demonstrated that the MNA-SF was comparable or even marginally superior than the MNA-LF in predicting four-year mortality33. However, and of relevance in the busy clinical setting at geriatric wards, it is of note that administration of the modified MNA-LF is more time-consuming than the modified MNA-SF.
In the present study, all the different MNA versions, i.e., the modified MNA-SF, modified MNA-LF, standard MNA-SF, and standard MNA-LF, were able to predict six-month and one-year mortality. In contrast, in this study the NRS 2002 and the MUST, did not reveal such an ability to predict six-month and or one-year mortality. Diekmann et al. previously reported that the MNA had greater predictive power than the NRS 2002 and MUST for survival in a prospective longitudinal analysis with a follow-up period of up to one year in 200 nursing-home residents34. Koren-Hakim et al. reported that the MNA-SF, but not the NRS 2002 or MUST, was able to predict mortality during a 36-month follow-up period with measurement of nutritional status in 215 older people undergoing hip fracture surgery, with a mean age of 83.9 ± 6.09 years35. Donini et al. reported that the MNA-LF and MNA-SF had higher predictive value for mortality than the NRS 2002 and MUST in a study cohort of 246 institutionalised participants aged 76.5 ± 11 years32. In contrast, a Brazilian study of a cohort of 705 participants, which included 169 geriatric patients aged ≥ 65 years, found that the abilities of the MNA-SF and NRS 2002 to predict complications, prolonged hospital stay, and mortality in older patients were comparable36. Holst et al. studied a cohort of 233 hospitalised geriatric patients and found that neither the MNA-LF, nor the NRS 2002, nor the MUST was able to predict one-year mortality46.
Among the different malnutrition screening instruments evaluated here, only the modified MNA-LF displayed independent predictive value for six-month and one-year mortality after adjustment of the analysis for potential confounding factors, including age, sex, frailty status, comorbidity, and ADL disability burden. There might be an interaction between age, sex, frailty status, comorbidity and ADL disability on the one hand and malnutrition and the risk of malnutrition on the other in hospitalised geriatric patients with, along with each of these conditions, a potential impact on the mortality risk. Among the malnutrition screening instruments evaluated here, the modified MNA-LF may be the most objective and complex, and probably allows the predictive value of malnutrition and risk for malnutrition to be discriminated from the contributions of age, sex, frailty status, comorbidity and ADL disability to prediction of mortality. Nevertheless, with the exception of the NRS 2002, all the malnutrition screening instruments evaluated here had predictive power for six-month and one-year mortality after adjustment of the analysis for age and sex only. In line with the findings reported here, several other authors have reported that malnutrition has independent predictive value for mortality in different settings. Jiang et al. reported studies of a cohort of 437 patients with a median age of 81.0 (74.5–84.0) years in an acute geriatric ward showing that malnutrition as defined by the European Society for Clinical Nutrition and Metabolism (ESPEN) criteria, but not by the MNA, was an independent predictor of three-year mortality47. In a study of 131 patients aged ≥ 60 years, the Geriatric Nutritional Risk Index (GNRI) predicted three- and six-month mortality independent of potential confounding factors, including age, sex, and cancer48. In a retrospective study of 1170 participants aged ≥ 60 years living in a community dwelling setting, malnutrition was found to be an independent risk factor for seven-year mortality49. A prospective cohort study of 164 emergency department patients aged ≥ 75 years showed that malnutrition was a strong independent risk factor for short-term mortality in geriatric patients50. Liu et al. reported a prospective observational study including diabetes patients aged ≥ 65 years with a 2.8-year follow-up which showed that malnutrition was an independent predictor of mortality51. Another study of a cohort of 1306 participants aged ≥ 75 years showed that malnutrition as assessed on the MNA-SF was independently related to six-month mortality52. Correia et al. also noted that malnutrition was an independent risk factor of mortality16. Lilamand et al. reported that the MNA-SF predicted one-year mortality independently of potential confounding factors in a nursing home setting in a study of 773 older people with a mean age of 86.2 ± 7.5 years53. It should be noted that the setting on hospital geriatric wards may not be comparable to the setting in the community/home or nursing homes.
The six-month and one-year mortality rates in the present study in hospitalised geriatric patients-11.7% and 20.8% - are in line with mortality rates reported from other studies by ourselves and other authors in hospitalised geriatric patients. We have previously reported that 15.4% of 306 study participants died during the first six-month post-baseline examination in a cohort of hospitalised geriatric patients54,55. Dent et al. found a six-month mortality rate of 16% in 172 hospitalised geriatric patients56. A study by Drame et al. of 1306 acute hospitalised study participants aged ≥ 75 years found that a six-month mortality rate of 24.4%52. One of our own earlier studies yielded a one-year mortality of 20.3% in 304 hospitalised geriatric patients57,58. A study of 2033 geriatric in-patients recorded a one-year mortality of 24.9%59. Holst et al. reported a one-year mortality rate of 27% in 233 hospitalised geriatric patients46.
The mortality rates of individuals with malnutrition or at risk for malnutrition as evaluated by the modified MNA-SF and modified MNA-LF in the present study are similar to those in our previous studies using the malnutrition screening instruments identified above in hospitalised geriatric patients28.
This study has a number of strengths. It is the first prospective longitudinal analysis dissecting the ability of the modified MNA (modified MNA-SF and modified MNA-LF) as compared with the standard MNA-SF, MNA-LF, NRS 2002 and MUST to predict six-month and one-year mortality. Furthermore, we adjusted the analysis for potential confounding factors to analyse whether malnutrition as classified by the different screening instruments has independent predictive value for six-month and/or one-year mortality. Follow-up data were obtained at two time points (six months and one year post-baseline). The rate of loss to follow-up was very low. Only 0.4% of the 240 study participants could not be reached at the six-months and 1.7% at one year. Previous studies showed comparable percentages of loss to follow up at six months or one year54,57,58,60.
On average it takes 5–10 minutes to perform the MNA-SF and 10–20 minutes to perform the MNA-LF depending on individual patient characteristics. In the case the MMSE, GDS and Barthel Index are routinely applied in terms of the comprehensive geriatric assessment at the geriatric ward the assessor can save approximately up to five minutes by using the score of the MMSE, GDS and mobility item of the Barthel Index to operationalize the modified MNA-SF and modified MNA-LF. Clearly, in the case the MMSE, GDS and Barthel Index are not routinely applied in terms of the comprehensive geriatric assessment at the geriatric ward it would take longer to operationalize the modified MNA-SF and modified MNA-LF in comparison to the standard MNA-SF and standard MNA-LF as the MMSE, GDS and mobility item of the Barthel Index have to be performed in addition.
The study also has some limitations. The caring geriatric teams were not blinded to the results of all these scores of the comprehensive geriatric assessment tools at the time of admission and may have altered their care plans on the basis of these data. This may have altered mortality risk potentially confounding the observed results. It was a single-centre study of hospitalised patients on geriatric wards. It may therefore be misleading to extrapolate the findings to the geriatric wards of any other hospital or other clinical setting. All study participants were Caucasian, so the study results may not be transferable to other ethnic groups.
In conclusion, the modified MNA-SF and standard MNA-SF showed comparable abilities to predict six-month and one-year mortality in hospitalised geriatric patients. With the exception of the modified MNA-SF (there were no differences in the abilities of the modified MNA-LF and modified MNA-SF to predict six-month mortality), the modified MNA-LF gave better predictions of six-month and one-year mortality than all the other malnutrition screening instruments evaluated here. The various MNA versions evaluated in this study, i.e., the modified MNA-SF and MNA-LF and the standard MNA-SF and MNA-LF, and the MUST, but not the NRS 2002, were able to predict six-month and one-year mortality after adjustment of the analysis for age and sex. The modified MNA-LF, but none of the other malnutrition screening instruments evaluated here, had independent predictive value for six-month and one-year mortality after adjustment of the analysis for age, sex, frailty status, comorbidity and ADL disability burden. The modified MNA-SF and modified MNA-LF therefore emerge as potentially valuable tools for predicting mortality in hospitalised geriatric patients in whom the Barthel Index, MMSE and GDS are routinely applied in the course of standardised comprehensive geriatric assessment.
Data Availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Clinical Practice Committee. Definition of terms, style, and conventions used in A.S.P.E.N. (2010).
Mueller, C., Compher, C. & Ellen, D. M. A. S. P. E. N. clinical guidelines: Nutrition screening. assessment, and intervention in adults. JPEN 35, 16–24, https://doi.org/10.1177/0148607110389335 (2011).
Eglseer, D., Halfens, R. J. G., Schols, J. & Lohrmann, C. Dysphagia in Hospitalized Older Patients: Associated Factors and Nutritional Interventions. J Nutr Health Aging 22, 103–110, https://doi.org/10.1007/s12603-017-0928-x (2018).
Konturek, P. C., Herrmann, H. J., Schink, K., Neurath, M. F. & Zopf, Y. Malnutrition in Hospitals: It Was, Is Now, and Must Not Remain a Problem! Med Sci Monit 21, 2969–2975, https://doi.org/10.12659/msm.894238 (2015).
Morley, J. E. & Kraenzle, D. Causes of weight loss in a community nursing home. J Am Geriatr Soc 42, 583–585 (1994).
Little, M. O. Updates in nutrition and polypharmacy. Curr Opin Clin Nutr Metab Care 21, 4–9, https://doi.org/10.1097/mco.0000000000000425 (2018).
Pirlich, M. et al. The German hospital malnutrition study. Clin Nutr 25, 563–572, https://doi.org/10.1016/j.clnu.2006.03.005 (2006).
Norman, K., Pichard, C., Lochs, H. & Pirlich, M. Prognostic impact of disease-related malnutrition. Clin Nutr 27, 5–15, https://doi.org/10.1016/j.clnu.2007.10.007 (2008).
Kaiser, M. J. et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc 58, 1734–1738, https://doi.org/10.1111/j.1532-5415.2010.03016.x (2010).
Schrader, E., Grosch, E., Bertsch, T., Sieber, C. C. & Volkert, D. Nutritional and Functional Status in Geriatric Day Hospital Patients - MNA Short Form Versus Full MNA. J Nutr Health Aging 20, 918–926, https://doi.org/10.1007/s12603-016-0691-4 (2016).
Volkert, D., Saeglitz, C., Gueldenzoph, H., Sieber, C. C. & Stehle, P. Undiagnosed malnutrition and nutrition-related problems in geriatric patients. J Nutr Health Aging 14, 387–392 (2010).
Rolland, Y. et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging 12, 433–450 (2008).
Volkert, D. The role of nutrition in the prevention of sarcopenia. Wien Med Wochenschr 161, 409–415, https://doi.org/10.1007/s10354-011-0910-x (2011).
Bonnefoy, M. et al. Frailty and nutrition: searching for evidence. J Nutr Health Aging 19, 250–257, https://doi.org/10.1007/s12603-014-0568-3 (2015).
Dorner, T. E. et al. Association between nutritional status (MNA(R)-SF) and frailty (SHARE-FI) in acute hospitalised elderly patients. J Nutr Health Aging 18, 264–269, https://doi.org/10.1007/s12603-013-0406-z (2014).
Correia, M. I. & Waitzberg, D. L. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr 22, 235–239 (2003).
Caccialanza, R., Cereda, E. & Klersy, C. Malnutrition, age and inhospital mortality. Cmaj 183, 826, https://doi.org/10.1503/cmaj.111-2038 (2011).
Asiimwe, S. B., Muzoora, C., Wilson, L. A. & Moore, C. C. Bedside measures of malnutrition and association with mortality in hospitalized adults. Clin Nutr 34, 252–256, https://doi.org/10.1016/j.clnu.2014.03.013 (2015).
Ulger, Z. et al. Malnutrition in Turkish nursing homes: a correlate of short term mortality. J Nutr Health Aging 17, 305–309, https://doi.org/10.1007/s12603-013-0016-9 (2013).
Kang, M. C. et al. Prevalence of Malnutrition in Hospitalized Patients: a Multicenter Cross-sectional Study. J Korean Med Sci 33, e10, https://doi.org/10.3346/jkms.2018.33.e10 (2018).
Rubenstein, L. Z., Harker, J. O., Salva, A., Guigoz, Y. & Vellas, B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 56, M366–372 (2001).
Kaiser, M. J. et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 13, 782–788 (2009).
Guigoz, Y., Vellas, B. & Garry, P. J. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev 54, S59–65 (1996).
Kondrup, J., Rasmussen, H. H., Hamberg, O. & Stanga, Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 22, 321–336 (2003).
Kondrup, J., Allison, S. P., Elia, M., Vellas, B. & Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin Nutr 22, 415–421 (2003).
Orlandoni, P. et al. Malnutrition upon Hospital Admission in Geriatric Patients: Why Assess It? Front Nutr 4, 50, https://doi.org/10.3389/fnut.2017.00050 (2017).
Cederholm, T. et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr 36, 49–64, https://doi.org/10.1016/j.clnu.2016.09.004 (2017).
Christner, S. et al. Evaluation of the nutritional status of older hospitalised geriatric patients: a comparative analysis of a Mini Nutritional Assessment (MNA) version and the Nutritional Risk Screening (NRS 2002). J Hum Nutr Diet 29, 704–713, https://doi.org/10.1111/jhn.12376 (2016).
Rockwood, K., Silvius, J. L. & Fox, R. A. Comprehensive geriatric assessment. Helping your elderly patients maintain functional well-being. Postgrad Med 103, 247–249, 254–248, 264, https://doi.org/10.3810/pgm.1998.03.424 (1998).
Applegate, W., Deyo, R., Kramer, A. & Meehan, S. Geriatric evaluation and management: current status and future research directions. J Am Geriatr Soc 39, 2s–7s (1991).
Kiesswetter, E. et al. Prognostic differences of the Mini Nutritional Assessment short form and long form in relation to 1-year functional decline and mortality in community-dwelling older adults receiving home care. J Am Geriatr Soc 62, 512–517, https://doi.org/10.1111/jgs.12683 (2014).
Donini, L. M. et al. Mini-Nutritional Assessment, Malnutrition Universal Screening Tool, and Nutrition Risk Screening Tool for the Nutritional Evaluation of Older Nursing Home Residents. J Am Med Dir Assoc 17, 959.e911–958, https://doi.org/10.1016/j.jamda.2016.06.028 (2016).
Wang, J. Y. & Tsai, A. C. The short-form mini-nutritional assessment is as effective as the full-mini nutritional assessment in predicting follow-up 4-year mortality in elderly Taiwanese. J Nutr Health Aging 17, 594–598, https://doi.org/10.1007/s12603-013-0048-1 (2013).
Diekmann, R. et al. Screening for malnutrition among nursing home residents - a comparative analysis of the mini nutritional assessment, the nutritional risk screening, and the malnutrition universal screening tool. J Nutr Health Aging 17, 326–331, https://doi.org/10.1007/s12603-012-0396-2 (2013).
Koren-Hakim, T. et al. Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clin Nutr 35, 1053–1058, https://doi.org/10.1016/j.clnu.2015.07.014 (2016).
Raslan, M. et al. Comparison of nutritional risk screening tools for predicting clinical outcomes in hospitalized patients. Nutrition 26, 721–726, https://doi.org/10.1016/j.nut.2009.07.010 (2010).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198 (1975).
Yesavage, J. A. et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17, 37–49 (1982).
Podsiadlo, D. & Richardson, S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39, 142–148 (1991).
Mahoney, F. I. & Barthel, D. W. Functional Evaluation: The Barthel Index. Md State Med J 14, 61–65 (1965).
Nikolaus, T., Specht-Leible, N., Bach, M., Oster, P. & Schlierf, G. Social aspects in diagnosis and therapy of very elderly patients. Initial experiences with a newly developed questionnaire within the scope of geriatric assessment. Z Gerontol 27, 240–245 (1994).
Rockwood, K., Andrew, M. & Mitnitski, A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 62, 738–743 (2007).
Linn, B. S., Linn, M. W. & Gurel, L. Cumulative illness rating scale. J Am Geriatr Soc 16, 622–626 (1968).
Stratton, R. J. et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br J Nutr 92, 799–808 (2004).
Hanley, J. A. & McNeil, B. J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148, 839–843, https://doi.org/10.1148/radiology.148.3.6878708 (1983).
Holst, M. et al. Nutritional screening and risk factors in elderly hospitalized patients: association to clinical outcome? Scand J Caring Sci 27, 953–961, https://doi.org/10.1111/scs.12010 (2013).
Jiang, J. et al. Predicting long-term mortality in hospitalized elderly patients using the new ESPEN definition. Sci Rep 7, 4067, https://doi.org/10.1038/s41598-017-04483-1 (2017).
Abd-El-Gawad, W. M., Abou-Hashem, R. M., El Maraghy, M. O. & Amin, G. E. The validity of Geriatric Nutrition Risk Index: simple tool for prediction of nutritional-related complication of hospitalized elderly patients. Comparison with Mini Nutritional Assessment. Clin Nutr 33, 1108–1116, https://doi.org/10.1016/j.clnu.2013.12.005 (2014).
Ferreira, L. S. et al. Undernutrition as a major risk factor for death among older Brazilian adults in the community-dwelling setting: SABE survey. Nutrition 27, 1017–1022, https://doi.org/10.1016/j.nut.2010.11.008 (2011).
Gentile, S. et al. Malnutrition: a highly predictive risk factor of short-term mortality in elderly presenting to the emergency department. J Nutr Health Aging 17, 290–294, https://doi.org/10.1007/s12603-012-0398-0 (2013).
Liu, G. X. et al. Pilot study of the Mini Nutritional Assessment on predicting outcomes in older adults with type 2 diabetes. Geriatr Gerontol Int 17, 2485–2492, https://doi.org/10.1111/ggi.13110 (2017).
Drame, M. et al. Six-month outcome of elderly people hospitalized via the emergency department: the SAFES cohort. Rev Epidemiol Sante Publique 60, 189–196, https://doi.org/10.1016/j.respe.2011.11.004 (2012).
Lilamand, M. et al. The Mini Nutritional Assessment-Short Form and mortality in nursing home residents–results from the INCUR study. J Nutr Health Aging 19, 383–388, https://doi.org/10.1007/s12603-014-0533-1 (2015).
Ritt, M., Jager, J., Ritt, J. I., Sieber, C. C. & Gassmann, K. G. Operationalizing a frailty index using routine blood and urine tests. Clin Interv Aging 12, 1029–1040, https://doi.org/10.2147/cia.s131987 (2017).
Ritt, M. et al. A comparison of Frailty Indexes Based on a Comprehensive Geriatric Assessment for the Prediction of Adverse Outcomes. J Nutr Health Aging 20, 760–767, https://doi.org/10.1007/s12603-015-0644-3 (2016).
Dent, E., Chapman, I. M., Piantadosi, C. & Visvanathan, R. Performance of nutritional screening tools in predicting poor six-month outcome in hospitalised older patients. Asia Pac J Clin Nutr 23, 394–399, https://doi.org/10.6133/apjcn.2014.23.3.18 (2014).
Ritt, M., Ritt, J. I., Sieber, C. C. & Gassmann, K. G. Comparing the predictive accuracy of frailty, comorbidity, and disability for mortality: a 1-year follow-up in patients hospitalized in geriatric wards. Clin Interv Aging 12, 293–304, https://doi.org/10.2147/cia.s124342 (2017).
Ritt, M., Bollheimer, L. C., Sieber, C. C. & Gassmann, K. G. Prediction of one-year mortality by five different frailty instruments: A comparative study in hospitalized geriatric patients. Arch Gerontol Geriatr 66, 66–72, https://doi.org/10.1016/j.archger.2016.05.004 (2016).
Pilotto, A. et al. Comparing the prognostic accuracy for all-cause mortality of frailty instruments: a multicentre 1-year follow-up in hospitalized older patients. PLoS One 7, e29090, https://doi.org/10.1371/journal.pone.0029090 (2012).
Ritt, M. et al. Analysis of Rockwood et Al’s Clinical Frailty Scale and Fried et Al’s Frailty Phenotype as Predictors of Mortality and Other Clinical Outcomes in Older Patients Who Were Admitted to a Geriatric Ward. J Nutr Health Aging 19, 1043–1048, https://doi.org/10.1007/s12603-015-0534-8 (2015).
Acknowledgements
The present work was performed in fulfilment of the requirements for obtaining the degree ‘Dr. med.’ of the Friedrich-Alexander University Erlangen-Nuremberg, Germany (LB). We acknowledge support from Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the Open Access Publishing funding programme.
Author information
Authors and Affiliations
Contributions
Contribution to the conception or design (L.B., D.V., C.C.S., K.G.G. and M.R.), data collection (L.B., M.R. and K.G.G.), data analysis and interpretation (L.B. and M.R.), drafting the text (L.B. and M.R.), critical review of the text (L.B., D.V., C.C.S., K.G.G. and M.R.) and/or final approval of the version for submission of the manuscript to the journal (L.B., D.V., C.C.S., K.G.G. and M.R.).
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Becker, L., Volkert, D., Christian Sieber, C. et al. Predictability of a modified Mini- Nutritional- Assessment version on six-month and one-year mortality in hospitalized geriatric patients: a comparative analysis. Sci Rep 9, 9064 (2019). https://doi.org/10.1038/s41598-019-45452-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45452-0
This article is cited by
-
Comparing the validity of anthropometric measurements in identifying malnutrition status of older age people in Borena district, North Central Ethiopia: a cross_sectional study
BMC Geriatrics (2022)
-
Nutritional Assessment in Older Adults: MNA® 25 years of a Screening Tool & a Reference Standard for Care and Research; What Next?
The journal of nutrition, health & aging (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.