Abstract
Empathizing is defined as “the drive to identify another’s mental states and to respond to these with an appropriate emotion” and systemizing is defined as “the drive to the drive to analyze and construct rule-based systems”. While mean diffusivity (MD) has been robustly associated with several cognitive traits and disorders related with empathizing and systemizing, its direct correlation with empathizing and systemizing remains to be investigated. We undertook voxel-by-voxel investigations of regional MD to discover microstructural correlates of empathizing, systemizing, and the discrepancy between them (D score: systemizing − empathizing). Whole-brain analyses of covariance revealed that across both sexes, empathizing was positively correlated with MD of (a) an anatomical cluster that primarily spreads in the areas in and adjacent to the left dorsolateral prefrontal cortex, left anterior to the middle cingulate cortex, and left insula and (b) an anatomical cluster of the left postcentral gyrus and left rolandic operculum. The former overlaps with positive MD correlates of cooperativeness. The D score and systemizing did not show significant correlations. In conclusion, while increased MD has generally been associated with reduced neural tissues and possibly area function, higher empathizing and cooperativeness were commonly reflected by greater MD values in areas (a) that mainly overlap with areas that play a key role in emotional salience and empathy. In addition, higher empathizing was correlated with greater MD values in areas (b) that play a key role in the mirror neuron system.
Similar content being viewed by others
Introduction
Empathizing and systemizing are important cognitive traits as stronger systemizing and weaker1,2 empathizing characterize thinking patterns of males and individuals with autism spectrum conditions (ASCs)3,4. Empathizing is “the drive to identify another’s mental states and to respond to these with an appropriate emotion”1. On the other hand, systemizing is “the drive to the drive to analyze and construct rule-based systems”2. The D score is the discrepancy between systemizing and empathizing (systemizing–empathizing) and characterizes thinking patterns of males and ASCs5. Among the cognitive characteristics of ASCs, social cognition deficits such as those related to the theory of mind are believed to be associated with a lower level of empathizing6, whereas higher competence in engineering, math, physics, and spatial cognition are believed be associated with higher systemizing1,5.
Previously, we investigated regional gray and white matter volume (rGMV and rWMV, respectively) and white matter structural connectivity (fractional anisotropy: FA) associated with empathizing, systemizing, and D score7,8. In these studies, we hypothesized that empathizing was associated with the default mode network (DMN) and systemizing was associated with the external attention system (EAS). EAS is the network that is active during the externally directed attention-demanding task and consists of inferior parietal lobes, the dorsal part of the anterior cingulate cortex (ACC) and lateral prefrontal cortices (LPFCs) and so on9,10. DMN is a network that is deactivated during these tasks and is recruited during socially-related cognition. This network includes, the superior temporal sulcus, some areas within the lateral temporal cortex, areas within the posterior cingulate cortices, precuneus, and medial prefrontal cortices (mPFCs)9. The results were more complicated, for example, in the analyses of rWMV, despite little correlation between empathizing and systemizing, positive rWMV correlates of empathizing and negative rWMV correlates of systemizing substantially overlapped and were located in the white matter, adjacent to the DMN and other networks, such as the ventral medial prefrontal cortex, the right inferior frontal gyrus, bilateral temporal lobe, and the posterior cingulate cortex7. Furthermore, negative rGMV correlates of empathy were located not only in DMN areas such as the mPFC, precuneus, middle cingulate gyrus, and temporal pole but also in EAS areas such as the LPFC, superior parietal lobule, and ACC and subcortical areas such as the thalamus and the caudate. A significant positive correlation was found between systemizing and rGMV in the LPFC—the key node of EAS—as well as a negative correlation with the putamen and caudate8.
On the other hand, mean diffusivity (MD) of diffusion tensor imaging (DTI)11 measures microstructural brain properties. As we summarized previously12, lower MD is caused by “a greater density of cellular structures in tissues, such as capillaries, synapses, and macromolecular proteins,” as well as “changes in the shapes of neurons or glia and the directionality of tissue organization (e.g., by strengthening of the axonal or dendritic backbones and the surrounding tissues)”11,12,13. Therefore, an MD decrease is generally considered to reflect local functional augmentation, and higher individual cognitive competence is usually associated with a lower MD of the relevant areas14. Several studies have demonstrated a robust and characteristic correlation between the MD measurements of gray and white matter and individual cognitive differences, compared with volume and fractional anisotropy measures of DTI (fractional anisotropy reflects the myelination of white matter, the properties of the axon, the direction of tracts, etc)12,15,16. In addition, autistic subjects have been shown to exhibit a robustly elevated MD in extensive regions in the brain, which is suggestive of this measure’s relevance in autism17. Reduction, however, of certain tissue components such as synapses is associated with functional refining, whereas both greater and lesser cortical thickness and rGMV are associated with greater cognitive competence, depending on the conditions8,14. Similarly, an MD increase may be associated with a greater cognitive competence. Consistently, our previous study showed that the personality trait of cooperativeness, which is associated with social competence, was positively correlated with MD in areas close to the ACC, insula, and LPFC16.
Despite these data, MD correlates (including those of gray and white matter) of empathizing, systemizing, and D score have never been investigated. The purpose of this study was to investigate these issues. Given the importance of these psychological measures in ASDs and the unique ability of MD to reveal the neural bases of individual cognitive differences, it is vital to understand the association between MD and empathizing/systemizing/D score.
Based on the abovementioned background we set two hypotheses. One was that MD of DMN would be associated with empathizing and MD of EAS would be associated with systemizing. The other was that empathizing would positively correlate with MD in the areas between ACC and LPFC (the key node of EAS), similar to cooperativeness, which shares prosocial components with empathizing.
Methods
Subjects
The present study is a part of an ongoing project aiming to investigate the association between brain imaging, cognitive function, and aging. The descriptions in this subsection have been reproduced from our previous study, in which the exact same methods were used7,16,18,19,20. It included EQ, SQ measures and imaging data from 1332 healthy, right-handed individuals (774 men and 558 women). The mean age of the subjects was 20.8 years [standard deviation (SD), 1.8; age range: 18–27 years old]. The following descriptions were mostly reproduced from another study of ours from the same project using the exactly same methods regarding these issues21. All subjects were university students, postgraduates, or university graduates of less than one year’s standing. All subjects had normal vision and none had a history of neurological or psychiatric illness. Handedness was evaluated using the Edinburgh Handedness Inventory22.
Among the subjects of this study, data from 567 subjects were used in our previous study investigating the associations between empathizing/systemizing and rGMV8 and between FA and rWMV7, and data from 248 subjects were previously used to investigate the association between empathizing/systemizing and resting state functional connectivity23. Among the subjects of the present study, several participated also in our intervention studies (psychological data and imaging data recorded before the intervention were used in this study)24. Psychological tests and MRI scans not described in this study were performed together with those described in this study. The subjects were recruited by advertising the study on the bulletin boards of the Tohoku University or by emailing the information to potential subjects. Written informed consent was obtained from each subject. For nonadult subjects, written informed consent was obtained from their parents (guardians). This study was approved by the Ethics Committee of Tohoku University.
For the day of the cognitive tests and MRI scans, the subjects were instructed to get sufficient sleep, maintain their normal conditions, eat sufficient breakfast, and consume their usual amount of caffeinated foods and drinks. In addition, they were instructed to avoid alcohol on the night before the assessment.
Systemizing quotient (SQ) and empathy quotient (EQ) questionnaires
Japanese versions25 of the SQ and EQ questionnaires3,4 were administered. The following methods were reproduced from our previous study using the exact same method7,8,23,26. The EQ score was used as an index of empathizing, and the SQ score was used as an index of systemizing. These tests consist of 40 items for each quotient and 20 filler items that are not scored. The scales consist of self-descriptive statements scored on a four-point scale ranging from Strongly Disagree to Strongly Agree. Half the items are worded to produce an “agree” response and rest to produce a “disagree” response. Items are randomized to avoid a response bias. Each strong systemizing/empathizing response is awarded 2 points, and each slightly systemizing/empathizing response is awarded 1 point (i.e., each item is scored as 2, 1, or 0), resulting in a range of total scores from 0–80 for each quotient.
The D score was calculated according to a previous study27. The raw SQ and EQ scores were standardized by subtracting the population mean from the score then dividing it by the maximum possible score: S = (raw SQ score − population mean of the raw SQ score)/80 and E = (raw EQ score − population mean of the raw EQ score)/80. For this computation, we used the estimated population means (EQ: mean = 33.4, SQ: mean = 22.7 within the whole sample) derived from a previous study’s large sample (N = 1250) of Japanese university students with an almost equal number of men and women25. This procedure was performed according to our previous studies8. The discrepancy between systemizing and empathizing was then quantified as D = (S − E)/2. The greater the D score in a positive direction, the stronger one’s systemizing relative to one’s empathizing. D scores close to zero represent an equal drive to systemize and empathize.
The questionnaire comprised the psychometric properties described below. Some studies have reported empathizing and systemizing as largely independent. However, a weak negative correlation between them has been reported by several studiese.g.28, whereas others failed to find such correlatione.g.25. Individuals with autism spectrum conditions have been found to exhibit higher SQ scores and lower EQ scores than controls25. Similarly, male individuals exhibit higher SQ scores than female individuals who, in turn, present higher EQ scores than males29. Students of humanities also show higher EQ scores than students of science who, in turn, present higher SQ scores than those studying humanities29. Additionally, actors were found to have higher EQ scores30. EQ is positively correlated with both the size of an individual’s social network31 and their performance on a face perception task32. The Autism Spectrum Quotient (AQ) is a measure of autistic traits. Although that measure was not collected in this project, it is well explained by the model including both EQ and SQ (more than 75% of the variance)28, whereas the AQ score is strongly and significantly correlated with the D score (r = 0.69)33. These findings have demonstrated the criterion-related validity of the present questionnaire. The internal consistencies of both EQ and SQ, calculated in a previous, large sample study, were 0.86 and 0.88, respectively, demonstrating the reliability of this questionnaire.
The Japanese version of the questionnaires was validated by Prof. Akio Wakabayashi, Prof. Baron-Cohen, and others25,34. In the Japanese version, the patterns of male EQ and SQ scores (vs. female EQ and SQ scores), ASC group (vs. controls), and science majors (vs. humanities majors) were similar to those of the original version25,34. The present study’s participants’ EQ score was lower than that of the previous study’s control sample3,4, but similar to the EQ scores shown by the previous study’s Japanese university students25,34.
The following are examples of items found on the SQ–EQ questionnaires:
“I can tune into how someone else feels rapidly and intuitively” (EQ)
“I am good at predicting how someone will feel” (EQ)
“I am fascinated by how machines work” (SQ)
“If I were buying a stereo, I would want to know about its precise technical features” (SQ)
The timing for the subjects to answer the questionnaires was not fixed along the project but was set within two months before or after MRI scans, except in rare cases, such as that of subjects having to postpone the experiments for a while due to illness. Since empathizing and systemizing are individual traits, they are not supposed to be influenced by the timing.
Assessment of psychometric measures of general intelligence
Raven’s Advanced Progressive Matrix35, which is often shown to be the measure most correlated with general intelligence and thus the best measure of general intelligence35, was used to assess intelligence and adjust for the effect of general intelligence on MD. For additional details on administration of Raven’s Advanced Progressive Matrix, refer to our previous studies36,37. The descriptions in this subsection were mostly reproduced from our previous study using the exact same method7.
Assessment of cooperativeness
To measure cooperativeness, we used a Japanese version38 of the Temperament Character Inventory39.
Image acquisition
MRI data acquisition was performed using a 3T Philips Achieva scanner. The descriptions in this subsection have been mostly reproduced from our previous study that used the exact same methods40. All data was obtained in our facility, using a single scanner (Institute of Development, Aging and Cancer, Tohoku University). Diffusion-weighted data were acquired using a spin-echo EPI sequence (TR = 10293 ms, TE = 55 ms, big delta (Δ) = 26.3 ms, little delta (δ) = 12.2 ms, FOV = 22.4 cm, 2 × 2 × 2 mm3 voxels, 60 slices, SENSE reduction factor = 2, number of acquisitions = 1). The diffusion weighting was isotropically distributed along 32 directions (b value = 1,000 s/mm2). Additionally, three images with no diffusion weighting (b value = 0 s/mm2) (b = 0 images) and one b = 0 image were acquired from 1207 and 125 subjects, respectively, using a spin-echo EPI sequence (TR = 10293 ms, TE = 55 ms, FOV = 22.4 cm, 2 × 2 × 2 mm3 voxels, 60 slices). When three b = 0 images were obtained, the average of the three images was generated in the console and used for the following preprocessing procedure. From the collected images, FA maps and MD maps were calculated using the commercially available diffusion tensor analysis package on the MR consol. For more details, see Supplemental Methods.
Preprocessing of imaging data
Preprocessing and analysis of imaging data were performed using SPM8 implemented in Matlab. The descriptions in this subsection have been mostly reproduced from our previous study that used the exact same methods41. Basically, we normalized MD images of subjects with previously validated7 diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL)-based registration process method to give images with 1.5 × 1.5 × 1.5 mm3 voxels, then tissues that are not likely to be gray or white matter were carefully removed and smoothed by convolving them with an isotropic Gaussian kernel of 8-mm full width at half maximum. For details, see Supplemental Methods.
Statistical analysis of MD
In the whole brain analyses, we used voxel-wise analysis of covariance (ANCOVA), with sex difference as a grouping factor (using the full factorial option of SPM). The descriptions in this subsection were mostly reproduced from our previous study using the same method7,8,23,41.
In D score analyses, age, RAPM score, the number of b = 0 images, and D score were covariates. In the analyses of the EQ and SQ scores, age, RAPM score, the number of b = 0 images, EQ score, and SQ score were covariates. In analyses of cooperativeness, age, RAPM score, and cooperativeness were covariates (in the sample from which the cooperativeness score was gathered, single b = 0 images were obtained from few subjects and these subjects were excluded in the analyses of the cooperativeness score). We performed three different whole-brain ANCOVAs.
In these analyses, age, RAPM score, and target variables (D/EQ/SQ/cooperativeness scores) were modeled so that each covariate had a unique relationship with MD for each sex (using the interactions option in SPM), which enabled investigation of the effects of interactions between sex and each covariate. On the other hand, the number of b = 0 images was not modeled in this manner, and a common effect of the number of b = 0 images on MD was assumed for both sexes (in analyses of cooperativeness, this covariate did not exist). In these analyses, the centering option was used for centering the all covariates. The main effects of the target variables (D/EQ/SQ/cooperativeness scores) (contrasts of [the effects of the target variables (D/EQ/SQ/cooperativeness scores) for males and females] were [1 1] or [−1 −1]) and the interaction between sex and the target variables (D/EQ/SQ/cooperativeness scores) (contrasts of [the effect of the target variables (D/EQ/SQ/cooperativeness scores) for males, the effect of the target variables (D/EQ/SQ/cooperativeness scores) for females] were [−1 1] or [1 −1]) were assessed using t-contrasts. Analysis was limited to the gray and white matter masks, which comprise areas highly likely to be gray or white matter (as described in Supplemental Methods).
Sex differences in the MD correlates of empathizing and systemizing were investigated, as a previous study showed both commonalities and differences in the brain structural and functional connectivity correlates of empathizing7,8,23. General intelligence control is a standard procedure and general intelligence is included as a covariate to exclude the possibility of associations between MD and empathizing or systemizing, explained in terms of the associations between general intelligence and MD (combined with those between general intelligence and empathizing or systemizing).
The anatomical labels of significant clusters of major white matter fibers presented in the Results section were determined using the ICBM DTI-81 Atlas (http://www.loni.ucla.edu/).
A multiple comparison correction of the cross-sectional analyses was performed using threshold-free cluster enhancement (TFCE)42, with randomized (5,000 permutations) nonparametric permutation testing via the TFCE toolbox (http://dbm.neuro.uni-jena.de/tfce/). We applied the threshold of an FWE corrected P < 0.05.
Ethical approval
This study was approved by the Ethics Committee of Tohoku University. All experiments were performed in accordance with declaration of Helsinki.
Results
Behavioral data
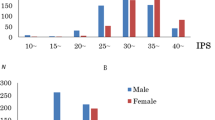
The mean EQ scores of males and females were 30.24 (SD, 9.64; range 7–66) and 34.68 (SD, 9.86; range 12–67), respectively. The mean SQ scores of males and females were 28.44 (SD, 8.59; range 6–57) and 21.60 (SD, 7.36; range 8–54), respectively. The mean D scores of males and females were 0.0556 (SD, 0.0698; range −0.1644 to 0.2981) and −0.0149 (SD, 0.0700; range −0.2206 to 0.1919), respectively. The mean, SD, and range of all psychological variables is presented in Table 1.
For all subjects, simple regression analyses showed significant (a) positive correlation between EQ and SQ scores, (b) negative correlation between EQ and D scores, (c) negative correlation between EQ and RAPM scores, (d) positive correlation between EQ score and cooperativeness, (d) positive correlation between SQ and D scores, (e) positive correlation between SQ and RAPM scores, (f) positive correlation between D and RAPM scores, and (g) negative correlation between D score and cooperativeness.
For male subjects, simple regression analyses showed significant (a) positive correlation between EQ and SQ scores, (b) negative correlation between EQ and D scores, (c) positive correlation between SQ and D scores, (d) positive correlation between SQ and RAPM scores, (e) positive correlation between SQ score and cooperativeness, (f) positive correlation between D and RAPM scores, and (g) negative correlation between D score and cooperativeness.
For female subjects, simple regression analyses showed (a) positive correlation between EQ and SQ scores, (b) negative correlation between EQ and D scores, (c) negative correlation between EQ and RAPM scores, (d) positive correlation between EQ score and cooperativeness, (d) positive correlation between SQ and D scores, (e) positive correlation between SQ and RAPM scores, (f) positive correlation between D and RAPM scores, (g) negative correlation between D score and cooperativeness, and (h) negative correlation between RAPM and cooperativeness scores. For statistical values, see Table 2.
Figure 1 presents the data of distributions of EQ and SQ scores. Figure 2 presents the data of distributions of D scores.
These results can be summarized as follows:
-
(a)
In both sexes, D scores (discrepancy between systemizing and empathizing [systemizing − empathizing]) was positively correlated with SQ score and negatively correlated with EQ score, as expected from the definition of D score.
-
(b)
In both sexes, cooperativeness was positively correlated with EQ score and negatively correlated with D score, which is consistent with the pro-social characteristics of cooperativeness as described in the Introduction.
-
(c)
In both sexes, RAPM score and SQ score were positively correlated, consistent with the nature of systemizing (the drive to analyze the rules that govern a system)1.
-
(d)
In both sexes, EQ score and SQ score were positively correlated, although only weakly so.
-
(e)
In females, EQ score and cooperativeness were negatively correlated with RAPM score, suggesting sex-specific characteristics of empathizing and cooperativeness.
Effects of EQ, SQ, and D scores on MD
ANCOVA involving both EQ and SQ scores revealed a significant overall positive effect (regardless of sex) of the EQ score on MD in the anatomical cluster that spreads in the areas in and adjacent to the left dorsolateral prefrontal cortex, left ACC, and the left insula; in the anatomical cluster of the left postcentral gyrus and left rolandic operculum; and in the anatomical cluster of the left middle cingulate gyrus (Fig. 3, Table 3). There were no other significant effects of EQ and SQ scores, interaction between sex and the EQ score, and interaction between sex and the SQ score on MD. ANCOVA involving the D score revealed no significant effects of the D score or of the interaction between D score and sex on MD.
The main positive effects (regardless of sex) of the EQ score across both sexes. (a,c) The results shown were obtained using a threshold of threshold-free cluster enhancement (TFCE) of P < 0.05, based on 5000 permutations. The results were corrected at the whole-brain level. Regions with significant correlations are overlaid on a “single subject” T1 image of SPM8. The color represents the strength of the TFCE value. (a) Regions with negative main positive effects of EQ scores on MD across both sexes were observed in the areas in and adjacent to the left dorsolateral prefrontal cortex, left ACC, and left insula. (b) Scatter plot of the associations between EQ scores and mean MD values for this cluster of (a). (c) Regions with positive main positive effects of EQ scores on MD across both sexes were observed in the areas of the left postcentral gyrus and left rolandic operculum. (d) Scatter plot of the associations between EQ scores and mean MD values for this cluster of (b).
Effects of cooperativeness on MD
ANCOVA involving cooperativeness revealed a significant overall positive effect (regardless of sex) of the cooperativeness on MD in the anatomical cluster that spreads in the areas in and adjacent to the left dorsolateral prefrontal cortex, left ACC, and the left insula; in the anatomical cluster that spreads in the areas in and adjacent to the right dorsolateral prefrontal cortex, the right ACC, and right insula; in the anatomical cluster that spreads in and adjacent to the left lateral and medial parietal lobes as well as in the anatomical cluster that spreads in and adjacent to the right lateral and medial parietal lobes (Fig. 4, Table 4).
The main positive effects (regardless of sex) of cooperativeness score across both sexes. (a) The results shown were obtained using a threshold of threshold-free cluster enhancement (TFCE) of P < 0.05, based on 5000 permutations. The results were corrected at the whole brain level. Regions with significant correlations are overlaid on a “single subject” T1 image of SPM8. The color represents the strength of the TFCE value. Regions with negative main positive effects of cooperativeness scores on MD across both sexes were observed in the areas in and adjacent to the gray and white matter areas of bilateral frontal and parietal areas. (b–d) Scatter plot of the associations between EQ scores and mean MD values for the cluster spreading mainly across the posterior areas of the right hemisphere (b), mean MD values for the cluster spreading mainly across the left hemisphere (c), mean MD values for the cluster spreading mainly across the anterior areas of the right hemisphere (d).
There was a substantial overlap between the cluster of overall positive effects of the EQ score and the cluster of overall positive effects of cooperativeness in the areas in and adjacent to the gray and white matter areas of the left dorsolateral prefrontal cortex, left ACC, and left insula.
Discussion
To the best of our knowledge, this is the first study to successfully reveal MD correlates of empathizing. Our results showed that both sexes present a positive correlation between empathizing and the MD of an anatomical cluster primarily adjacent to the left dorsolateral prefrontal cortex, left anterior and middle cingulate cortex, and left insula; the anatomical cluster of the left postcentral gyrus and left rolandic operculum; and the anatomical cluster of the left middle cingulate gyrus. Further, cooperativeness is positively correlated with MD of the bilateral anatomical clusters that spread between the anterior and middle cingulate cortex, medial prefrontal cortex, insula lateral prefrontal cortex as well as the bilateral anatomical clusters that spread between the medial parietal cortex and lateral parietal cortex. These data support our second hypothesis that predicted an overlap between MD correlates of empathizing and those of cooperativeness in the area between the dorsal part of the ACC, the insula, and the lateral prefrontal cortex. However, the hypothesized involvement of DMN areas in empathizing and the involvement of EAS areas in systemizing were not supported, suggesting that MD correlates have unique characteristics. The results suggest that empathizing is reflected in microstructural properties in areas that overlap with areas that play key roles in emotional salience and empathy as well as with areas that play key roles in the mirror neuron system, as discussed below.
Overall, previous studies have generally suggested that increased MD is associated with reduced neural tissues and possibly function. As described similarly in our previous study15, decreased MD has been suggested to reflect various cellular and cytoarchitectonic changes resulting in higher tissue density in various tissue components, such as synapses, macromolecular proteins, capillaries, and spines; changes in the properties of myelin, axon and membrane; shape alterations of glia or neurons; or enhanced tissue organization11,13. However, MD is not specifically sensitive to any one of them11,13. Therefore, MD decrease is thought to reflect tissue and functional adaptation increase14. Consistently, a greater motivational state is associated with lower MD of the subcortical areas involved in motivation, such as the putamen and pallidum15, and greater performance IQ is associated with lower MD of the extensive areas across the whole brain areas14. However, lower MD can indicate blood flow decreases and in certain cases, functional adaptation is seemingly reflected in an increase in MD43. Therefore, whether lower MD signifies an adaptive condition cannot be definitely concluded. In addition, while in autistic subjects, MD is robustly and extensively elevated and ASCs is characterized with lower empathizing17, in this study, lower empathizing is associated with lower MD. Therefore, the neural mechanisms behind the variations in tendencies of ASCs in the normal sample and the neural mechanisms behind autism may be different.
There was an overlap of positive MD correlates of empathizing and cooperativeness in the anatomical cluster between the dorsal part of ACC, the lateral prefrontal cortex, and the insula. The anterior insula and the dorsal-anterior/anterior-midcingulate cortex play central roles in responses in the domain of various pleasant and disgusting feelings. Moreover, those regions play a central role in the subjective experiences and adaptive responses toward the predicted and actual states, both those within oneself and in others. Empathy constitutes a special case among these general cognitive processes44,45. Therefore, augmentation of these regional functions is possibly reflected in greater MD due to increased default cerebral blood flow. Other mechanisms may contribute to empathizing and they form the observed correlations herein. Alternatively, the anterior cingulate and anterior insula form the salience network46, which is thought to integrate interoceptive information with emotional salience46,47. Greater functioning of these areas is thought to be associated with generalized anxiety46 and social anxiety48. Thus, as we noted previously16, we speculate that a greater amount of tissues in this pathway may be associated with social anxiety, which in turn may prohibit prosociality that likely plays a key role in empathizing and cooperativeness. However, because this overlap of MD correlates was extensive, and this area is adjacent to other networks, such as EAS, positive MD correlates in this area may reflect other cognitive factors, such as relatively weakened function of EAS, or increased cerebral blood flow to these areas. Therefore, future studies need to elucidate the nature of increased MD in this area. Furthermore, in relation to the observed findings in the insula, a previous study has shown that a greater score in a measure of affective empathy (personal distress, defined as a focus on having aversive emotional feelings when witnessing another’s pain or anguish) was associated with a greater magnetization transfer measure49. This measure captures not only the effects of macromolecules, predominantly myelin content, but also the effects of other cell components that facilitate myelination and overall myeloarchitectural integrity49. Since a lower measure of magnetization transfer and a greater MD are associated with advanced aging in similar areas50, the present findings regarding a positive correlation between empathizing and MD in the insular and contingent areas may be parallel to the previous findings regarding a negative correlation between a measure of affective empathy and the magnetization transfer measure.
There was no significant correlation between MD and systemizing. Our previous study showed that among seven major personalities of temperament and character inventory, cooperativeness showed positive associations with MD in bilateral areas between ACC, lateral prefrontal cortex, and insula16. However, other six personalities that showed substantial correlation with cognitive components of motivation were correlated with MD measurements in limited areas related to the dopaminergic system, including the putamen, pallidum, caudate and as well as contingent areas and the thalamus16. Further, we investigated MD correlates of mood states using by the Profile of Mood states15. However, we found robust significant negative correlation between motivational state (state vigor) and MD in the thalamus, putamen, pallidum, and contingent areas15. Also, there was no significant correlation between other mood states and MD. Therefore, although there are a variety of cognitive components in these traits of temperament and character inventory and states of Profile of Mood states, it seems that only MD of the limited areas show correlation with limited cognitive components of these traits and states and perhaps systemizing does not include these cognitive components. Future studies are needed to elucidate whether these (lack of correlation between MD and cognitive differences that do not include certain limited cognitive components) are applied to other states and traits and the mechanism behind this phenomenon.
Empathizing was positively correlated with the MD of the left postcentral gyrus and the Rolandic operculum areas. These regions overlap with areas of the key nodes of the mirror neuron system51. The involvement of these regions in empathizing may be consistent with the view that the mirror neuron system facilitates the understanding of the intentions of others and plays an important role in empathy52,53, as well as with the finding that reduced rGMV in these areas, which may be caused by advanced synaptic pruning, is associated with greater empathizing8. Depending on the situational context and the information available in the environment (such as the perceived fairness of another person or group membership and the similarities between oneself and others), empathic responses have been suggested to involve a co-recruitment of mirror neuron networks and regions involved in the theory of mind or mentalizing54,55,56.
Finally, cooperativeness showed significant positive correlation with MD in anatomical clusters that spread in and adjacent to the left lateral and medial parietal lobes, as well as in the anatomical cluster that spreads in and adjacent to the right lateral and medial parietal lobes, which were not observed in areas showing a significant positive correlation between empathizing and MD. Functional implications of these correlates are unclear because of lack of previous studies showing specific MD correlates in these areas, which are widespread and the contingent areas are associated with multiple functions57. However, one possibility is that posterior MD correlates around the posterior cingulate cortex (PCC) may relate to disrupted functions that inhibit prosociality, similar to the anterior MD correlates of cooperativeness, namely contentious interpersonal orientation, aggression, and anger. This is because the anterior part of PCC is functionally associated with negative emotions, such as anger, fear, and pain58. We have previously suggested an association between contentious interpersonal orientation and this area’s structural properties, given the correlation of PCC’s regional gray matter density with traits such as the hostile behaviors displayed by Type A personalities and competitive achievement motivation (i.e., the desire to manage and succeed in difficult tasks, directed at the pursuit of social prestige by defeating and outperforming others)59,60. Further, while a lack of serotonin plays a key role in aggression61, reduced serotonin in the PCC is associated with unfriendliness and greater social aggression in primates62. However, this is speculative and future studies are needed to better understand the impact of a greater MD in PCC among young normal adults.
Previous studies have investigated regional gray matter, white matter volume, and fractional anisotropy and resting state functional connectivity that is associated with empathizing, systemizing, and D score7,8,23,63. Each of these imaging measures can provide unique information about the brain, but MD measures reveal unique information that other techniques cannot provide. For example, the state and traits that are associated with cognitive components of motivation showed robust association with MD in the putamen and pallidum which play a key role in motivation15,16 and we previously showed that fatigue was positively correlated with MD in the basal ganglia; however, the amount of regional gray matter in these areas failed to show such associations64. As described above, cooperativeness showed a robust association with MD in and near areas that play key roles in emotional salience and anxiety16. But, to our knowledge, it is unknown whether different imaging techniques show association with cooperativeness in the same areas. Therefore, by using this unique measurement, we have elucidated the neural bases of empathizing.
In the present study, relatively small correlation coefficients were found between the mean MD values in the significance cluster and empathizing or systemizing (r < 0.11). In studies with large samples of young, normal individuals, relatively weak correlations (r < 0.2) between individual cognitive differences and neuroimaging measures are a universal phenomenon (i.e., N > several hundreds), regardless of the type of imaging measures21,65,66,67,68. This also holds true for associations between representative imaging measures and cognitive abilities, such as associations between gray matter volume or cortical thickness and general intelligence measures or working memory performance, and associations between white matter volume and processing speed. Therefore, the low correlation coefficients obtained in this study do not indicate a low relevance of the observed associations. It is noteworthy that in whole-brain imaging analyses with small samples, overfitting usually causes an extreme effect size overestimation69.
In conclusion, while increased MD is generally associated with decreased neural tissues and possibly function of an area, higher empathizing and cooperativeness was reflected by greater MD measurements of the areas in and adjacent to the left anterior and middle cingulate cortex, left lateral prefrontal cortex, and left insula. These areas mainly overlapped areas that play a key role in empathy and emotional salience. In addition, higher empathizing was reflected in greater MD of the left postcentral gyrus and left Rolandic operculum areas, which are overlapped with the areas of the mirror neuron system.
References
Baron-Cohen, S. The essential difference: The truth about the male and female brain. (Perseus Books Group, New York, 2003).
Baron-Cohen, S., Richler, J., Bisarya, D., Gurunathan, N. & Wheelwright, S. The systemizing quotient: an investigation of adults with Asperger syndrome or high–functioning autism, and normal sex differences. Philosophical Transactions of the Royal Society of London B: Biological Sciences 358, 361–374 (2003).
Baron-Cohen, S., Richler, J., Bisarya, D., Gurunathan, N. & Wheelwright, S. The systemizing quotient: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 358, 361–374 (2003).
Baron-Cohen, S. & Wheelwright, S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders 34, 163–175 (2004).
Baron-Cohen, S., Knickmeyer, R. C. & Belmonte, M. K. Sex differences in the brain: implications for explaining autism. Science 310, 819–823 (2005).
Krill, A. L., Platek, S. M. & Wathne, K. Feelings of control during social exclusion are partly accounted for by empathizing personality. Personality and individual differences 45, 684–688 (2008).
Takeuchi, H. et al. White matter structures associated with empathizing and systemizing in young adults. Neuroimage 77, 222–236 (2013).
Takeuchi, H. et al. Regional gray matter volume is associated with empathizing and systemizing in young adults. PLoS ONE 9, e84782 (2014).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The Brain’s Default Network. Annals of the New York Academy of Sciences 1124, 1–38 (2008).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience 3, 201–215 (2002).
Beaulieu, C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed. 15, 435–455 (2002).
Takeuchi, H., & Kawashima, R. Mean diffusivity in the dopaminergic system and neural differences related to dopaminergic system. Current neuropharmacology, 16(4), 460–474 (2018).
Sagi, Y. et al. Learning in the fast lane: new insights into neuroplasticity. Neuron 73, 1195–1203 (2012).
Takeuchi, H. et al. Impact of videogame play on the brain’s microstructural properties: Cross-sectional and longitudinal analyses. Mol. Psychiatry 21, 1781–1789 (2016).
Takeuchi, H. et al. Mean diffusivity of basal ganglia and thalamus specifically associated with motivational states among mood states. Brain Struct. Funct. 1–11 (2016).
Takeuchi, H. et al. Mean diffusivity of globus pallidus associated with verbal creativity measured by divergent thinking and creativity-related temperaments in young healthy adults. Hum. Brain Mapp. 36, 1808–1827 (2015).
Groen, W. B., Buitelaar, J. K., Van Der Gaag, R. J. & Zwiers, M. P. Pervasive microstructural abnormalities in autism: a DTI study. Journal of psychiatry & neuroscience: JPN 36, 32 (2011).
Takeuchi, H. et al. Regional gray matter density is associated with morningness–eveningness: Evidence from voxel-based morphometry. Neuroimage 117, 294–304 (2015).
Takeuchi, H. et al. White matter structures associated with emotional intelligence: Evidence from diffusion tensor imaging. Hum. Brain Mapp. 34, 1025–1034 (2013).
Takeuchi, H. et al. Differences in gray matter structure correlated to nationalism and patriotism. Scientific reports 6, article 29912 (2016).
Takeuchi, H. et al. Degree centrality and fractional amplitude of low-frequency oscillations associated with Stroop interference. Neuroimage 119, 197–209 (2015).
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Takeuchi, H. et al. Association between resting-state functional connectivity and empathizing/systemizing. Neuroimage 99, 312–322 (2014).
Takeuchi, H. et al. Effects of Multitasking-Training on Gray Matter Structure and Resting State Neural Mechanisms. Hum. Brain Mapp. 35, 3646–3660 (2014).
Wakabayashi, A. et al. Empathizing and systemizing in adults with and without autism spectrum conditions: cross-cultural stability. J. Autism Dev. Disord. 37, 1823–1832 (2007).
Takeuchi, H. et al. Creativity measured by divergent thinking is associated with two axes of autistic characteristics. Frontiers in psychology 5, Article 921, 1–8 (2014).
Goldenfeld, N., Baron-Cohen, S. & Wheelwright, S. Empathizing and systemizing in males, females and autism. Clinical Neuropsychiatry 2, 338–345 (2005).
Wheelwright, S. et al. Predicting autism spectrum quotient (AQ) from the systemizing quotient-revised (SQ-R) and empathy quotient (EQ). Brain Research 1079, 47–56 (2006).
Wakabayashi, A. et al. Development of short forms of the Empathy Quotient (EQ-Short) and the Systemizing Quotient (SQ-Short). Personality and individual differences 41, 929–940 (2006).
Nettle, D. Psychological profiles of professional actors. Personality and individual differences 40, 375–383 (2006).
Stileman, E. In Department of Psychology, Vol. Undergraduate (University of Edinburgh, Edinburgh (2007).
Penton-Voak, I. S., Allen, T., Morrison, E. R., Gralewski, L. & Campbell, N. Performance on a face perception task is associated with empathy quotient scores, but not systemizing scores or participant sex. Personality and individual differences 43, 2229–2236 (2007).
Groen, Y., Fuermaier, A., Den Heijer, A., Tucha, O. & Althaus, M. The empathy and systemizing quotient: The psychometric properties of the Dutch version and a review of the cross-cultural stability. J. Autism Dev. Disord. 45, 2848–2864 (2015).
Wakabayashi, A., Baron-Cohen, S. & Wheelwright, S. Individual and gender differences in Empathizing and Systemizing: Measurement of individual differences by the Empathy Quotient (EQ) and the Systemizing Quotient (SQ). Japanese Journal of Psychology 77, 271–277 (2006).
Raven, J. Manual for Raven’s progressive matrices and vocabulary scales. (Oxford Psychologists Press, Oxford, 1998).
Takeuchi, H. et al. White matter structures associated with creativity: Evidence from diffusion tensor imaging. Neuroimage 51, 11–18 (2010).
Takeuchi, H. et al. Regional gray matter volume of dopaminergic system associate with creativity: Evidence from voxel-based morphometry. Neuroimage 51, 578–585 (2010).
Kijima, N. et al. Cloninger-no-kishitsu-to-seikaku-no-7inshimodel-oyobi-nihongoban [Cloninger’s seven-factor model of temperament and character and Japanese version of Temperament and Character Inventory (TCI)]. Seishinka-shindangaku [Archives of Psychiatric Diagnosis and Clinical Evaluation] 7, 379–399 (1996).
Cloninger, C. R., Svrakic, D. M. & Przybeck, T. R. A psychobiological model of temperament and character. Arch. Gen. Psychiatry 50, 975–990 (1993).
Takeuchi, H. et al. Creative females have larger white matter structures: evidence from a large sample study. Hum. Brain Mapp. 38, 414–430 (2017).
Takeuchi, H. et al. Shorter sleep duration and better sleep quality are associated with greater tissue density in the brain. Scientific reports 8, 5833 (2018).
Smith, S. M. & Nichols, T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44, 83–98 (2009).
Takeuchi, H. et al. Working memory training impacts the mean diffusivity in the dopaminergic system. Brain Struct. Funct. 220, 3101–3111 (2015).
Bernhardt, B. C. & Singer, T. The neural basis of empathy. Annu. Rev. Neurosci. 35, 1–23 (2012).
Decety, J. & Lamm, C. Human empathy through the lens of social neuroscience. The scientific World journal 6, 1146–1163 (2006).
Seeley, W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience 27, 2349–2356 (2007).
Taylor, K. S., Seminowicz, D. A. & Davis, K. D. Two systems of resting state connectivity between the insula and cingulate cortex. Hum. Brain Mapp. 30, 2731–2745 (2009).
Liao, W. et al. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage 52, 1549–1558 (2010).
Allen, M. et al. Insula and somatosensory cortical myelination and iron markers underlie individual differences in empathy. Scientific Reports 7, 43316 (2017).
Draganski, B. et al. Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ). Neuroimage 55, 1423–1434 (2011).
Cattaneo, L. & Rizzolatti, G. The mirror neuron system. Arch. Neurol. 66, 557–560 (2009).
Rizzolatti, G. & Craighero, L. Mirror neuron: a neurological approach to empathy. Neurobiology of human values, 107–123 (2005).
Iacoboni, M. & Dapretto, M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience 7, 942–951 (2006).
Singer, T. et al. Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162 (2004).
Meyer, M. L. et al. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Soc. Cogn. Affect. Neurosci. 8, 446–454 (2012).
Sessa, P. & Meconi, F. Perceived trustworthiness shapes neural empathic responses toward others’ pain. Neuropsychologia 79, 97–105 (2015).
Cavanna, A. E. & Trimble, M. R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583 (2006).
Vogt, B. A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 6, 533–544 (2005).
Takeuchi, H. et al. Amygdala and cingulate structure is associated with stereotype on sex-role. Scientific reports 5, article 14220 (2015).
Takeuchi, H. et al. Regional gray matter density is associated with achievement motivation: evidence from voxel-based morphometry. Brain Struct. Funct. 219, 71–83 (2014).
Siever, L. Neurobiology of aggression and violence. A. J. Psychiatry 165, 429–442 (2008).
Yokoyama, C., Kawasaki, A., Hayashi, T. & Onoe, H. Linkage Between the Midline Cortical Serotonergic System and Social Behavior Traits: Positron Emission Tomography Studies of Common Marmosets. Cereb. Cortex 23, 2136–2145 (2013).
Sassa, Y. et al. The correlation between brain gray matter volume and empathizing and systemizing quotients in healthy children. Neuroimage 60, 2035–2041 (2012).
Nakagawa, S. et al. Basal ganglia correlates of fatigue in young adults. Scientific reports 2016, article 21386 (2016).
Takeuchi, H. et al. Global associations between regional gray matter volume and diverse complex cognitive functions: evidence from a large sample study. Scientific Reports 7, article 10014 (2017).
Magistro, D. et al. The Relationship between Processing Speed and Regional White Matter Volume in Healthy Young People. PLoS ONE 10, e0136386 (2015).
Schilling, C. et al. Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Mol. Psychiatry 18, 624–630 (2012).
Takeuchi, H. et al. General intelligence is associated with working memory-related brain activity: new evidence from a large sample study. Brain Struct. Funct. Epub ahead of print (2018).
Vul, E., Harris, C., Winkielman, P. & Pashler, H. Reply to comments on “puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition”. Perspect. Psycholo. Sci. 4, 319–324 (2009).
Maldjian, J. A., Laurienti, P. J. & Burdette, J. H. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21, 450–455 (2004).
Maldjian, J. A., Laurienti, P. J., Kraft, R. A. & Burdette, J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002).
Acknowledgements
We thank Yuki Yamada for operating the MRI scanner, Haruka Nouchi for conducting the psychological tests, all other assistants for helping with the experiments and the study, and the study participants, and all our other colleagues at IDAC, Tohoku University for their support. This study was supported by JST/RISTEX, JST/CREST, a Grant-in-Aid for Young Scientists (B) (KAKENHI 23700306), and a Grant-in-Aid for Young Scientists (A) (KAKENHI 25700012) from the Ministry of Education, Culture, Sports, Science, and Technology. This study was supported by JST/RISTEX, JST/CREST, a Grant-in-Aid for Young Scientists (B) (KAKENHI 23700306) and a Grant-in-Aid for Young Scientists (A) (KAKENHI 25700012) from the Ministry of Education, Culture, Sports, Science, and Technology.
Author information
Authors and Affiliations
Contributions
H.T., Y.T. and R.K. designed the study. H.T., A.S., R.N., Y.K., S.N., C.M.M., K.I., R.Y., Y.Y., S.H., T.A., Y.S., K.S., T.N., S.I., S.Y., Y.S. and M.D. collected the data. H.T. analyzed the data and prepared the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takeuchi, H., Taki, Y., Nouchi, R. et al. Empathizing associates with mean diffusivity. Sci Rep 9, 8856 (2019). https://doi.org/10.1038/s41598-019-45106-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45106-1
This article is cited by
-
Rapid neuroplasticity changes and response to intravenous ketamine: a randomized controlled trial in treatment-resistant depression
Translational Psychiatry (2023)
-
Mercury levels in hair are associated with reduced neurobehavioral performance and altered brain structures in young adults
Communications Biology (2022)
-
Sensory processing sensitivity and axonal microarchitecture: identifying brain structural characteristics for behavior
Brain Structure and Function (2022)
-
Macro- and micro-structural cerebellar and cortical characteristics of cognitive empathy towards fictional characters in healthy individuals
Scientific Reports (2021)
-
Childhood socioeconomic status is associated with psychometric intelligence and microstructural brain development
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.