Abstract
Associative memory (AM) deficits are common in neurodegenerative disease and novel therapies aimed at improving these faculties are needed. Theta band oscillations within AM networks have been shown to be important for successful memory encoding and modulating these rhythms represents a promising strategy for cognitive enhancement. Transcranial alternating current stimulation (TACS) has been hypothesized to entrain and increase power of endogenous brain rhythms. For this reason, we hypothesized that focal delivery of theta band electrical current, using high-definition TACS, would result in improved AM performance compared to sham stimulation or transcranial direct current stimulation (TDCS). In this pilot study, 60 healthy subjects were randomized to receive high definition TACS, high definition TDCS, or sham stimulation delivered to the right fusiform cortex during encoding of visual associations. Consistent with our hypothesis, improved AM performance was observed in the TACS group, while TDCS had no effect. However, TACS also resulted in improved correct rejection of never seen items, reduced false memory, and reduced forgetting, suggesting the effect may not be specific for AM processes. Overall, this work informs strategies for improving associative memory and suggests alternating current is more effective than direct current stimulation in some contexts.
Similar content being viewed by others
Introduction
Memory for the relationship or association between two items (associative memory; AM) is critical for normal everyday functioning. In this type of memory, individual experiences, objects, or words are linked together directly, or through spatial, temporal, or other kinds of relationships. Associative memory is central to episodic memory abilities1,2. Examples include remembering a person’s name or remembering the details of where you met a specific person. Associative memory can be considered verbal, such as when remembering a name which is associated with a face, or visual, such as when remembering the link between two visual objects. Difficulties with associative memory can result in disabling cognitive impairment, like those most readily seen in Alzheimer’s Disease3. Encoding of associative information occurs in distributed brain networks involving the left inferior frontal cortex, bilateral fusiform cortex, bilateral medial temporal lobe, bilateral premotor cortex, and bilateral posterior parietal cortex4,5,6. The modality of information to be remembered influences the relative extent of activation within this distributed network. For example, in a large meta-analysis examining neural regions involved in the subsequent memory effect of verbal and non-verbal(visual) information, it was found that the fusiform cortex was preferentially involved in the encoding of successful visual associative memory4. While this region is well known for its involvement in encoding face stimuli7, these meta-analytical results support a more general involvement of the fusiform region in visual memory encoding. Indeed, the inferior occipital-temporal region, of which the fusiform cortex is a part of, has long been hypothesized to have critical mnemonic functions8. Other research points to a role of the fusiform region in encoding of specific and general visual features9, and a role in the development of visual expertise10. Supporting a more general role in visual mnemonic functions, the fusiform region is also commonly implicated in disorders of cognition such as Alzheimer’s Disease11 and Parkinson’s Disease Dementia12,13,14. Along with the structure of associative brain networks, it is important to consider the neural dynamics which contribute to successful memory. In particular, theta band oscillations within the associative memory network are thought to play a critical role in the encoding of memories15,16,17,18,19, with a lateralization of these frequency effects seen in the right temporal lobe16.
Transcranial electrical current stimulation (TES) delivers electrical activity into the brain via electrodes attached to the scalp20, and has recently been used to modulate cognitive performance in a wide-variety of paradigms21. The electrical current is typically delivered in a relatively non-focal manner using a pair of electrodes placed on the scalp (1 × 1 electrode configuration). However, multi-electrode configurations (refered to as High Definition; HD) have been shown to result in a more focal electrical field distribution22,23. Typical HD electrode set-ups utilize a 4 × 1 ring configuration, where one center electrode is surrounded by 4 return electrodes. More recent multi-channel devices (MxN HD) with individual control of current intensity at each electrode allow for unique combinations of electrode locations combined with current optimization algorithms to more focally target brain regions24.
Modulation of associative memory with TES has been mostly limited to application of direct current (TDCS) paradigms during verbal associative memory tasks. Stimulation using 1 × 1 TDCS applied to the left dorsolateral prefrontal cortex (DLPFC)25,26 or left inferior frontal gyrus (IFG)27 during encoding of a face-name task improved memory performance. These positive results are contrasted by the findings of Gaynor et al.28 and Leach et al.29. Gaynor et al. found impaired memory performance when 1 × 1 anodal TDCS was applied to the left DLPFC during the encoding of semantically unrelated word pairs. Leach et al. applied 1 × 1 TDCS to the inferior frontal gyrus in older adults during the encoding of a face-name task and found increased false memory. While these studies show promise, their inconsistencies suggest new avenues should be explored. Indeed, a recent meta-analysis assessing the effect of TDCS on episodic memory found inconsistent and small effects. Still, the studies that used recall tasks and longer duration of stimulation showed enhancing effects of anodal tDCS30. Further, TDCS studies that have targeted posterior brain regions show promise for modulating both item31,32,33 and associative memory34,35,36.
In contrast to TDCS, an alternative form of TES involves the delivery of an alternating current, typically with a sinusoidal waveform.Transcranial alternating current stimulation (TACS) delivers oscillatory electrical activity into the brain via the same electrode set-up utilized in TDCS37. At the cellular level, it is thought that TACS sinusoidally alters the transmembrane potential, an effect which is magnified through synaptically connected neurons38. This gives rise to what is considered the primary mechanism, which is an amplification and entrainment of endogenous neuronal oscillations39,40,41,42. TACS can also have widespread effects on neuronal networks41,43,44 and may induce after-effects lasting up to 70 minutes if delivered for prolonged periods of time45. Theta TACS has previously been used to improve reaction times during performance of a visual memory task, though this study did not investigate whether memory performance (i.e number of correctly identified items) was modulated46. In a direct comparison of TDCS vs TACS on working memory performance, it was observed that TACS improved reaction time for hits compared to TDCS47. This offers some evidence that TACS might be more efficacaous in improving cognitive abilities as compared to TDCS.
Given the conflicting results obtained with TDCS, the importance of the fusiform region and theta oscillations in successful visual memory, and previous research suggesting TACS can modulate memory46 and may be more efficacaous then TDCS47, the present study utilized a strategy aimed at amplifying endogenous theta power in the fusiform region during memory encoding with MxN HD-TACS. Stimulation of the fusiform region has been shown to modulate face perception48 and facial working memory49, but has not been applied in an associative memory paradigm. We hypothesized that HD-TACS would improve visual associative memory performance compared to TDCS and sham stimulation. We included an active control group, applying anodal HD-TDCS, to investigate the necessity of the theta rhythm. To our knowledge, this study is the first to directly compare the effects of HD-TACS to those of HD-TDCS applied to the visual associative cortex on the performance of an associative memory task. This may guide future clinical therapies using non-invasive brain stimulation for memory enhancement.
Methods
Design
This is a single blind, between group, randomized, and sham controlled pilot trial assessing the difference between theta (6 Hz) HD-TACS, anodal HD-TDCS, and sham stimulation on visual associative memory performance.
Subjects
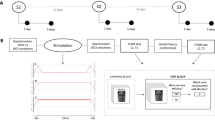
60 healthy young adults (18–45) were recruited from the university environment and underwent informed consent to participate in this protocol, which was reviewed and approved by the University of Calgary’s Conjoint Health Research Ethics Board. All methods were carried out in accordance with institutional ethical standards and with the relevant guidelines and regulations. Inclusion criteria included age between 18 and 45, no history of neurological or psychiatric disease, no recreational drug use, and no other contraindication to transcranial electrical stimulation. Participants were screened before each experiment to ensure they met these criteria and were reimbursed $40 for their participation. All experimental sessions occurred between the hours of 0730 am and 1400 pm. Subjects were randomized into one of three groups: TACS (6 Hz), anodal TDCS, or sham stimulation. One subject was excluded for failing to follow instructions, resulting in 59 subjects (TACS = 19; TDCS = 21; Sham = 19). A permutation in randomisation occurred, with one subject initially randomized to the sham group receiving TDCS due to experimenter error. Data was analysed as treated (Fig. 1). Prior to beginning the protocol, subjects completed a survey which included sleep and fatigue ratings (Supplementary Information I). Demographic information is displayed in Table 1.
Memory task
A visual associative memory task was designed in-house, called the Face and Scene Task (FAST). Previous studies have used similar face-scene tasks to investigate associative memory50,51,52, and have shown activity in the fusiform cortex increases for correct memory52 and declines with age51. In FAST, pictures of a face and outdoor scene are displayed side by side on a computer screen. Faces are of various ethnicities and ages, and all display a neutral expression. Scene pictures were drawn from an online public database (www.pixabay.com), while face pictures were drawn from the Park Aging Mind Laboratory Face Database53. Subjects were asked to remember the association between the Face and Scene, and were explicitly told their memory would be tested following the memorization/encoding phase. Picture pairs were presented for six seconds each, with a three second inter-stimulus interval. Subjects viewed 27 picture pairs during the encoding phase. This number of picture pairs was chosen based on pilot data suggesting an optimal trade-off between minimzing the ceiling effect and ensuring subjects were not responding at chance levels. Following the encoding phase, subjects completed a distraction task where they evaluated a series of simple arithmetic expressions. Twenty-five simple math expressions were presented on the screen, and subjects were instructed to identify whether they were correct or not. This distraction phase was implemented to limit recency effects while being sufficiently distinct to not engage cognitive processes involved in the FAST task54. Immediately following the distraction phase, the recognition phase commenced. In this phase, 27 picture pairs, consisting of the same picture pairs the subjects had memorized (called ‘together’ pairs), were randomly presented with 27 ‘lure’ pairs (consisting of a face and scene previously seen but not in the correct pairing) and 27 new pairs (consisting of a face and scene which the subjects were not exposed to during encoding). Lure pairs always consisted of both a face and scene which had previously been presented. Subjects were required to determine whether the pictures pairs were previously seen ‘together’, ‘not together’ or ‘never seen’, respectively (Fig. 2A). A maximum limit of ten seconds per picture pair was given to perform the recognition task. The primary outcomes for analysis were Correct Associative Memory and Incorrect Associative Memory. Correct Associative Memory was the proportion of responses which correctly identifies ‘together’ pairs and ‘lure’ pairs. Incorrect Associative Memory was defined as the proportion of answers which misidentifies ‘lure’ pairs as being previously seen together and ‘together’ pairs as being previously seen, but not in the same pairing. Three other memory outcomes were analyzed as secondary outcomes: Correct Rejection, False Memory, and Forgetting. Correct Rejection occurred when subjects correctly identified never seen picture pairs, False Memory occurred when subjects identified never seen picture pairs as being previously seen (together or not together), and Forgetting occurred when subjects answered ‘never seen’ to pictures which had previously been seen (Fig. 2B). Each subject performed two different versions of the task, once without stimulation (baseline performance) and once with stimulation during encoding (stimulation performance). The versions had the exact same format, but each had a unique set of picture pairs. The order of the two versions were counter-balanced between participants, though stimulation always occurred during the second presentation to mitigate influence of stimulation after-effects45. Primary outcomes were compared between groups on this second administration of the task. To familiarise subjects with the task, a training phase was administered prior to the baseline performance, in which a separate, abbreviated version of the task was performed with five encoding pairs and 15 recognition pairs (five ‘together’, five ‘lure’, and five never seen pairs). Twenty-four hours after the intitial memory testing, subjects returned to test for any prolonged effects of stimulation. On this visit, subjects were asked to perform the exact same recognition tests (without encoding) that they had performed the day prior. The same memory outcomes were calculated based on their performance. The study protocol is represented in Fig. 3.
(A) Face and Scene Task (FAST): 27 encoding pairs are displayed sequentially, followed by a distraction task and then the recognition phase. In the recognition phase, 81 pairs are shown in random order with 1/3 consisting of ‘Together’ (Old) Pairs, 1/3 ‘Not-together’ (Lure) Pairs, and 1/3 ‘Never Seen’ (New) Pairs. (B) Memory Outcomes: Five memory outcomes were derived depending on the subjects response.
Study Protocol. Subjects undergo a brief training phase, followed by the complete FAST task to measure baseline performance. Subjects subsequently completed an alternate version of the FAST Task, with stimulation (TACS/TDCS/Sham) applied during the encoding phase. They returned 24 hours later to complete the recognition phase of the recognition test.
Stimulation
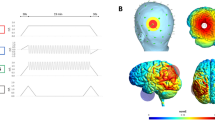
HD-TES was administered using the Soterix MxN TES device (Soterix Medical Inc., New York, USA). Electrode positions were selected using HD-Targets software (Soterix Medical Inc., New York, USA) which uses a finite-element model of a template adult brain to estimate the current distribution. This modelling software has previously shown good correspondence with other complex computational current models55. While modelling was performed only for TDCS, these models should also be applicable for TACS56,57. Stimulation sites were chosen to result in the highest focality within the right fusiform cortex. Based on this modelling, electrodes were placed at FP1, P2, P3, PO7, and P10 (Fig. 4). The same montage was used for TDCS, TACS, and sham stimulation. P10 acted as the anode, delivering 2 mA of current (peak to baseline), which was returned to the remaining electrodes based on the optimized current modelling by the HD-Targets software. The current balance at each return electrode was as follows: FP1 = −0.62 mA; P2 = −0.99 mA; P3 = −0.14 mA, and PO7 = −0.24 mA. Conductive gel (HD-GEL™, Soterix Medical Inc, New York, USA) was used to optimize conductivity and minimize impedance. While the specific imedence for each electrode was not documented, they were kept below 20kOhm. Stimulation time was set to ten minutes, with a 30 second ramp-up and ramp-down time for the active conditions. During sham stimulation, the current was ramped up to 2 mA over 30 seconds, prior to being ramped down over the next 30 seconds to 0.06 mA, where it remained for the following 9 minutes. At the end of the stimulation session, the current was again ramped up to 2 mA over 30 seconds. This procedure is commonly used to blind participants in TES studies27,58,59. For the first five minutes of stimulation, subjects were asked to relax and let their mind wander. For the second five minutes of stimulation, subjects performed the encoding phase of the FAST task. This delay prior to commencing the memory task was included because previous research has suggested significant effects of tDCS on cortical excitability following five minutes of stimulation60. To assess tolerability with each stimulation paradigm, all participants completed an adverse effect survey after the first 60 seconds of stimulation, where they rated their subjective experience of itching, burning, tingling, and discomfort on a scale of 1–5. The average of these scores constituted the overall side-effect profile (Supplementary Information II). Each subject was also asked which type of stimulation they received. This data was coded such that the answers were grouped into one of three categories: Active, Sham, or Unknown.
High definition Transcranial Electrical Stimulation (HD-TES). (A) Soterix MxN HD-TES system and the HD-Targets software were used to define the optimal electrode montage to focally stimulate the right fusiform cortex. (B) Finite element Modelling based on a normal adult brain template (HD-Targets software) demonstrating a high focality of electric field in the right fusiform cortex (model based on anodal direct current stimulation).
Memory encoding survey
After completion of the protocol, subjects filled out an encoding strategy survey assessing subjective ratings of memory confidence and attention to the face or scene. (Supplementary Information III).
Statistics
All data analyses were performed with MATLAB (MathWorks®, MA, USA). Normality of data was assessed with one sample Kolmogrov-Smirinov tests. To assess for our primary outcomes of stimulation induced changes in memory, and to account for the repeated measures nature of the data and the intersubject variability in performance at baseline, we utilized linear mixed effect models with a random effect of subject and fixed effects of condition (baseline and stimulation condition) and group (TACS, TDCS, and Sham). This was performed with MATLAB’s function fitlme, which estimates parameters of the model using maximum likelihood estimation. We were primarily interested in the interaction terms of condition and group, which gave insight into whether the dependent variable (memory scores) changed as a function of condition and group. TACS was used as the reference group given our aim of directly comparing TACS with TDCS and Sham. We used a separate model for each of our memory outcomes. Secondarily, were were interested in exploring whether any effects would persist at 24 hours, without any additional encoding. To examine this we performed similar linear mixed models for each memory outcome on the delayed follow-up. Finally, we calculated the sensitivity index (d’) as a measure of participants ability to discriminate between signal and noise61 (Supplementary Information IV).
Age, education, side-effect profile, memory encoding confidence, and subject ratings of focus to the face or scene showed non-normal distributions and were compared with Kruskal-Wallis tests, followed by Mann-Whitney U tests when appropriate. To test for equal proportions of gender within each group, a χ2 test was utilized. To formally test for blinding, we also used a χ2 test. We assessed for equal proportions of subjects in each treatment arm who responded with ‘Active’, ‘Sham’ or ‘Unknown’ when directly asked which type of stimulation they recieved. Significance was set at p < 0.05 for all analyses.
Results
Demographics
There was no significant difference between groups in age, gender, education, fatigue, or stress ratings (Table 1). None of these variables were linearly related to our primary memory outcomes (p > 0.05).
Side-effect profile
There was no significant difference in overall stimulation side-effect profile reported by the subjects, suggesting blinding was effective. Importantly, subjects were unaware of which stimulation condition they received when directly asked (χ2 = 5.157, p = 0.2716). However, analysis of the individual side-effects revealed a group difference in the subjective rating of ‘tingling’ (Table 2). Post-hoc testing demonstrated this resulted from subjects receiving TACS reporting higher ratings than Sham (U = 450, p = 0.017). There was no significant difference between TACS and TDCS in the tingling rating. TACS also caused 47% of subjects to report feelings of a ‘bouncing/shaking visual field’ which consistently went away prior to commencement of the encoding phase. This effect was not reported in the TDCS or sham condition. To investigate this effect further, we examined post-stimulation correct associative memory scores between those in the TACS group who experienced the side-effect (n = 9; mean score +/− SEM = 47.60 +/− 2.00) and those that did not (n = 10; 48.33 +/− 1.28) with a Mann-Whitney U Test. No significant difference was observed (U = 89.0, p = 0.949). All subjects tolerated stimulation.
Memory outcomes
We performed separate linear mixed effect models for each of the memory outcomes, with TACS as the reference group (Fig. 5). The model for our primary outcome, Correct Associative Memory, demonstrated a significant main effect of condition (β = 4.368, t(112) = 3.117, p = 0.002) and a significant condition*group interaction for TDCS (β = −4.035, t(112) = −2.085, p = 0.0393). The condition*group interaction for Sham was not significant (β = −2.85, t(112) = −1.46, p = 0.147). Incorrect Associative Memory was not related to any of the fixed effects tested. The model for Correct Rejection demonstrated a significant condition*group interaction for TDCS (β = −2.015, t(112) = −2.046, p = 0.043) but not for sham ((β = −1.26, t(112) = −1.25, p = 0.213). False Memory also showed a significant condition*group interaction for TDCS (β = 1.90, t(112) = 1.99, p = 0.0493), but not Sham (β = 1.05, t(112) = 0.982, p = 0.286). The model for Forgetting demonstrated a significant main effect of condition ((β = −1.79, t(112) = −2.70, p = 0.0079), a significant condition*group interaction for TDCS (β = 2.17, t(112) = 2.38, p = 0.0191) and a significant condition*group interaction for Sham (β = 1.95, t(112) = 2.08, p = 0.0397). Details, including average memory scores for each group, can be found in Table 3. The models assessing the 24 hour delayed performance for Correct Associative Memory demonstrated a signficiant main effect of condition (β = 3.21, t(112) = 2.00, p = 0.0474), and a condition*group trend for TDCS (β = −4.02, t(112) = −1.82, p = 0.0717). There were no other significant effects for any of the additional models at the delayed time point (Fig. 6). Sensitivity index calculations were consistent with these results (Supplementary Information IV).
Immediate Memory Performance. TACS is the reference group. (A) Correct Associative Memory: Significant condition*group interaction, with TACS demonstrating improved memory performance compared to TDCS. (B) Incorrect Associative Memory: No difference between groups. (C) False Memory: Significant condition*group interaction, with TACS demonstrating less errors compared to TDCS. (D) Forgetting: Significant condition*group interaction, with TACS demonstrating demonstrating less errors compared to TDCS and compared to Sham. (E) Correct Rejection: Significant condition*group interaction, with TACS demonstrating improved rejection compared to TDCS.
Memory encoding strategy
There was no difference between groups in memory confidence (H(2) = 1.4, p = 0.4955) or subjective reports of attention to the face (H(2) = 3.85, p = 0.1458) or scene (H(2) = 0.62, p = 0.7323).
Discussion
Associative memory deficits are commonly experienced during aging and are a prominent feature of many neurodegenerative diseases. Novel therapies inspired by an understanding of memory network physiology are needed. Previous attempts at improving associative memory using TES have targeted primarily the lateral frontal (DLPFC/IFG) cortex using direct current stimulation with conflicting results. The lateral frontal cortex is involved in a broad range of executive and high-level cognitive processes62 and may not be the ideal target for modulating associative memory directly. Attempts to modulate episodic memory have shown inconsistencies, though there may be a moderator effect of task type (recall), stimulation duration, and parietal location30. We have chosen to target the fusiform region due to fMRI studies suggesting a critical role in the encoding of successful visual associative memory4. Six Hz alternating current was chosen based on the well-known role of the theta rhythm in memory performance15,16,17,18,19, while the right side was selected based on previous literature specifically implicating right temporal theta activity in successful memory encoding16. One previous study targeted the right fusiform cortex with 1 × 1 anodal TDCS during a working memory task involving facial recognition49. In that study, stimulation enhanced demanding face working memory performance, suggesting that non-invasive brain stimulation of the fusiform cortex was possible. While the role of the fusiform cortex in face7 and object63 perception/encoding is well appreciated, this region (and more generally the inferior temporal-occipital cortex) has long been suspected of having critical mnemonic functions8, and has been consistently implicated in the successful encoding of visual associations4. This is consistent with our finding of improved visual associative memory performance. However, it is possible that the effects of stimulating this region specifically improved the encoding of faces. This possibility is supported by the observation of improved correct rejection, which is a memory process relient on item memory. We have also observed reduced false memory and reduced forgetting, suggesting a more general memory or perceptual process may have been modulated. We cannot refute this possibility with our data set, and alternative tasks will need to be utilized in the future to conclusively demonstrate an effect specific to associative memory. Despite this, the data suggest that oscillatory activity is critical for the beneficial memory results, which is consistent with the communication through coherence hypothesis64,65,66,67. According to this hypothesis, effective communication and information processing in brain networks is mediated through coherent oscillations between neuronal groups within those networks. Transcranial alternating current stimulation, through its hypothesised mechanism of amplifying power and entraining endogenous brain rhythms, may improve communication and information processing in neural networks. Consequently, it may be hypothesized that when TACS, at an appropriate rhythm, is targeted towards a node of a cognitive network (as done in this study), improved neural communication within the network, and ultimately improved cognitive abilities, may be expected. Our results are consistent with this reasoning.
In addition, this is the first study to directly compare HD-TACS and HD-TDCS using a MxN electrode configuration. The results suggest that TACS may be more effective than TDCS in some contexts, likely due to the reasons proposed above. Anodal TDCS had no effect on memory performance, consistent with the findings of a recent systematic review30. However, in that study longer duration of stimulation or the use of a recall (vs a recognition task here) were shown to moderate the effect size of TDCS stimulation, suggesting that TDCS may still be efficacious in some circumstances. Perhaps due to our relatively small smaple size, we did not observe a difference between TACS and sham stimulation on our primary outcome. However, we observed consistent trends in the same direction as TACS vs TDCS for all the memory outcomes. Furthermore, we did observe a statistical difference between TACS and sham on forgetting, supporting the claim that TACS improves memory performance.
By using the current modelling within HD-Targets software, we chose a unique electrode montage which maximizes the focality of current within the right fusiform region. Compared to traditional 1 × 1 TES, this minimizes the chances of stimulating confounding brain structures. Furthermore, previous literature targeting the dorsal anterior cingulate68, and recent computational modelling work, suggests that targeting deep cortical brain structures is possible with unique electrode configurations such as that utilized here69. The amplitude used in our TACS group is higher than that used most frequently in the TACS literature34. The optimal amplitude for TACS is not known, and given the lack of literature directly comparing TACS to TDCS, it is unclear which amplitudes are most comparable between the modalities. In the only other study to our knowledge performing a direct comparisons between TACS and TDCS, the TACS amplitude was chosen to be 2 mA peak to peak, resulting in a maximum amplitude which is half that of a 2 mA TDCS configuration. Nonetheless, these authors also report a beneficial effect of TACS, suggesting that perhaps lower amplitudes may also be efficaous47.
Several limitations must be considered when interpreting these results. Firstly, a post-hoc power analysis suggested this study was underpowered. Using the online toolbox GLIMMPSE70, the observed power of this study was demonstrated to be 0.493. Next, firm conclusions about the anatomical or frequency specificity of the observed effect cannot be made from this trial as no alternative region or frequency was tested. Indeed, while the computational modelling suggested the maximal intensity was focused in the fusiform cortex, it also suggested other regions of the temporal and temporal-occipital lobe received similar current intensities (Fig. 4). Alternatively, there is a possibility our unique electrode configuration resulted in the simulatenous modulation of frontal, parietal, and inferior temporal regions. This interpretation, while less likely, would suggest that the behavioural effects arose through the modulation of distributed brain regions at a theta rhythm. Therefore, we cannot conclude with certainty which region is responsible for the observed effects.
Despite the overall side-effect profile being equal between groups, post-hoc analysis suggested the TACS group experienced a higher level of a ‘tingling’ sensation compared to sham. This group also experienced, in 47% of cases, a visual side effect which to our knowledge has not been reported in the literature. This was consistently described as a ‘bouncing’ or ‘shaking’ visual field and went away prior to the commencement of the task. We cannot rule out a level of heightened attention resulting from these differences. However, we believe this is highly unlikely because no difference in correct associative memory performance was observed between those who experienced the visual side effect and those that did not. Further, there was no significant difference in side-effect profile between TACS and TDCS. Next, the extension of these results to aging and diseased populations should be considered speculative, as these biological factors can influence the result of transcranial electrical stimulation on cognitive performance25. Finally, we cannot rule out entrainment of brain activity resulting from peripheral nerve stimulation, though this limitation exists for most non-invasive brain stimulation research.
In summary, we have demonstrated that HD-TACS, delivered in a focal manner to the right fusiform region at a theta frequency during the encoding of visual associations, resulted in improved associative memory performance. By directly comparing HD-TACS to HD-TDCS, we find that the alternating current is crucial in the beneficial results observed. The effect occurred after a single 10-minute application of stimulation and a trend towards group differences was observed when memory was re-tested at 24 hours. This provides preliminary support for the use of high definition transcranial alternating current stimulation paradigms for memory enhancement, while also providing evidence for the role of visual association cortex in associative memory. Further research, investigating the effect of this paradigm in aging and diseased populations, testing the effect of different frequencies and anatomical locations, using longer or multiple stimulation sessions, and testing different associative memory paradigms, will be required.
Data Availability
The data that support the finding of this manuscript are available from the corresponding author, upon reasonable request.
References
Squire, L. R. Memory systems of the brain: A brief history and current perspective. Neurobiol. Learn. Mem. 82, 171–177 (2004).
Suzuki, W. A. Making new memories: The role of the hippocampus in new associative learning. In Annals of the New York Academy of Sciences, https://doi.org/10.1196/annals.1379.007 (2007).
Reitz, C., Brayne, C. & Mayeux, R. Epidemiology of alzheimer disease. Nat. Rev. Neurol. 7, 137–152 (2011).
Kim, H. Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. Neuroimage 54, 2446–2461 (2011).
Wagner, A. D., Shannon, B. J., Kahn, I. & Buckner, R. L. Parietal lobe contributions to episodic memory retrieval. Trends Cogn. Sci. 9, 445–453 (2005).
Eichenbaum, H. Prefrontal–hippocampal interactions in episodic memory. Nat. Rev. Neurosci. 18, 547–558 (2017).
Kuskowski, M. A. & Pardo, J. V. The role of the fusiform gyrus in successful encoding of face stimuli. Neuroimage 9, 599–610 (1999).
Miyashita, Y. Inferior temporal cortex: Where visual perception meets memory. Annu. Rev. Neurosci. 16, 263 (1993).
Garoff, R. J., Slotnick, S. D. & Schacter, D. L. The neural origins of specific and general memory: the role of the fusiform cortex. Neuropsychologia 43, 847–859 (2005).
McGugin, R. W., Gatenby, J. C., Gore, J. C. & Gauthier, I. High-resolution imaging of expertise reveals reliable object selectivity in the fusiform face area related to perceptual performance. Proc. Natl. Acad. Sci. 109, 17063 LP–17068 (2012).
Whitwell, J. L. et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology 70, 512 LP–520 (2008).
Burton, E. J., McKeith, I. G., Burn, D. J., Williams, E. D. & O’Brien, J. T. Cerebral atrophy in Parkinson’s disease with and without dementia: A comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 127, 791–800 (2004).
Pagonabarraga, J. et al. Pattern of regional cortical thinning associated with cognitive deterioration in parkinson’s disease. PLoS One 8, e54980 (2013).
Biundo, R. et al. Anatomical correlates of cognitive functions in early parkinson’s disease patients. PLoS One 8, e64222 (2013).
Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 29, 169–195 (1999).
Osipova, D. et al. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J. Neurosci. 26, 7523 LP–7531 (2006).
Nakahara, K. et al. Associative-memory representations emerge as shared spatial patterns of theta activity spanning the primate temporal cortex. Nat. Commun. 7, 1–9 (2016).
Portoles, O., Borst, J. P. & Vugt, M. K. Van. Characterizing synchrony patterns across cognitive task stages of associative recognition memory. Eur. J. Neurosci. 1–11, https://doi.org/10.1111/ejn.13817 (2017).
Köster, M., Finger, H., Graetz, S., Kater, M. & Gruber, T. Theta-gamma coupling binds visual perceptual features in an associative memory task. Sci. Rep. 8, 17688 (2018).
Fertonani, A. & Miniussi, C. Transcranial electrical stimulation: What we know and do not know about mechanisms. Neuroscientist 23, 109–123 (2017).
Coffman, B. A., Clark, V. P. & Parasuraman, R. Battery powered thought: Enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage 85, 895–908 (2014).
Villamar, M. F. et al. Technique and Considerations in the Use of 4x1 Ring High-definition Transcranial Direct Current Stimulation (HD-tDCS). J. Vis. Exp. https://doi.org/10.3791/50309 (2013).
Alam, M., Truong, D. Q., Khadka, N. & Bikson, M. Spatial and polarity precision of concentric high-definition transcranial direct current stimulation (HD-tDCS). Phys. Med. Biol. 61, 4506–4521 (2016).
Dmochowski, J. P., Datta, A., Bikson, M. & Su, Y. Optimized multi-electrode stimulation increases focality and intensity at target. J. Neural Eng. 8 (2011).
Leach, R. C., McCurdy, M. P., Trumbo, M. C., Matzen, L. E. & Leshikar, E. D. Differential age effects of transcranial direct current stimulation on associative memory. Journals Gerontol. Ser. B 00, 1–11 (2018).
Leshikar, E. D. et al. Transcranial direct current stimulation of dorsolateral prefrontal cortex during encoding improves recall but not recognition memory. Neuropsychologia 106, 390–397 (2017).
Matzen, L. E., Trumbo, M. C., Leach, R. C. & Leshikar, E. D. Effects of non-invasive brain stimulation on associative memory. Brain Res. 1624, 286–296 (2015).
Gaynor, A. M. & Chua, E. F. tDCS over the prefrontal cortex alters objective but not subjective encoding. Cogn. Neurosci. 8, 156–161 (2017).
Leach, R. C., McCurdy, M. P., Trumbo, M. C., Matzen, L. E. & Leshikar, E. D. Transcranial stimulation over the left inferior frontal gyrus increases false alarms in an associative memory task in older adults. Heal. aging Res. https://doi.org/10.1097/01.HXR.0000491108.83234.85 (2016).
Galli, G., Vadillo, M. A., Sirota, M., Feurra, M. & Medvedeva, A. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) on episodic memory. Brain Stimul. 12, 231–241 (2019).
Pergolizzi, D. & Chua, E. F. Transcranial direct current stimulation over the parietal cortex alters bias in item and source memory tasks. Brain Cogn. 108, 56–65 (2016).
Perceval, G., Martin, A. K., Copland, D. A., Laine, M. & Meinzer, M. High-definition tDCS of the temporo-parietal cortex enhances access to newly learned words. Sci. Rep. 7, 17023 (2017).
Jacobson, L., Goren, N., Lavidor, M. & Levy, D. A. Oppositional transcranial direct current stimulation (tDCS) of parietal substrates of attention during encoding modulates episodic memory. Brain Res. 1439, 66–72 (2012).
England, H. B., Fyock, C., Meredith Gillis, M. & Hampstead, B. M. Transcranial direct current stimulation modulates spatial memory in cognitively intact adults. Behav. Brain Res. 283, 191–195 (2015).
Bjekić, J. et al. The immediate and delayed effects of single tDCS session over posterior parietal cortex on face-word associative memory. Behav. Brain Res. 366, 88–95 (2019).
Bjekić, J., Čolić, M. V., Živanović, M., Milanović, S. D. & Filipović, S. R. Transcranial direct current stimulation (tDCS) over parietal cortex improves associative memory. Neurobiol. Learn. Mem. 157, 114–120 (2019).
Antal, A. & Paulus, W. Transcranial alternating current stimulation (tACS). Front. Hum. Neurosci. 7, 1–4 (2013).
Ali, M. M., Sellers, K. K. & Fröhlich, F. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J. Neurosci. 33, 11262 LP–11275 (2013).
Helfrich, R. F. et al. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr. Biol. 24, 333–339 (2014).
Tavakoli, A. V. & Yun, K. Transcranial alternating current stimulation (tACS) mechanisms and protocols. Front. Cell. Neurosci. 11, 1–10 (2017).
Weinrich, C. A. et al. Modulation of long-range connectivity patterns via frequency-specific stimulation of human cortex. Curr. Biol. 27, 3061–3068.e3 (2017).
Zaehle, T., Rach, S. & Herrmann, C. S. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS One 5, e13766 (2010).
Bächinger, M. et al. Concurrent tACS-fMRI reveals causal influence of power synchronized neural activity on resting state fMRI connectivity. J. Neurosci. 37, 4766–4777 (2017).
Moisa, M., Polania, R., Grueschow, M. & Ruff, C. C. Brain network mechanisms underlying motor enhancement by transcranial entrainment of gamma oscillations. J. Neurosci. 36, 12053–12065 (2016).
Kasten, F. H., Dowsett, J. & Herrmann, C. S. Sustained aftereffect of α-tACS lasts up to 70 min after stimulation. Frontiers in Human. Neuroscience 10, 245 (2016).
Polanía, R., Nitsche, M. A., Korman, C., Batsikadze, G. & Paulus, W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr. Biol. 22, 1314–1318 (2012).
Röhner, F. et al. Modulation of working memory using transcranial electrical stimulation: a direct comparison between TACS and TDCS. Frontiers in Neuroscience 12, 761 (2018).
Rangarajan, V. et al. Electrical stimulation of the left and right human fusiform gyrus causes different effects in conscious face perception. J. Neurosci. 34, 12828–12836 (2014).
Brunyé, T. T., Moran, J. M., Holmes, A., Mahoney, C. R. & Taylor, H. A. Non-invasive brain stimulation targeting the right fusiform gyrus selectively increases working memory for faces. Brain Cogn. 113, 32–39 (2017).
Becker, N. et al. Structural brain correlates of associative memory in older adults. Neuroimage 118, 146–153 (2015).
Dennis, N. A. et al. Effects of aging on the neural correlates of successful item and source memory encoding. J. Exp. Psychol. Learn. Mem. Cogn. 34, 791–808 (2008).
Dennis, N. A., Johnson, C. E. & Peterson, K. M. Brain and cognition neural correlates underlying true and false associative memories. Brain Cogn. 88, 65–72 (2014).
Minear, M. & Park, D. C. A lifespan database of adult facial stimuli. Behav. Res. Methods, Instruments, Comput. 36, 630–633 (2004).
Ezzyat, Y. et al. Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat. Commun. 9 (2018).
Nikolin, S., Loo, C. K., Bai, S., Dokos, S. & Martin, D. M. Focalised stimulation using high definition transcranial direct current stimulation (HD-tDCS) to investigate declarative verbal learning and memory functioning. Neuroimage 117, 11–19 (2015).
Neuling, T., Wagner, S., Wolters, C. H., Zaehle, T. & Herrmann, C. S. Finite-element model predicts current density distribution for clinical applications of tDCS and tACS. Front. psychiatry 3, 83 (2012).
Ruffini, G. et al. Transcranial current brain stimulation (tCS): models and technologies. IEEE Trans. Neural Syst. Rehabil. Eng. 21, 333–345 (2013).
Pergolizzi, D. & Chua, E. F. Increased contextual cue utilization with tDCS over the prefrontal cortex during a recognition task. Brain Res. 1655, 1–9 (2017).
Lara, G. Ade et al. Perturbation of theta-gamma coupling at the temporal lobe hinders verbal declarative memory. Brain Stimul. 11, 509–517 (2018).
Nitsche, M. A. & Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527, 633–639 (2000).
Stanislaw, H. & Todorov, N. Calculation of signal detection theory measures. Behav. Res. Methods, Instruments, Comput. 31, 137–149 (1999).
Tanji, J. & Hoshi, E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol. Rev. 88, 37–57 (2008).
Haist, F., Lee, K. & Stiles, J. Individuating faces and common objects produces equal responses in putative face-processing areas in the ventral occipitotemporal cortex. Front. Hum. Neurosci. 4, 1–15 (2010).
Fries, P. Rhythms for cognition: communication through coherence. Neuron 88, 220–235 (2015).
Varela, F., Lachaux, J. P., Rodriguez, E. & Martinerie, J. The brainweb: Phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239 (2001).
Bressler, S. L. & Menon, V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 14, 277–290 (2010).
Sarnthein, J., Petsche, H., Rappelsberger, P., Shaw, G. L. & von Stein, A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl. Acad. Sci. USA 95, 7092–7096 (1998).
Onoda, K., Kawagoe, T., Zheng, H. & Yamaguchi, S. Theta band transcranial alternating current stimulations modulates network behavior of dorsal anterior cingulate cortex. Sci. Rep. 7, 3607 (2017).
Huang, Y. & Parra, L. C. Can transcranial electric stimulation with multiple electrodes reach deep targets? Brain Stimul. https://doi.org/10.1101/382598 (2018).
Kreidler, S. M. et al. GLIMMPSE: Online Power Computation for Linear Models with and without a Baseline Covariate. J. Stat. Softw. 54, i10 (2013).
Acknowledgements
We would like to acknowledge Jordan Huang for assistance with data collection and recruitment, and Iris Kathol for administrative support. We would also like to acknowledge Meng Wang for statistical support. This work was funded by a National Sciences and Engineering Research Council Discovery grant (213374), the Tourmaline Oil Chair in Parkinson’s Disease, the Canada Research Chair in non-motor symptoms of Parkinson’s disease to OM. SL is the recipient of a graduate student award from Parkinson Canada, the Donald and Louise Burns Graduate Scholarship in Dementia, and a CIHR doctoral award. LG is funded by the Non-Invasive Neurostimulation Network (N3) at the University of Calgary. There was no role of funding sponsors in the design or implementation of this study.
Author information
Authors and Affiliations
Contributions
S.L. conceived the idea, collected the data, performed the analysis, and wrote the manuscript. L.S. collected the data, wrote portions of the Methods section, helped in the preparation of all Figures, and critically reviewed the manuscript. T.A. contributed to the design of the memory task. O.M. supervised the project at each stage and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lang, S., Gan, L.S., Alrazi, T. et al. Theta band high definition transcranial alternating current stimulation, but not transcranial direct current stimulation, improves associative memory performance. Sci Rep 9, 8562 (2019). https://doi.org/10.1038/s41598-019-44680-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44680-8
This article is cited by
-
A meta-analysis showing improved cognitive performance in healthy young adults with transcranial alternating current stimulation
npj Science of Learning (2023)
-
Theta tACS impairs episodic memory more than tDCS
Scientific Reports (2023)
-
High-definition transcranial direct current stimulation (HD-tDCS) of the left middle temporal gyrus (LMTG) improves mathematical reasoning
Brain Topography (2023)
-
Effects of online parietal transcranial electric stimulation on associative memory: a direct comparison between tDCS, theta tACS, and theta-oscillatory tDCS
Scientific Reports (2022)
-
Performance after training in a complex cognitive task is enhanced by high-definition transcranial random noise stimulation
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.