Abstract
As an intractable health threat, neuropathic pain is now a key problem in clinical therapy, which can be caused by lesions affecting the peripheral nervous systems. 1,8-cineole is a natural monoterpene cyclic ether present in eucalyptus and has been reported to exhibit anti-inflammatory and antioxidant effects. Research has shown that 1,8-cineole inhibits P2X3 receptor-mediated neuropathic pains in dorsal root ganglion. The P2X2 and P2X3 receptors participate in the transmission of algesia and nociception information by primary sensory neurons. In the present study, We thus investigated in the spinal cord dorsal horn whether 1,8-cineole inhibits the expression of P2X2 receptor-mediated neuropathic pain. This study used rats in five random groups: group of chronic constriction injury(CCI) with dimethysulfoxide control (CCI + DMSO); group of CCI; sham group(Sham); group of CCI treated with a low dose 1,8-cineole (CCI + 50 mg/kg); group of CCI with a high dose (CCI + 100 mg/kg). We observed the effects of 1,8-cineole on thermal withdrawal latency (TWL) and mechanical withdrawal threshold (MWT). We examined P2X2 receptors mRNA change in rat spinal cord dorsal horn by In situ nucleic acid hybridization(ISH) and Quantitative realtime polymerase chain reaction (qRT-PCR) methods. Western Blotting and Immunohistochemical staining methods were used to observe P2X2 receptor protein expressions in the rat spinal cord dorsal horn. It demonstrated that oral administration of 1,8-cineole inhibits over-expression of P2X2 receptor protein and mRNA in the spinal cord and dorsal horn in the CCI rats. And the study explored new methods for the prevention and treatment of neuropathic pain.
Similar content being viewed by others
Introduction
It is known that Neuropathic pain can arise from lesions which affect the central or peripheral nervous systems. At present, the most important clinical treatment for neuropathic pain is chemical drug therapy. However, due to the complex mechanism of neuropathic pain, there is still no drug that is effective for all neuropathic pain diseases. Now the commonly used therapeutic drugs include anti-epileptic drugs, antidepressants, NMDA antagonists, and opioid analgesics, etc1,2. However, their long-term use has side effects that cannot be ignored. For example, anti-epileptic drug gabapentin is currently used in the treatment of neuropathic pain, producing a good effect on the central and peripheral neuropathic pain, but it is easy to cause dizziness, lethargy, and peripheral edema, wherefore its long-term use can cause the risk of movement disorders and secondary infections. Therefore, based on new molecular targets, finding an analgesic that is powerful, safe, effective and low tolerant has become a hot topic in pain treatment research.
1,8-cineole (1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane, also known as eucalyptol), is a monoterpene present in many plant essential oils such as from rosemary and eucalyptus3. It exhibits anti-inflammatory and antioxidant effects according to reports4,5,6. This material was used to treat sinusitis, chronic rhinitis and bronchitis as well as asthma7. It could reduce the spread of infectious bacteria according to some studies8,9. It inhibits prostaglandin and cytokine by stimulating monocytes in vitro10 and also possesses good transdermal permeation for many drugs11,12. Patch clamp techniques were used in our previous studies13, which showed that cineole can activate TRPA1 channels of dorsal root ganglia (DRG) and consequently, in the substantia gelatinosa neurons, it can increase the spontaneous excitatory postsynaptic currents. This suggests that 1,8-cineole is a potential candidate for neurodegenerative diseases.
Currently, most studies usually consider these ion channels: channel of acid-sensing ion, channel of TRP and channel of ATP-gated ion. For extracellular ATP, in the nerve systems, it is generally regarded as either a crucial co-neurotransmitter or sole neurotransmitter in most nerves14,15. It is also known that the P2 family of receptors covers G protein-coupled receptor (P2Y receptor) with more diverse agonist profiles and ATP-gated ion channel (P2X purinergic receptor), which function as a media for nucleotides signalling16,17. The P2X receptor opens responding to ATP binding as a nonselective cation channel, which allows for rapid ion flows (Ca2+, Na+, K+) across the membrane. The extracellular ATP interacts with P2X receptors and consequently stimulates the sensory neurons18,19,20,21. P2X2/3 subtypes use primary sensory neurons while transmitting the nociception and algesia information22,23. The selective P2X2/3 and P2X3 receptor antagonists, in experimental pain models, effectively reduce neuropathic pain24,25,26. The CCI rats behave analogously with the neuropathic pain conditions of human beings, which were therefore used as the neuropathic pain model27.
Our previous studies demonstrated that, for CCI rats, in the L4-5 DRG, the down-regulation of P2X3 receptor expressions are decreased by 1,8-cineole treatment28. In the spinal cord, P2X2 receptor expression was highest in the dorsal horn, with significant neuronal labeling in the ventral horn and intermediolateral cell column29. However, in the spinal cord dorsal horn, it is not clear whether 1,8-cineole could inhibit the expression of P2X2 receptor-mediated neuropathic pain. Therefore, in the current work, that question was investigated regarding the effects of oral administration of 1,8-cineole.
Results
Behavioural assessment of mechanical withdrawal threshold and thermal withdrawal latency
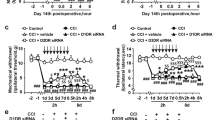
In the CCI group, MWT and TWL became lower than those in the group of Sham (P < 0.05) seven days after the operation and then remained until day 14. In the group of CCI and group of CCI + DMSO, MWT and TWL were not statistically different (P > 0.05). The groups of CCI with 1,8-cineole treatment demonstrated higher MWT and TWL than the group of CCI at 7 and 14 days (p < 0.05). The group of CCI + 50 mg/kg, compared with its 100 mg/kg counterpart, exhibited an increase (P < 0.05) (Fig. 1).
Mechanical withdrawal threshold and thermal withdrawal latency in CCI rats treated with 1,8-cineole. In the CCI + DMSO and CCI groups, the TWL and MWT were decreased than the Sham group; compared to the group of CCI, the two groups of CCI + 100 mg/kg and CCI + 50 mg/kg demonstrated less decreased mechanical and thermal values. The group of CCI + 100 mg/kg had a more pronounced analgesic effect by 1,8-cineole compared with its 50 mg/kg counterpart. &P < 0.05 vs. CCI + 50 mg/kg group, #P < 0.05 vs. CCI group, *P < 0.05 vs. Sham group. All compared to the corresponding time point (least significant difference test and one-way analysis of variance). n = 6 rats in each group, mean ± SD.
Effects of 1,8-cineole on P2X2 mRNA expression in spinal cord dorsal horn of CCI rats
The groups of CCI and CCI + DMSO demonstrated higher P2X2 receptor mRNA expression than the Sham group (P < 0.05); while the former two groups were not different statistically (P > 0.05). The groups of CCI with 1,8-cineole treatment showed lower P2X2 mRNA expression levels than the group of CCI (P < 0.05). Additionally, compared to the group of CCI + 50 mg/kg, its 100 mg/kg counterpart had lower expression levels of P2X2 mRNA (P < 0.05) (Fig. 2).
Expression of P2X2 receptor mRNA in spinal cord dorsal horn of 1,8-cineole-treated CCI rats. Relative P2X2 receptor mRNA expression 7 and 14 days following the surgery. In the groups of CCI + DMSO and CCI, the expression was increased than the Sham group; in the two groups of CCI + 100 mg/kg and CCI + 50 mg/kg, the expression was less pronounced. &P < 0.05 vs. CCI + 50 mg/kg group, #P < 0.05 vs. CCI group, *P < 0.05 vs. Sham group. All compared to the corresponding time point (least significant difference test and one-way analysis of variance). n = 6 rats in each group, mean ± SD.
In situ hybridization results
The P2X2 receptor mRNA expression was not changed in the Sham group at 7 and 14 days after the surgery; in the group of CCI, however, the expression was increased significantly comparatively (P < 0.05). For the two groups of CCI + 100/CCI + 50 mg/kg, significantly lower P2X2 expression demonstrated compared to the CCI group (P < 0.05), though the two groups still showed higher expression than the Sham group. Within the two groups of CCI + 100/CCI + 50 mg/kg, the former exhibited a less pronounced increase (P < 0.05). However, the P2X2 receptor mRNA expression was not different significantly among the CCI + DMSO and CCI group (P > 0.05) (Fig. 3).
P2X2 mRNA expression in spinal cord dorsal horn. 1 and 2 respectively corresponds to 7 and 14 days after the surgery. (A) to (E) respectively represents the group of the Sham, CCI, CCI + DMSO, CCI + 50 mg/kg, and CCI + 100 mg/kg. In the two groups of CCI + 100 mg/kg and CCI + 50 mg/kg, P2X2 mRNA showed less upregulation; in the CCI + DMSO and CCI groups, an increased P2X2 mRNA expression was showed compared to the Sham group. &P < 0.05 vs. CCI + 50 mg/kg group, #P < 0.05 vs. CCI group, *P < 0.05 vs. Sham group. All compared to the corresponding time point (least significant difference test and one-way analysis of variance). n = 6 rats in each group, mean ± SD.
Effect of 1,8-cineole on P2X2 receptor expression in spinal cord dorsal horn of CCI rats
The expression of spinal cord dorsal horn P2X2 receptor was detected by immunohistochemistry. In the groups of CCI + DMSO and CCI, the samples had increased staining intensities for P2X2 receptor than the group of Sham (P < 0.05). In the two groups administrated with 1,8-cineole, in comparison with the group of CCI, the ratio of P2X2-positive cells decreased (P < 0.05). Within the two groups of CCI + 100/CCI + 50 mg/kg, the former had a significantly decreased ratio of P2X2-immuno labelled cells than the latter (P < 0.05) (Fig. 4).
P2X2 immunoreactivity in spinal cord dorsal horn of 1,8-cineole-treated CCI rats. 1 and 2 respectively corresponds to 7 and 14 days after the surgery. (A) to (E) respectively represents the group of the Sham, CCI, CCI + DMSO, CCI + 50 mg/kg, and CCI + 100 mg/kg. P2X2 receptor immunoreactivity, observed mainly in the cytoplasm 7 and 14 days after the surgery, was shown in yellow-brown. In the group of Sham and the two groups of CCI + 100 mg/kg and CCI + 50 mg/kg, less strong immunore activity was showed, while the groups of CCI and CCI + DMSO were distinctly stained. The mean optical density of P2X2 immunoreactive cells was showed in the following statistical figure. &P < 0.05 vs. CCI + 50 mg/kg group, #P < 0.05 vs. CCI group, *P < 0.05 vs. Sham group. All compared to the corresponding time point (least significant difference test and one-way analysis of variance). n = 6 rats in each group, mean ± SD, Scale bar: 50 um.
Effect of 1,8-cineole on P2X2 protein expression in spinal cord dorsal horn of CCI rats
In this study, western blotting was used to detect the expression of P2X2 protein in spine cord. In comparison with the group of Sham, the CCI group demonstrated significantly enhanced P2X2 protein expression stain values in the spine cord and dorsal horn (P < 0.05). But the CCI group and CCI + DMSO group were not statistically different (P > 0.05). The 1,8-cineole administrated groups demonstrated lower relative levels of P2X2 protein than the CCI group (P < 0.05). Additionally, the group of CCI + 100 mg/kg demonstrated less elevated expression levels of P2X2 protein compared with its 50 mg/kg counterpart (P < 0.05) (Fig. 5).
Expression of P2X2 protein in spinal cord dorsal horn of CCI rats treated with 1,8-cineole. In the two groups of CCI + 100 mg/kg and CCI + 50 mg/kg, P2X2 protein showed less upregulation; in the CCI + DMSO and CCI groups, an increased P2X2 protein expression was showed compared with the Sham group. &P < 0.05 vs. CCI + 50 mg/kg group, #P < 0.05 vs. CCI group, *P < 0.05 vs. Sham group. All compared to the corresponding time point (least significant difference test and one-way analysis of variance). n = 6 rats in each group, mean ± SD.
Discussion
Generally speaking, three orders of neurons are involved in the superfical sensory pathway of trunk and limbs. The first-order neurons with their nuclei in the dorsal root ganglia, carry sensations from the exteroceptors located in the skin of trunks and limbs and enter the posterolateral sulcus of the spinal cord. The axons of second-order neurons in the posterior horns travels up to the ventral posterolateral nucleus (VPL) of the thalamus (the third-order neurons). The trunk and limb receptors sense various external stimuli and convert this stimuli into electrical signals, the primary afferent fibers conduct this electrical signals to the dorsal horn, which is then transmitted by the ascending fibers of the spinal cord to the more advanced nerve center after being transmitted to the dorsal horn by the DRG. The central processes of the horn neurons and the DRG neurons form a primary synapse30,31,32, in which the spinal cord dorsal horn have a role of relaying and processing sensory information. Therefore, for the understanding of the mechanism of neuropathic pain, the spinal and dorsal horn may be a key part.
The CCI model has peripheral and central sensitization characteristics that are very similar to the clinical features of chronic neuropathic pain33. This allows for the wide use of this model in neuropathic pain related studies. Its core is to ligature the sciatic nerve with a chrome gut whilst not affecting the blood supply. In our experiments, there was no dyskinesia and autophagy in the hind limbs of the CCI group. In addition, the sensitivity of each CCI rat to mechanical pain and heat sensitive pain was increased at 7 and 14 days after the surgery, i.e. the pain threshold is lowered, indicating that it is a successful model. For neuropathic pain, both cell electrophysiology and immunohistochemistry showed significantly increased P2X2 receptor expression, indicating that the neuropathic pain is closely related to the P2X2 receptor expression24,25,26.
According to the results of immunohistochemistry, in situ hybridization, Western blotting or qRT-PCR in this study, each CCI group demonstrated significantly increased expressions of P2X2 receptor mRNA and protein. The groups of CCI with 1,8-cineole administration showed a significant decrease (P < 0.05) than the group of CCI and the group of CCI + DMSO. The CCI + 100 mg/kg group showed significantly lower P2X2 receptor expression than the CCI + 50 mg/kg group (P < 0.05), while the CCI group and CCI + DMSO group were basically the same concerning P2X2 receptor and mRNA expression. The reason why the 100 mg/kg dose group achieved better therapeutic results than its 50 mg/kg counterpart is probably because of the first-step elimination effect after the oral administration. We referred to the relevant literature34 and took many pre-experiments, in which we found that if the dose of 1,8-cineole given to the CCI rats is too low, the treatment effect is not obvious; while if the dose is too high, repeated vomiting and even death would occur in the rats. Finally, we chose 50 mg/kg and 100 mg/kg as the reference doses in the treatment. However, the optimal therapeutic dose of 1,8-cineole needs to be established by further studies investigating the toxicity and side effects of this drug.
This study indicates that, for rats having neuropathic pain, in their spinal cord dorsal horn, the over-expression of P2X2 receptor protein and mRNA can be reduced by 1,8-cineole. We speculate that the possible mechanism works in these procedures: ① Oral administration of 1,8-cineole works faster and more effectively; ② Around the lesion, a better microenvironment is created by 1,8-cineole owing to its anti-inflammatory and antiseptic effects6,7; β 1,8-cineole relieves structural damage to the other cells (such as Schwann cells, etc.) whilst also promoting the restoration of the injured sciatic nerves28.
For the treatment of neuropathic pain, 1,8-cineole may become a new analgesic, though further verifications are needed concerning its mechanisms. For example, the route of administration of 1,8-cineole, the optimal dose for therapeutic effects, as well as the drug metabolism rate of 1,8-cineole in the body still need to be further explored. Additionally the influence of P2X2 receptor on neuropathic pain remains unclear, i.e., whether there are other affecting signal channels. With a deeper understanding of 1,8-cineole in the field of analgesia, it is expected that, in the near future, it will become a major drug in the treatment of neuropathic pain.
Methods
Statement
(1) All experiments, including methods and operations were approved by the Animal Care and Ethics Committee of the Medical School of Nanchang University, China. (2) All experimental methods were performed in accordance with guidelines and regulations of the Ethics Committee of the Medical School of Nanchang University, China.
Animals, drugs and drug administration
For the experiment rats, the Jiangxi Province Traditional Chinese Medicine University Laboratory Animal Science Department provided 120 SD rats, male and female, each with a weight between 200–250 g. The animal certificate has the code SCXK2006-0001. The Committee for Ethical Use of Medical School of the Nanchang university, China. approved all the experiments as per relative international codes. On a clean work bench, the DMSO and 1,8-cineole, both at 2% v/v, were prepared. Five random groups of rats: group of CCI + DMSO (CCI, DMSO administrated), group of CCI (CCI, no administration), group of Sham (sciatic nerves isolated, not constricted), group of CCI + 50 mg/kg (CCI, 50 mg/kg/d 1,8-cineole administrated) and group of CCI + 100 mg/kg (CCI, 100 mg/kg/d 1,8-cineole administrated). The intragastrical administration of 1,8-cineole or equal quantity of DMSO was made daily from the first postoperative day. Each of the above groups were subdivided into two groups, with 12 rats per group, respectively for 7 or 14 days treatment and time point.
Model production
With CCI model established25, 1% sodium pentobarbital (40 mg/kg, i.p.) was used to anesthetize the rats. The left sciatic nerve in the rat thigh was ligated 1 mm apart using 4–0 catgut, with an appropriate intensity not affecting the nerve blood supply. This procedure, except the ligation of nerve injury, was applied to the group of Sham.
Behavioral test
At 7 and 14 days after the surgery, all the groups of rats were placed in a VonFrey pain threshold measuring glass instrument, the bottom of which was made of barbed wire. The device was placed on a horizontal surface, let the rats adapt to this tool for 15–20 minutes at normal temperatures and in a quiet environment. Von Frey filaments and BME-410C Thermal Paw Stimulation System (Aesthesio, Danmic, CA, USA) were used respectively for the tests of TWL and MWT. The experiments were performed three times with the average value to be obtained and investigated35,36.
qRT-PCR
PBS was used to isolate and immediately wash the rat spinal cord dorsal horn lumbosacral enlargement. A FastQuant RT Kit with gDNase (Tiangen Biotech Co., Beijing, China) was used to synthesize cDNA with total RNA of 2 µg. TRNzol Universal Reagent (Beijing Tiangen) was used to prepare the total RNA samples. Primer Express 3.0 (Applied Biosystems, Inc., Foster City, CA, USA) was used to design the primers. The sequences were defined in the following way: P2X2 receptor, forward 5′-GGTGGTAGTGCCGTTTATCT-3′, reverse 5′-AAGGGCGGTGTCATTGGA-3′; β-actin, forward 5′-AAGATCCTGACCGAGCGTGG-3′, reverse 5′-CAGCACTGTGTTGGCATAGAGG-3′. The ΔΔCT method was used to quantify the gene expression, with the threshold cycle denoted by CT. The relative levels of the target genes normalized to those of the samples with the lowest CT were reported as 2−ΔΔCT. A SuperReal PreMix Plus (SYBR Green) was used to perform quantitative PCR in an ABI PRISM® 7500 Sequence Detection System (Applied Bio-systems, Inc.).
In situ nucleic acid hybridization
Firstly 1% sodium pentobarbital (40 mg/kg, i.p.) was used to deeply anaesthetize the rats and then physiological saline, 4% paraformaldehyde and 0.1% diethylpyrocarbonate (DEPC) were perfused through the ascending aorta successively. Afterwards, 4% paraformaldehyde with 0.1% DEPC was used to incubate the tissues overnight at 4 °C. On the next day, sucrose solutions of 15% and 30% at 4 °C were sequentially used for overnight dehydration. A cryostat was used to slice the tissues into sections of 10 µm in thickness before being placed on poly-L-lysine covered glass slides. These sections were then incubated for 10 minutes in 0.4% Triton X-100/PBS after they were washed using 0.01 mol/L PBS for 3 × 5 min. Protease K (5.0 μg/ml) in PBS was then used to incubate the sections at 37 °Cfor 10 minutes. 4% paraformaldehyde was used to fix the sections for 5 minutes to stop the protease activity before being washed off the fixative by 2 × 3 min washes with PBS. Sodium chloride of 0.6 mol/L and sodium citrate of 0.06 mol/L (2 × SSC) were then successively used to wash the sections for totally 10 min. To allow for hybridization, a humid chamber at 37 °C was used to keep the coverslip covered smear for 30 minutes. Pre-hybridization buffer was used to wash the sections for three times before incubating these firstly at 60 °C for 30 minutes and then at 45 °C for two hours, respectively with a hybridization buffer containing 5 pM of each probe and with a humid chamber. Finally wash buffer and rinse buffer were used to respectively twice wash and rinse the smear before it was dried in the darkness.
Immunohistochemical staining
4% paraformaldehyde was used to perfuse and fix the spine cords of the rats and then post fix these for additional 24 h before being transferred to 15% and 30% sucrose solutions at 4 °C for overnight dehydration. The segments of the spine cord lumbosacral enlargement were longitudinally cut into serial sagittal frozen sections of 10-um in thickness on a cryostat after being embedded in optimal cutting temperature compounds. The frozen slides were washed twice with PBS every five minutes after being dried for 10 minutes at room temperatures in the air. Rabbit anti-P2X2 antibody (1:500; Abcam) was then used to incubate the sections at 4 °C overnight after these were blocked for 15 minutes at room temperatures using 10% normal goat serum (Boster, Wuhan, China). An additional incubation for 90 minutes at 37 °C was then performed using horseradish peroxidase-labelled goat anti-rabbit IgG (1:100; Boster). After that, PBS was used to rinse the slices for three times at 5-minute intervals, which were then dehydrated, permeabilized, and mounted after being developed using 3,3′-diaminobenzidine and stained using hematoxylin. From each rat, three sections were obtained, and for each section light microscope (Olympus, Tokyo, Japan) was used to randomly select three non-overlapping fields.
Western blotting
The spinal cord dorsal horn lumbosacral enlargement was homogenized in RIPA lysis buffer containing a protease inhibitor (100 mg/mL phenylmethylsulfonyl fluoride; 1 mg/mL aprotinin; 0.1% sodium dodecyl sulfate (SDS); 1% Nonidet P-40; 0.02%sodium deoxycholate; 150 mMNaCl; and 50 mM Tris-Cl, pH 8.0). Collect the supernatant after centrifuging the homogenate at 12,000 rpm for 10 min. A 6× loading buffer was used to dilute the supernatant, which was then heated for five minutes at 95 °C. A gel electrophoresis system of Bio-Rad 10% SDS polyacrylamide was used to separate the proteins from the samples that contain the same quantity of protein (30 µg) and to transfer the proteins to polyvinylidene fluoride membranes. 5% non-fat dried milk was used to block the membrane for two hours at room temperatures in 1× Tris-buffered saline containing Tween-20 (TBST). A rabbit Polyclonal anti-P2X2 antibody(1:500; Abcam) and a mouse monoclonal anti-β-actin antibody (1:5,000, ABclonal) were then used to incubate the membrane at 4 °C in blocking buffer overnight. A 1× TBST and a horseradish peroxidase-conjugated secondary antibody (1:5,000, ABclonal) (goat anti-rabbit IgG or goat anti-mouse IgG) were used to wash the membranes for 3 × 10 min and incubate these for one hour at room temperatures respectively in blocking buffer. After a second time washing, a Bio-Rad system and enhanced chemiluminescence were then used to visualize the labeled proteins. To quantify the band intensity, Image-Pro Plus software was adopted. The relative band intensities of the target proteins were normalized to those of the respective β-actin internal controls37.
Statistical analysis
The software of SPSS 19.0 (SPSS, Chicago, IL, USA) was used to analyze the data, which are expressed as the mean ± SD. One-way analysis of variance ANOVA was used to explore the intergroup differences and the least significant difference as a post hoc test, in which P < 0.05 was regarded as significant statistically.
Date Availability
All data generated or analysed during this study are included in this published article.
References
Benoliel, R., Tal, M. & Eliav, E. Effects of topiramate on the chronic constriction injury model in the rat. J Pain. 7, 878–883 (2006).
Burnstock, G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 110, 433–454 (2006).
Yadav, N. & Chandra, H. Suppression of inflammatory and infection responses in lung macrophages by eucalyptus oil and its constituent 1,8-cineole: Role of pattern recognition receptors TREM-1 and NLRP3, the MAP kinase regulator MKP-1, and NFκB. PLoS One. 12, e0188232 (2017).
Ryu, S., Park, H., Seol, G. H. & Choi, I. Y. 1,8-Cineole ameliorates oxygen-glucose deprivation/reoxygenation-induced ischaemic injury by reducing oxidative stress in rat cortical neuron/glia. J Pharm Pharmacol. 66, 1818–1826 (2014).
Caceres, A. I. et al. Transient Receptor Potential Cation Channel Subfamily M Member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br J Pharmacol. 174, 867–879 (2017).
Khan, A. et al. 1,8-cineole (eucalyptol) mitigates inflammation in amyloid Beta toxicated PC12 cells: relevance to Alzheimer’s disease. Neurochem Res. 39, 344–352 (2014).
Yang, Z. et al. Anti-infectious bronchitis virus (IBV) activity of 1,8-cineole: effect on nucleocapsid (N) protein. J Biomol Struct Dyn. 28, 323–330 (2010).
Kim, K. Y., Lee, H. S. & Seol, G. H. Eucalyptol suppresses matrix metalloproteinase-9 expression through an extracellular signal-regulatedkinase-dependent nuclear factor-kappa B pathway to exert anti-inflammatory effects in an acute lung inflammation model. J Pharm Pharmacol. 67, 1066–1074 (2015).
Juergens, U. R., Stober, M., Schmidt-Schilling, L., Kleuver, T. & Vetter, H. Antiinflammatory effects of eucalyptol (1.8-cineole) in bronchial asthma: inhibition of arachidonic acid metabolism in human blood monocytes ex vivo. Eur J Med Res. 3, 407–412 (1998).
Ahad, A., Aqil, M. & Ali, A. Investigation of antihypertensive activity of carbopol valsartan transdermal gel containing 1,8-cineole. Int J Biol Macromol. 64, 144–149 (2014).
Ahad, A., Aqil, M., Kohli, K. & Sultana, Y. Mujeeb M. Design, formulation and optimization of valsartan transdermal gel containing iso-eucalyptol as novel permeation enhancer: preclinical assessment of pharmacokinetics in Wistar albino rats. Expert Opin Drug Deliv. 11, 1149–1162 (2014).
Zhang, Y.-ling et al. 1,8-cineole decreases neuropathic pain probably via a mechanism mediating P2X3 receptor in the dorsal root ganglion. Neurochemistry International. 121, 69–74 (2018).
Khakh, B. S. & North, R. A. Neuromodulation by extracellular ATP and P2X receptor in the CNS. Neuron. 76, 51–69 (2012).
Burnstock, G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 27, 166–176 (2006).
Adriaensen, D., Brouns, I. & Timmermans, J. P. Sensory input to the central nervous system from the lungs and airways: A prominentrole for purinergic signalling via P2X2/3 receptors. Auton Neurosci. 191, 39–47 (2015).
Kellenberger, S. & Grutter, T. Architectural and functional similarities between trimeric ATP-gated P2X receptors andacid-sensing ion channels. J Mol Biol. 427, 54–66 (2015).
Gao, Y. et al. Effect of emodin on neuropathic pain transmission mediated by P2X2/3 receptor of primary sensory neurons. Brain Res Bull. 84, 406–413 (2011).
Peverini, L., Beudez, J., Dunning, K., Chataigneau, T. & Grutter, T. New Insights Into Permeation of Large Cations Through ATP-gated P2X Receptor. Front Mol Neurosci. 11, 265 (2018).
Li, X. et al. Intrathecal leptin inhibits expression of the P2X2/3 receptors and alleviates neuropathic pain induced by chronic constriction sciatic nerve injury. Mol Pain. 9, 65 (2013).
Zhao, S. et al. Effect of nanoparticle-encapsulated curcumin on HIV-gp 120-associated neuropathic pain induced by the P2X3 receptor in dorsal root ganglia. Brain Res Bull. 135, 53–61 (2017).
Rao, S. et al. The effect of sinomenine in diabetic neuropathic pain mediated by the P2X3 receptor in dorsal root ganglia. Purinergic Signal. 13, 227–235 (2017).
Chen, D. J. et al. Microencapsulated Schwann cell transplantation inhibits P2X3 receptor in dorsal root ganglia and neuropathic pain. Neural Regen Res. 13, 1961–1967 (2018).
Fu, H., Li, F., Thomas, S. & Yang, Z. Hyperbaric oxygenation alleviates chronic constriction injury (CCI)-induced neuropathic pain and inhibits GABAergic neuron apoptosis in the spine cord. Scand. J Pain. 17, 330–338 (2017).
Zhang, Y. L. et al. 1,8-cineole decreases neuropathic pain probably via a mechanism mediating P2X3 receptor in the dorsal root ganglion. Neurochem Int. pii: S0197-0186(18)30282-1 (2018).
Kanjhan, R. et al. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol. 8,407(1), 11–32 (1999).
Shypshyna, M. S., Veselovsky, N. S., Myasoedov, N. F., Shram, S. I. & Fedulova, S. A. Effect of Peptide Semax on Synaptic Activity and Short-Term Plasticity of Glutamatergic Synapses of Co-Cultured Dorsal Root Ganglion and Dorsal Horn Neurous. Fiziol Zh. 61, 48–55 (2015).
Antal, M., Papp, I., Bahaerguli, N., Veress, G. & Vereb, G. Expression of hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 in axon terminals of peptidergic nociceptive primary sensory neurons in the superficial spinal dorsal horn of rats. Eur J Neurosci. 19, 1336–1342 (2004).
Wang, L. X. & Wang, Z. J. Animal and cellular models of chronic pain. Adv Drug Deliv Rev. 55, 949–965 (2003).
Liapi, C. et al. Antinociceptive properties of 1.8-Cineole and beta-pinene, from the essential oil of Eucalyptus camaldulensis leaves, in rodents. Planta Med. 73, 1247–1254 (2007).
Chen, C. J. et al. Identification of key genes and pathways associated with neuropathic pain in uninjured dorsal root ganglion by using bioinformatic analysis. J Pain Res. 10, 2665–2674 (2017).
Lee, J. et al. 1,8-cineole prevents UVB-induced skin carcinogenesis by targeting the aryl hydrocarbon receptor. Oncotarget. 8, 105995–106008 (2017).
Bernier, L. P., Ase, A. R. & Séguéla, P. P2X receptor channels in chronic pain pathways. Br J Pharmacol 175, 2219–2230 (2018).
Kasuya, G. et al. Structural insights into the competitive inhibition of the ATP-gated P2X receptor channel. Nat Commun. 8, 876 (2017).
Shypshyna, M. S. & Veselovs’kyĭ, M. S. Characteristics of sensory neurotransmission in co-culture of neurons from the dorsal root ganglion and dorsal horn spine cord in rats. Fiziol Zh. 56, 26–36 (2010).
Zhang, Y. L. et al. Microencapsulated Schwann cell transplantation inhibits P2X2/3 receptor overexpression in a sciatic nerve injury rat model with neuropathic pain. Neurosci Lett. 676, 51–57 (2018).
Lin, J. Q., Luo, H. Q., Lin, C. Z., Chen, J. Z. & Lin, X. Z. Sodium hydrosulfide relieves neuropathic pain in chronic constriction injured rats. Evid Based Complement Alternat Med. 2014, 514898 (2014).
Wang, W. S. et al. Electroacupuncture and A-317491 depress the transmission of pain on primary afferent mediated by the P2X3 receptor in rats with chronic neuropathic pain states. J Neurosci Res. 92, 1703–1713 (2014).
Acknowledgements
This study is perfommed with the full support and in the guidance of Professor Zeng-xu Liu. Each link of this study has devoted a lot of efforts of Professor Zeng-xu Liu from topic selection, concept, revision and final presentation. We are very grateful to Associate Professor Bao-lin Yang and Ya-ling Zhang from Nanchang University in China for their valuable technical guidance. We thank the helps of Qing Li, Xiang-dong Wang and all members from Medical Office. These studies were supported by grants from the National Natural Science Foundation of China (81760418 and 81260190) and Natural Science Foundation of Jiangxi Province (20151BAB205022 and 20181BAB205061). The Science and Technology Research Project of Jiangxi Education Department (GJJ13159) and the Science and Technology Program of the Department of Health of Jiangxi Province (20173010).
Author information
Authors and Affiliations
Contributions
All authors listed above have contributed sufficiently to be included as authors. Zeng-xu Liu: designed experiments; Xiao-bo Zheng, Ya-ling Zhang: carried out experiments and wrote the manuscript; Qing Li, Xiang-dong Wang, Yi-guo Liu: carried out experiments; Ya-ling Zhang, Bao-lin Yang, Gao-chun Zhu, Zhong-fa Zhou, Yun Gao: analyzed experimental results; All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, Xb., Zhang, Yl., Li, Q. et al. Effects of 1,8-cineole on neuropathic pain mediated by P2X2 receptor in the spinal cord dorsal horn. Sci Rep 9, 7909 (2019). https://doi.org/10.1038/s41598-019-44282-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44282-4
This article is cited by
-
Protective role of citronellol on antioxidant enzymes and oxidative damage induced by gentamicin in experimental nephrotoxic rats
Molecular Biology Reports (2024)
-
Dorsal root ganglia P2X4 and P2X7 receptors contribute to diabetes-induced hyperalgesia and the downregulation of electroacupuncture on P2X4 and P2X7
Purinergic Signalling (2023)
-
Transcriptome Analysis Reveals the Anti-Tumor Mechanism of Eucalyptol Treatment on Neuroblastoma Cell Line SH-SY5Y
Neurochemical Research (2022)
-
Blockage of HCN Channels Inhibits the Function of P2X Receptors in Rat Dorsal Root Ganglion Neurons
Neurochemical Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.