Abstract
A shortage of conventional medicine during the American Civil War (1861–1865) spurred Confederate physicians to use preparations of native plants as medicines. In 1863, botanist Francis Porcher compiled a book of medicinal plants native to the southern United States, including plants used in Native American traditional medicine. In this study, we consulted Porcher’s book and collected samples from three species that were indicated for the formulation of antiseptics: Liriodendron tulipifera, Aralia spinosa, and Quercus alba. Extracts of these species were tested for the ability to inhibit growth in three species of multidrug-resistant pathogenic bacteria associated with wound infections: Staphylococcus aureus, Klebsiella pneumoniae, and Acinetobacter baumannii. Extracts were also tested for biofilm and quorum sensing inhibition against S. aureus. Q. alba extracts inhibited growth in all three species of bacteria (IC50 64, 32, and 32 µg/mL, respectively), and inhibited biofilm formation (IC50 1 µg/mL) in S. aureus. L. tulipifera extracts inhibited biofilm formation (IC50 32 µg/mL) in S. aureus. A. spinosa extracts inhibited biofilm formation (IC50 2 µg/mL) and quorum sensing (IC50 8 µg/mL) in S. aureus. These results support that this selection of plants exhibited some antiseptic properties in the prevention and management of wound infections during the conflict.
Similar content being viewed by others
Introduction
Antibiotic resistance in pathogenic microbes poses a significant threat to human health1; antibiotics are critical not only in treating bacterial diseases but also in enabling surgery and other procedures with high risks of infection. Given the great genetic diversity and capacity for evolution present in bacteria, a rise in antibiotic resistance is an inevitable response to antibiotic use. For example, in 1940, even before penicillin was widely used, penicillin resistance was observed. Any single antibiotic, then, is not a permanent solution but another step in the struggle against infection.
Several factors complicate the relationship between antibiotics and bacteria. For example, the innate immune system plays a role in fighting infections with or without the use of antibiotics. Further, commensal members of the microbiome may compete with pathogenic bacteria or may themselves become pathogenic under certain circumstances. Relevant to this study, bacterial community effects such as biofilms and quorum sensing produce resistance and virulence phenotypes not necessarily observed in vitro2,3. Biofilms are extracellular mixtures of polysaccharides and proteins that can physically protect bacterial populations from antibiotics and immune responses2,4. Consequently, biofilms are associated with chronic infections, especially in the cases of indwelling medical devices and implants, and there is currently a lack of effective treatments for these conditions4. Quorum sensing is a system by which toxin production or other pathogenic activity is initiated when extracellular communication indicates achievement of a threshold population of bacteria. Inhibition of quorum sensing and biofilm formation, then, can be therapeutic but not bactericidal3. In the absence of new antibiotics, multidrug-resistant infections may be treatable by administering biofilm inhibitors or quorum quenchers to increase the vulnerability of bacteria to the immune system or conventional antibiotics.
The natural product compositions investigated in this study are plant extracts used during the American Civil War (1861–1865), a period of history in which infections were treated without the use of modern antibiotics and before the emergence of germ theory. The accepted definition of antiseptic was “tonic useful to prevent external or internal mortification”5. Union General Ulysses S. Grant once famously demanded that onions be sent to him before he would move his army. While soldiers certainly used onions in their cooking, we know now that antimicrobial agents such as ajoene and allicin found in garlic and onions have an impact on quorum sensing and biofilm to disrupt infections6,7. At the time, they were used to treat powder burns.
During the latter half of the war, a Union blockade8 prevented the Confederacy from importing sufficient amounts of conventional medicines such as quinine, morphine, and chloroform (Fig. 1)9. Francis Porcher, a botanist, was commissioned to find and catalogue plants native to the southeastern US that could be used as medicines in their place5. Porcher compiled a book of his findings, including 37 plant species to be used as antiseptics, treating gangrene and other infections5. From this research, Samuel Moore, the Confederate Surgeon General, published a field guide of native plant medicines to be used by battlefield physicians, including methods of collection, preparation, and administration10. Infection was a leading cause of death for soldiers in the Civil War and was often treated with amputation11. It may be hoped that Porcher’s work with medicinal plants saved many lives and limbs.
Natural products—compounds produced by living organisms—are used directly as medicine by an estimated 4 billion people for whom traditional medicine is a primary healthcare source12. Approximately 25% of modern drugs are derived from natural products used in traditional medicine13. Plants in particular produce a large variety of secondary metabolites to interact with their environments, and some of these serve to control local microbes by encouraging or inhibiting bacterial growth and/or function.
Many of the plant species Porcher described as antiseptic have not been tested for antibiotic activity, particularly for adjuvant activity (biofilm and/or anti-virulence properties) or activity against multidrug-resistant bacteria. The aim of this study was to examine the potential efficacy of the plants used to stave off infection during the Civil War. While the majority of drugs on the market today are synthetic, many are still derived from natural products; a review of new drugs from 1981–2014 found that only two new approved novel chemical entities (NCEs), sorafenib and ataluren, were created through de novo combinatorial chemistry13. Searching natural products for NCEs may be a more effective tactic, especially when systems of traditional medicine and historical pharmacopoeias are available to use as heuristics.
One benefit of natural product extracts as antibiotic agents over single-compound drugs is that due to the presence of dozens to thousands of compounds, they can exhibit multiple mechanisms of activity, potentially making it more difficult for resistance to develop. For example, English oak (Quercus robur L., Fagaceae) bark was found to exert its quorum quenching activity via two distinct mechanisms14.
In this study, samples of three species from Porcher’s book were selected for investigation: white oak (Quercus alba L., Fagaceae), devil’s walking stick (Aralia spinosa L., Araliaceae), and tulip tree (Liriodendron tulipifera L., Magnoliaceae). We hypothesized that, given the historic use of these plants as antiseptics, their extracts may inhibit growth, biofilm production, and/or quorum sensing in pathogenic bacteria that affect the skin and soft tissue structures. Multidrug-resistant bacteria were used in all experiments to examine the potential use of these plant compounds to combat emerging resistance in species commonly found in wound infections today.
Results
Extract yield
Extraction in MeOH yielded six crude extracts, representing Q. alba bark and galls, A. spinosa leaves, and L. tulipifera leaves, root inner bark, and branch bark (Table 1). Extract yield was highest (27.1% of dry mass) in extract 620 (Q. alba galls). Other crude extracts had yields ranging from 8–11%. Masses of partitions and fractions of crude extracts varied from <0.1% to 4% relative to dry plant matter (Supplementary Table S1). Partitions were labelled B, C, D, and E for solvents hexane, ethyl acetate, n-butanol, and water, respectively; non-tannin fractions were labelled F1 and tannin fractions were labelled F2. The non-tannin fraction of L. tulipifera leaves (616-F1) was more than 10 times as massive as the tannin fraction, suggesting that tannin content is not high in L. tulipifera leaves. The tannin and non-tannin fractions of Q. alba bark were similar in mass.
Growth inhibition
All 19 crude extracts, partitions, and fractions were tested for growth inhibition of S. aureus, A. baumannii, K. pneumoniae, and P. aeruginosa (Table 2). Reported here are minimum concentrations of extract that achieved 50% inhibition (IC50) and 90% inhibition (MIC or IC90). Extracts from L. tulipifera and Q. alba were shown to be most active in inhibition of S. aureus growth (IC50 64 μg/mL in each case). Q. alba extracts 619, 619-F2, and 620 displayed inhibition of A. baumannii (IC50 64, 32, and 32 μg/mL, respectively) and K. pneumoniae (IC50 128, 64, and 32 μg/mL).
Extracts which displayed strong activity against S. aureus, A. baumannii, K. pneumoniae, and P. aeruginosa (619, 619-F2, and 620) were tested for growth inhibition of S. epidermidis and additional strains of A. baumannii and K. pneumoniae. All three of these Q. alba extracts inhibited growth in the strains of A. baumannii (IC50 32–256 μg/mL), but not in the additional K. pneumoniae strains tested (Table 3). Q. alba extracts 619 and 619-F2 were found to inhibit growth of S. epidermidis (IC50 256 and 64 μg/mL, respectively).
Biofilm inhibition
Extracts from all species inhibited S. aureus biofilm formation (IC50 1–256 µg/mL). Figure 2 shows biofilm inhibition across serial dilutions of the most active extracts. Q. alba extract 619-F2 displayed the greatest biofilm inhibition (IC50 1 µg/mL). Some extracts, such as L. tulipifera extract 616-F1 and A. spinosa extract 618B, displayed little growth inhibition activity against S. aureus but strongly inhibited biofilm formation (IC50 32 and 2 µg/mL, respectively). Full biofilm inhibition data is reported in Supplementary Table S2.
Quorum sensing inhibition
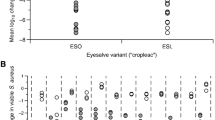
Transcription of S. aureus agr types I, II, and III was inhibited by several Civil War extracts (Fig. 3). L. tulipifera extract 617 C, A. spinosa extract 618 C, and Q. alba extract 619-F1 exhibited the most activity in these assays, primarily against agr III (IC50 16, 32, and 16 μg/mL, respectively). No extracts demonstrated inhibition of agr IV transcription. Full quorum sensing inhibition data is reported in Supplementary Table S3.
Quorum sensing inhibition of S. aureus by Civil War samples. (A) Screen of all samples at 64 µg/mL. OD represents S. aureus growth and FLD represents expression of the agr gene. (B) Serial dilution of active samples from 0.5 to 64 µg/mL. Only 224C-F2, the control, showed activity against agr IV at sub-inhibitory concentrations for growth.
Cytotoxicity
Of the 19 extracts studied, 13 were recognized to have potential antibiotic activity and were tested with human keratinocytes (HaCaT) to counter test for cytotoxicity. L. tulipifera root bark extracts 617 and 617 C displayed high levels of cytotoxicity (IC50 16 μg/mL in each case). Q. alba extracts displayed no significant cytotoxicity at test concentrations (2–256 μg/mL). Figure 4 displays cytotoxicity across serial dilutions of samples tested; IC50 and IC90 values are reported in Supplementary Table S4.
Chemical analysis
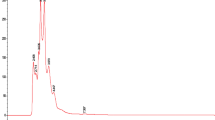
Q. alba extracts 619-F2 and 620 were selected for chemical analysis because of their strong antibacterial activity both in growth inhibition and in adjuvant assays and because of their lack of toxicity towards human cells. Initial HPLC indicated a wealth of early eluting compounds, so the chromatographic conditions were adjusted for LC-FTMS to achieve greater separation in that region. LC-FTMS revealed that 619-F2 and 620 have few compounds in common (Fig. 5).
Analysis of LC-FTMS revealed 22 peaks in 619-F2 and 24 peaks in 620 with ≥1% peak area. Of these peaks, 16 and 10 respectively were putatively matched with known Quercus spp. Compounds (Fig. 6). Only three compounds were found in both 619-F2 and 620, 6, 41, and 42 with m/z of 466.0306, 367.2866, and 367.2866 respectively.
Putative compounds from fraction 619-F2 and extract 620 identified from database searches (7) isomers of procyanidin: procyanidin C1, procyanidin C2, procyanidin T2, procyanidin T3, (8a) catechin-gallocatechin-4,8-dimer, (8b) catechin-gallocatechin-6′,8-dimer, (8c) gallocatechin-catechin-6′,8-dimer, (8d) potengriffioside A and tiliroside, (8e) prodelphinidin C, (8f) (2 R,2′R,3 S,3′S,4 R)-[2′-(3,4-dihydroxyphenyl)-3,3′,4,4′-tetrahydro-2-(3,4,5 trihydroxyphenyl)-4,6′-Bi-2H-1-benzopyran]-3,3′,5,5′,7,7′-hexol, (15a) isomers of procyanidin B: procyanidin B1, procyanidin B2, procyanidin B3, procyanidin B4, procyanidin B5, procyanidin B6, procyanidin B7, procyanidin B8, (15b) catechol-catechol-6′,8-dimer, (15c) echinacin, (24a) isomers of procyanidin B 3-O-gallate: procyanidin B1 3-O-gallate, procyanidin B2 3-O-gallate, procyanidin B3 3-O-gallate, (24b) procyanidin B2 3′-O-gallate, (28) epicatechin gallate, (30) isocryptomerin (2) castalin and vescalin, (12a) casuariin, (12b) pedunculagin, (14a) castacrenin A, (14b) castacrenin B, (14c) castacrenin C, (14d) leiocarposide, (29) ellagic acid, (32a) 2, 19, 23-trihydroxy-3-[(3, 4, 5-trihydroxybenzoyl) oxy]-β-D-glucopyranosyl ester (2α, 3β, 4α)-urs-12-en-28-oic acid and 2, 19, 23-trihydroxy-3-[(3, 4, 5-trihydroxybenzoyl) oxy]-α-D-glucopyranosyl ester (2α, 3β, 4α)-urs-12-en-28-oic acid, (32b) quercotriterpenoside I, (32c) quercotriterpenoside II, (32d) quercotriterpenoside III, (32e) quercotriterpenoside VI, (40a) arjugenin, (40b) belleric acid, (40c) sericic acid, (40d) 2α,19,23-trihydroxyursolic acid, (40e) 2,3,23,24-tetrahydroxy-(2α,3β)-urs-12-en-28-oic acid.
Discussion
Extracts of L. tulipifera, A. spinosa, and Q. alba displayed inhibitory activity against bacteria that cause skin and soft tissue infections, substantiating their use as antiseptics during the American Civil War. These medicinal plants may be useful in modern medicine as treatments for antibiotic-resistant bacteria. Of particular interest are 618B and 620 as S. aureus biofilm inhibitors and 619, 619-F2, and 620 as growth inhibitors of carbapenem-resistant Klebsiella pneumoniae.
While a 1947 survey of antibacterial properties of plants found no activity in A. spinosa and L. tulipifera15, the positive results in this experiment may be explained by differences in a number of factors. The previous study used H2O extracts whereas this experiment used MeOH extracts15; L. tulipifera bark was historically prepared for treatment by dissolving in EtOH5, which produces an extraction profile similar to MeOH16. Additionally, given the role of endophytic microorganisms in the synthesis of secondary metabolites, the chemical composition of plant extracts can vary based on differences in the plant microbiome17. Other possible sources of variation include collection date and location, assay method, and extract concentration tested. Finally, given the variability in how different laboratories may perform one type of extraction, results can vary between related studies. For example, of two studies that evaluated Aralia nudicaulis root (a traditional Native American remedy ingredient) for growth inhibition of mycobacteria, only one reported moderate antibacterial effects while the other reported little activity18,19.
In his report, Porcher recommended the entire genus Quercus as a source of antiseptics5. This activity is confirmed not only by the results of the experiments reported herein, but also by multiple other studies showing antibiotic effects by Quercus spp. extracts20,21,22,23,24. A European herbal remedy referred to as Quercus cortex (originating from Q. robur, Q. petrea, and Q. pubescens bark) has shown weak antibacterial and quorum sensing inhibition effects25. Acorn extract from a variety of oaks has shown inhibition of both Gram-positive and Gram-negative bacteria26.
However, the activity of various Quercus spp. extracts is far from uniform. For example, the Q. alba gall extract (620) in this study inhibited growth of drug-resistant K. pneumoniae whereas a study of Q. infectoria galls found no significant inhibition of drug-resistant K. pneumoniae24.
Antibacterial activity in oak extracts is frequently attributed to tannins27, compounds that typically interfere with biological processes by binding to proteins28. In Quercus, tannin content is typically highest in galls, with a reported 70% tannin content in Q. infectoria galls27. In this experiment, higher activity in 620 (gall crude extract) over 619 (bark crude extract) and 619-F2 (bark tannin fraction) over 619-F1 (bark non-tannin fraction) suggests that Q. alba’s growth inhibitory activity is due to tannins. However, quorum sensing inhibition by 619-F1 suggests that additional compounds could contribute to the antibacterial activity of crude oak extract, the medicine used during the Civil War.
LC-FTMS analysis of 619-F2 and 620 confirmed the existence of a variety of tannins in both extracts (Supplementary Tables S6 and S7). Of particular interest are ellagitannin isomers, 2, found in 620; as well as related ellagitannins 12a and 12b. Ellagitannins have been reported to have antibiotic activity against antibiotic-resistant S. aureus9. While only three MS peaks were found in common between 619-F2 and 620, both extracts are rich in tannins. 619-F2 is enriched in procyanidin condensed tannins and 620 contains many ellagitannins and triterpenes.
Tannins have been shown to inhibit growth in a wide range of bacteria, fungi, and viruses. Suggested mechanisms of action include inactivation of microbial enzymes, inhibition of membrane transport, and sequestering essential metal ions in complexes28. Tannins may also act as biofilm inhibitors by binding to matrix proteins29. However, tannins have also been found to bind with digestive enzymes and nutrients such as proteins and starches, and as such are generally considered as anti-nutritive; a variety of animals have shown gastrointestinal distress and decreased growth when fed on high-tannin diets28. Because of this nondiscriminatory binding, external applications of Q. alba extracts would be preferable to internal or systemic applications; Porcher recommended that powdered oak bark be applied in a wash for gangrene and a poultice for wounds5.
Leaves of several Quercus species (Q. cerris, Q. ilex, Q. virginiana, Q. incana) have also shown antibacterial properties, including biofilm and quorum sensing inhibition20,22,30. One future research direction could be to compare the antibacterial properties of Q. alba leaves with the activity identified in bark and gall extracts.
While A. spinosa has several reported uses in traditional medicine31, it has not frequently been studied for medicinal properties. The most notable results of this experiment for A. spinosa are significant biofilm inhibition by 618B (leaf hexane partition) and quorum sensing inhibition by 618 C (leaf ethyl acetate partition). The presence of these adjuvant properties rather than simple growth inhibitory activity in A. spinosa leaves may explain the 1947 report of no significant antibiotic activity in A. spinosa15.
Other Aralia species have exhibited antibacterial activity in roots18 and aerial parts (flowers, leaves, and stems)32, including biofilm inhibition by A. cachemirica32. In his list, Porcher also ascribed antiseptic activity to A. racemosa5.
L. tulipifera has been widely studied and its various parts have exhibited a variety of medicinal effects including antibacterial33, anti-malarial34, and anti-cancer35,36 activity. The other species of Liriodendron, L. chinense, is used in Chinese traditional medicine and has been shown to have antibacterial effects37. Additionally, an extract from a hybrid of L. tulipifera and L. chinense has been shown to exhibit inhibition of biofilm production and quorum sensing38.
In the present study, L. tulipifera extracts demonstrated activity in the inhibition of growth, biofilm production, and quorum sensing. However, the root bark extract (617), which is generally more bioactive than the leaf extract (616) and branch bark extract (621) in our models, displayed significant mammalian cytotoxicity (IC50: 16 µg/mL). It may therefore be ill-suited for medicinal use, or at least dose-limited. A study of L. tulipifera for antiplasmodial activity also found high cytotoxicity in active fractions but it has been suggested that, given the use of L. tulipifera in traditional medicine, toxicity may not be problematic in vivo at therapeutic doses34. Porcher recommended root bark as the medicinal part of L. tulipifera to be harvested5; perhaps preparation techniques or dosage made the potency/toxicity trade-off worthwhile in a wartime context. Interestingly, Porcher also suggested L. tulipifera bark as a substitute for Cinchona bark in malaria treatment, an application supported by recent research34.
Perhaps the most notable L. tulipifera extract with low toxicity is 616-F1 (leaf non-tannin fraction), which displayed little growth inhibition but significant biofilm and quorum sensing inhibition—an adjuvant effect similar to the A. spinosa extracts tested.
Further study should focus on bioassay-guided fractionation, a recursive process of fractionation and bioassay to identify individual active compounds and synergistic relationships. Of the extracts tested, 616-F1, 618B, 618 C, 619-F2, and 620 exhibit the most promise for antibiotic NCEs and are good candidates for this process. Specifically, the HPLC methods developed for 619-F2 and 620 could be used to produce further fractions with adaptation to preparative liquid chromatography.
In vivo testing of the antibacterial properties of extracts active in vitro is also a logical next step in this research. Given the potential of some of these extracts as adjuvants rather than direct antibiotics, they may be tested as adjuvants with existing, FDA-approved antibiotics for the potentiation of antibacterial activity in wound infections.
Finally, given the activity seen in the extracts tested in this study, it may be worthwhile to investigate the antibacterial properties of other plants recorded as antiseptics in Porcher’s book. In total, 37 plant species were described as having antiseptic applications5. As the global spread of antibiotic-resistant strains of bacteria continues, it is increasingly important to consider all possible sources of new, and perhaps old, treatments.
Methods
Plant material
Samples of Liriodendron tulipifera, Aralia spinosa, and Quercus alba were identified and collected in May 2015 from Lullwater Preserve on the Emory University campus in Atlanta, Georgia. Leaves were gathered manually and a handsaw was used to cut segments of roots and branches for bark collection. Vouchers (Accession numbers 20338-20341) were deposited in the Emory University Herbarium (GEO) in Atlanta and digital copies of the specimens are accessible for viewing online via the SERNEC web portal39. Samples were dried and ground into powder by either a Wiley mill equipped with a 2 mm mesh or coffee grinder (Table 1).
Extraction, partitioning, and fractionation
All ground material (Table 1) was sonicated in MeOH (1 g/10 mL). After 20 minutes the sample was filtered sequentially with Whatman filter paper 8 and 2, and then fresh MeOH was added to the plant material for a second round of sonication. The two filtrates were combined and dried in vacuo at ≤40 °C. The resulting residue was suspended in H2O, frozen, and lyophilized. The dried extract was collected and 20 mg of each extract was dissolved in DMSO (10 mg/mL) for biological testing.
Extracts 617 and 618 were suspended in H2O (1 g/10 mL) and were sequentially partitioned in hexane, ethyl acetate, and n-butanol, yielding 4 partitions. Extracts 616 and 619 were dissolved in 95% ethanol (1 g/2 mL and 1 g/3 mL, respectively), loaded on a Sephadex LH-20 column (25 g, 32 × 2.5 cm), and sequentially eluted with 95% ethanol (300 mL), 70% acetone (300 mL), and 100% acetone (150 mL) to yield three fractions. All partitions and fractions were dried in vacuo, resuspended in H2O, frozen and lyophilized before being dissolved in DMSO (10 mg/mL) for biological testing.
Bacterial strains and growth conditions
In this study, six strains of Staphylococcus aureus (UAMS1, UAMS929, NRS385, AH1747, AH1677, AH430, AH1872), one strain of Staphylococcus epidermidis (NRS101), three strains of Klebsiella pneumoniae (NR-15410, NR-15411, NR-15412), eight strains of Acinetobacter baumannii (AB5075, NR-17786, AR-BANK#0035, AR-BANK#0037, AR-BANK#0045, AR-BANK#0300, OIFC143, H72721), and one strain of Pseudomonas aeruginosa (AH071) were used (Supplementary Table S5). To create liquid cultures for all assays, strains were grown overnight in tryptic soy broth (TSB) with constant shaking (230 rpm). All strains were maintained on Tryptic Soy Agar (TSA) and tested in Cation-Adjusted Mueller-Hinton Broth (CAMHB).
Growth inhibition assays
Assays were carried out under CLSI M100-S23 guidelines40. A working culture was created by standardizing liquid culture using a BioTek Cytation3 and inoculating into CAMHB to a concentration of 5.0 × 105 CFU/mL. Working culture was added to extracts and controls in 96-well microtiter plates (Grenier-Bio 655-185) such that each well contained a total volume of 0.2 mL. Vehicle controls and antibiotic controls (ampicillin, kanamycin, and vancomycin for Staphylococcus spp. assays, gentamicin, tetracycline, and meropenem for other species, 0.5 to 64 µg/mL) were included for each strain. Extracts and vehicle were tested at a concentration range of 2.0 to 256 µg/mL, using 2-fold serial dilution. Plates were incubated at 37 °C, with S. aureus, S. epidermidis, and P. aeruginosa for 18 hours and A. baumannii and K. pneumoniae for 22 hours. Optical density (OD600) was measured using a BioTek Cytation3 plate reader at initial and final time points, to account for extract colour. The IC50 for growth was defined as the lowest concentration at which an extract displayed ≥50% inhibition and MIC (IC90) at ≥90% inhibition.
Extracts active against multidrug-resistant A. baumannii (OIFC143) and K. pneumoniae (NR-15410) were tested for growth inhibition of S. epidermidis and additional strains of A. baumannii and K. pneumoniae.
Biofilm inhibition assays for S. aureus
Biofilm inhibition of S. aureus was performed as described previously41. Briefly, supplemented TSB with 3% NaCl, 0.5% dextrose, and 2% human plasma was used in 96-well microtiter plates (Falcon 35–1172). Working cultures of UAMS-1 (wt) and UAMS-929 (isogenic ΔsarA mutant of UAMS-1) were standardized to a concentration of 5 × 105 CFU/mL and the final well volume was 0.2 mL. Extracts were assessed at sub-IC50 concentrations for growth, ranging from 2.0 to 256 µg/mL. The vehicle and positive control, 220D-F2, were assessed from 2.0 to 256 µg/mL. All experiments were incubated statically at 37 °C for 22 hours. Optical density (OD600) was measured using a BioTek Cytation3 plate reader at initial and final time points, to account for extract colour. Biofilms were rinsed twice with 1X PBS, fixed with 100% EtOH, and stained with crystal violet. The dry stain was eluted with ethanol, diluted in PBS, and quantified at 595 nm using a BioTek Cytation 3 plate reader. The MBIC50 (minimum biofilm inhibitory concentration) was defined as the lowest concentration at which an extract displayed ≥50% inhibition and MBIC90 at ≥90% inhibition.
Quorum quenching assays for S. aureus
Examination of the quorum quenching potential of extracts against S. aureus was conducted as previously described3. Briefly, all agr fluorescent reporter strains were maintained in chloramphenicol (10 µg/mL) supplemented TSA and TSB. The assay was conducted in tissue culture-treated clear bottom, black-sided 96-well microtiter plates (Costar 3603) with a final well volume of 0.2 mL. Extracts were assessed at sub-MIC50 concentrations, ranging from 0.5 to 64 µg/mL. Vehicle and positive control, 224C-F2, were also assessed from 0.5 to 64 µg/mL. Plates were incubated at 37 °C in a humidified chamber, shaking at 1200 rpm (Stuart SI505). OD (600 nm) and fluorescence (493 nm excitation, 535 nm emission) readings were taken at initial (0 hr) and final (22 hr) time points.
Cytotoxicity assays
Human immortalized keratinocytes (HaCaT) were maintained and used to examine the cytotoxicity of the active extracts with an LDH cytotoxicity assay (G-Biosciences, St. Louis, MO) as previously described3. Briefly, the cell culture was standardized to 4 × 104 cells/mL using a hemocytometer and 0.2 mL added per well in a tissue culture treated 96-well microtiter plate (Falcon 35–3075). Plates were incubated for 48 hours to allow for seeding, and then cells were exposed to fresh media with treatment. Extracts and vehicle were serially diluted 2-fold (2–256 μg/mL) and were processed 24 hours post-treatment following manufacturer’s protocol for chemical induced cytotoxicity.
Chemical analysis
HPLC methods were adapted from Mämmelä42 and were performed on an Agilent 1260 Infinity system running OpenLab CDS ChemStation (Agilent Technologies, Santa Clara, CA, USA) with an Agilent Zorbax Eclipse XDB-C18 (250 × 4.6 mm, 5 µm) column with compatible guard column at 35 °C. A gradient elution consisting of mobile phases (A) 1% formic acid in H2O and (B) 1.0% formic acid in MeOH at 1.0 mL/min beginning at 95:5 A:B for 9 min, then following a linear gradient to 0:100 A:B at 69 min, which was held for 9 min, before returning to initial conditions to equilibrate the column. Extracts were prepared for HPLC at 10 mg/mL in DI H2O with an injection volume of 10 µL.
The liquid chromatography-Fourier transform mass spectrometry (LC-FTMS) analysis was performed using a Shimadzu SIL-ACHT and Dionex 3600 SD HPLC pump with a modification of the previous method. A 10 µL injection at ambient temperature with (A) 1.0% formic acid in H2O and (B) 1% formic acid in MeOH at a flow rate of 1.0 mL/min. Initial conditions were 95:5 (A:B) and held for 9 min, changing to 38:62 (A:B) using a linear gradient at 85 min, and then 100% B at 109 min, which was held for 10 min before returning to initial conditions to equilibrate the column. The data was acquired in MS1 mode scanning from a m/z of 150–1500 on a Thermo Scientific LTQ-FT Ultra MS in negative ESI mode and processed with Thermo Scientific Xcalibur 2.2 software (San Jose, CA). The capillary temperature was 275.0 °C, nitrogen was the sheath gas at a flow of 60, source voltage and current 5.0 kV and 100.0 µA, and the capillary voltage −19.0 V.
Using SciFinder Scholar (Chemical Abstracts Service, Columbus, OH, USA) all reported compounds from the genus Quercus were searched for matches to the LC-FTMS accurate mass data for each peak. The resulting putative compounds for samples 619-F2 and 620 are listed in Supplementary Tables S6, S7.
Data Availability
All data generated or analysed during this study are included in this published article and its Supplementary Information Files.
References
Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. (Atlanta, GA, 2013).
Stewart, P. S. & William Costerton, J. Antibiotic resistance of bacteria in biofilms. Lancet 358, 135–138, https://doi.org/10.1016/S0140-6736(01)05321-1 (2001).
Quave, C. L. et al. Castanea sativa (European Chestnut) leaf extracts rich in ursene and oeanene derivatives block Staphylococcus aureus virulence and pathogenesis without detectable resistance. Plos One 10, e0136486, https://doi.org/10.1371/journal.pone.0136486 (2015).
Wu, H., Moser, C., Wang, H. Z., Hoiby, N. & Song, Z. J. Strategies for combating bacterial biofilm infections. Int J Oral Sci 7, 1–7, https://doi.org/10.1038/ijos.2014.65 (2015).
Porcher, F. P. Resources of the Southern Fields and Forests. (Evans & Cogswell, 1863).
Sharifi-Rad, J. et al. Plants of the genus Allium as antibacterial agents: From tradition to pharmacy. Cell Mol Biol 62, 57–68 (2016).
Jakobsen, T. H. et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob Agents Chemother 56, 2314–2325, https://doi.org/10.1128/AAC.05919-11 (2012).
Office of the Historian United States Department of State. The Blockade of Confederate Ports, 1861–1865, https://history.state.gov/milestones/1861-1865/blockade (2017).
Lipińska, L., Klewicka, E. & Sójka, M. The structure, occurrence and biological activity of ellagitannins: a general review. Acta Sci Pol Technol Aliment 13, 289–299, https://doi.org/10.17306/J.AFS.2014.3.7 (2014).
Moore, S. P. Standard Supply Table of the Indigenous Remedies for Field Service. (Richmond, 1863).
U.S. National Library of Medicine. Maimed Men- Life and Limb: The Toll of the American Civil War, https://www.nlm.nih.gov/exhibition/lifeandlimb/maimedmen.html (2011).
Ekor, M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 4, https://doi.org/10.3389/fphar.2013.00177 (2014).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79, 629–661, https://doi.org/10.1021/acs.jnatprod.5b01055 (2016).
Tolmacheva, A. A., Rogozhin, E. A. & Deryabin, D. G. Antibacterial and quorum sensing regulatory activities of some traditional Eastern-European medicinal plants. Acta Pharm 64, 173–186, https://doi.org/10.2478/acph-2014-0019 (2014).
Hayes, L. E. Survey of Higher Plants for Presence of Antibacterial Substances. Botanical Gazette 108, 408–414 (1947).
Xu, Bj & Chang, Skc A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci 72, S159–S166, https://doi.org/10.1111/j.1750-3841.2006.00260.x (2007).
Kusari, S., Hertweck, C. & Spitellert, M. Chemical Ecology of Endophytic Fungi: Origins of Secondary Metabolites. Chem Biol 19, 792–798, https://doi.org/10.1016/j.chembiol.2012.06.004 (2012).
Li, H. et al. Antimycobacterial natural products from endophytes of the medicinal plant Aralia nudicaulis. Nat Prod Commun 10, 1641–1642 (2015).
Webster, D. et al. Antimycobacterial screening of traditional medicinal plants using the microplate resazurin assay. Can J Microbiol 56, 487, https://doi.org/10.1139/W10-035 (2010).
Karioti, A. et al. Antimicrobial properties of Quercus ilex L. proanthocyanidin dimers and simple phenolics: Evaluation of their synergistic activity with conventional antimicrobials and prediction of their pharmacokinetic profile. J Agric Food Chem 59, 6412–6422, https://doi.org/10.1021/jf2011535 (2011).
Sati, S. C., Sati, N., Sati, O. P., Biswas, D. & Chauhan, B. S. Analysis and antimicrobial activity of volatile constituents from Quercus leucotrichophora (Fagaceae) bark. Nat Prod Res 26, 869–872, https://doi.org/10.1080/14786419.2011.564584 (2012).
Hobby, G. H. et al. Quercus cerris extracts limit Staphylococcus aureus biofilm formation. J Ethnopharmacol 144, 812–815, https://doi.org/10.1016/j.jep.2012.10.042 (2012).
Jamil, M., ul Haq, I., Mirza, B. & Qayyum, M. Isolation of antibacterial compounds from Quercus dilatata L. through bioassay guided fractionation. Ann Clin Microbiol Antimicrob 11, 11, https://doi.org/10.1186/1476-0711-11-11 (2012).
Wan Nor Amilah, Wa. W., Masrah, M., Hasmah, A. & Noor Izani, N. J. In vitro antibacterial activity of Quercus infectoria gall extracts against multidrug resistant bacteria. Trop Biomed 31, 680–688 (2014).
Deryabin, D. G. & Tolmacheva, A. A. Antibacterial and anti-quorum sensing molecular composition derived from Quercus cortex (Oak bark) extract. Molecules 20, 17093–17108, https://doi.org/10.3390/molecules200917093 (2015).
Mohebi, R. et al. In vitro and in vivo antibacterial activity of acorn herbal extract against some Gram-negative and Gram-positive bacteria. Roum Arch Microbiol Immunol 70, 149–152 (2011).
Chusri, S. & Voravuthikunchai, S. P. Detailed studies on Quercus infectoria Olivier (nutgalls) as an alternative treatment for methicillin-resistant Staphylococcus aureus infections. J Appl Microbiol 106, 89–96, https://doi.org/10.1111/j.1365-2672.2008.03979.x (2009).
Chung, K. T., Wong, T. Y., Wei, C. I., Huang, Y. W. & Lin, Y. Tannins and human health: a review. Crit Rev Food Sci Nutr 38, 421–464, https://doi.org/10.1080/10408699891274273 (1998).
Akiyama, H., Fujii, K., Yamasaki, O., Oono, T. & Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemother 48, 487–491, https://doi.org/10.1093/jac/48.4.487 (2001).
Sarwar, R. et al. Evaluation of antioxidant, free radical scavenging, and antimicrobial activity of Quercus incana Roxb. Front Pharmacol 6, https://doi.org/10.3389/fphar.2015.00277 (2015).
Brussell, D. E. Araliaceae species used for culinary and medicinal purposes in Niigata-ken, Japan. Econ Bot 58, 736–739, https://doi.org/10.1663/0013-0001(2004)058[0736:ASUFCA]2.0.CO;2 (2004).
Ali, F. et al. 4-epi-Pimaric acid: a phytomolecule as a potent antibacterial and anti-biofilm agent for oral cavity pathogens. Eur J Clin Microbiol 31, 149–159, https://doi.org/10.1007/s10096-011-1287-x (2012).
Miller, S. L., Villanueva, H. E., Palazzo, M. C., Wright, B. S. & Setzer, W. N. Seasonal variation and bioactivity in the leaf oil of Liriodendron tulipifera growing in Huntsville, Alabama. Nat Prod Commun 4, 839–843 (2009).
Graziose, R. et al. Antiplasmodial activity of aporphine alkaloids and sesquiterpene lactones from Liriodendron tulipifera L. J Ethnopharmacol 133, 26–30, https://doi.org/10.1016/j.jep.2010.08.059 (2011).
Moon, M. K. et al. Farnesyl protein transferase and tumor cell growth inhibitory activities of lipiferolide isolated from Liriodendron tulipifera. Arch Pharm Res 30, 299, https://doi.org/10.1007/BF02977609 (2007).
Doskotch, R. W. & El-Feraly, F. S. Antitumor agents II: Tulipinolide, a new germacranolide sesquiterpene, and costunolide. Two cytotoxic substances from Liriodendron tulipifera L. JPharmSci 58, 877–880, https://doi.org/10.1002/jps.2600580720 (1969).
Zeng, C.-z. & Liu, Z.-x. Bacteriostatic activity and stability of extracts from Liriodendron chinense. Hubei Agric Sci, 1222–1224 (2009).
Tan, X. et al. The investigation of inhibiting quorum sensing and methicillin-resistant Staphylococcus aureus biofilm formation from Liriodendron hybrid. Pak J Pharm Sci 28, 903–908 (2015).
SERNEC. Southeast Regional Network of Expertise and Collections, http://sernecportal.org/portal/collections/list.php?collector=Dettweiler&eventdate1=April%202015&eventdate2=July%202015&db=279;&page=1 (2017).
Cockerill, F. R. Performance standards for antimicrobial susceptibility testing: twenty-third informational supplement; M100 - S23. (CLSI, 2013).
Quave, C. L. et al. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. Plos One 7, e28737, https://doi.org/10.1371/journal.pone.0028737 (2012).
Mämmelä, P., Savolainen, H., Lindroos, L., Kangas, J. & Vartiainen, T. Analysis of oak tannins by liquid chromatography-electrospray ionisation mass spectrometry. J Chromatogr A 891, 75–83, https://doi.org/10.1016/S0021-9673(00)00624-5 (2000).
Acknowledgements
We thank Dr. Alexander Horswill for providing the agr reporter strains. Thanks to Dr. E. Jane Bradbury for help with plant identification and collection. This work was supported in part by the Howard Hughes Medical Institute Science Education Program award #52006923 to Emory University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Howard Hughes Medical Institute or Emory University. This work was also supported by a grant from the National Institutes of Health, National Center for Complementary and Integrative Health (R01 AT007052, PI: CQ) and National Institute of Allergy and Infectious Disease (R21 AI136563, PI: CQ). The content is solely the responsibility of the authors and does not necessarily reflect the officinal view of NCCIH, NIAID or NIH. The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
Author information
Authors and Affiliations
Contributions
M.D.: Collected the material; C.Q., D.Z. and J.L.: Conceived and designed the experiments; M.D., J.L., B.D., R.D. and K.N.: Performed the experiments; M.D. and C.Q.: Analyzed the data; M.D., J.L. and C.Q.: Prepared the draft; All authors proofread the final draft and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The research herein was conducted in absence of any competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dettweiler, M., Lyles, J.T., Nelson, K. et al. American Civil War plant medicines inhibit growth, biofilm formation, and quorum sensing by multidrug-resistant bacteria. Sci Rep 9, 7692 (2019). https://doi.org/10.1038/s41598-019-44242-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44242-y
This article is cited by
-
Botanical inhibitors of SARS-CoV-2 viral entry: a phylogenetic perspective
Scientific Reports (2023)
-
Targeting ESKAPE pathogens with anti-infective medicinal plants from the Greater Mpigi region in Uganda
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.