Abstract
The extrahepatic complications of primary biliary cholangitis (PBC) in Asian patients remain elusive. A 30-year cohort study of 150 Taiwanese PBC patients treated with ursodeoxycholic acid (UDCA) was conducted. Patients with alkaline phosphatase levels >1.67 × ULN after 1-year treatment were considered suboptimal responders. At baseline, of 150 patients (mean age: 53.75 years), 128 (85.3%) were females, and 34 (22.8%) had cirrhosis. The cumulative incidences of various incident events were all-cause mortality or liver transplantation: 46.7%; extrahepatic mortality: 24.5%; extrahepatic malignancies: 8.1%; hypertension: 46.2%; dyslipidemia: 44.1%; diabetes: 30.6%; hyperuricemia: 11.2%; acute coronary syndrome: 3.1%; cerebral vascular accident (CVA): 8.9%; autoimmune diseases: 16%; and osteoporosis: 20.9%. The 5- to 20-year cumulative incidences for all-cause mortality or liver transplantation and extrahepatic mortality were 16.2–41.3% and 3.1–11.9%, respectively. Baseline associations were age and alpha-fetoprotein levels with extrahepatic mortality, 80% due to sepsis; age with extrahepatic malignancies and hypertension; gender and hyperuricemia with CVA; and UDCA response with autoimmune disease. Conclusions: Sepsis accounted for most extrahepatic mortality in PBC patients, and the longer the follow-up was, the higher the extrahepatic/all-cause mortality ratio. Baseline age is crucial for incident extrahepatic events and only CVA shows gender-dimorphism; the association between UDCA response and autoimmune disease requires further investigation.
Similar content being viewed by others
Introduction
Primary biliary cholangitis (PBC), formerly called primary biliary cirrhosis1, predominantly affects middle-aged women2. It is a rare, chronic progressive cholestatic liver disease characterized by the autoimmune-mediated destruction of the small- and medium-sized intrahepatic bile ducts3. Without treatment, most PBC patients eventually develop hepatic fibrosis and may require liver transplantation during the late stage of the disease3. Currently, ursodeoxycholic acid (UDCA) and obeticholic acid (OCA) are the two approved therapies for PBC4. However, some patients treated with UDCA or OCA do not exhibit adequate responses, as 30–40% of PBC patients do not respond to UDCA5 and up to 60% of UDCA suboptimal responder do not show therapeutic effect to OCA6.
In addition to hepatic manifestations such as liver cirrhosis and hepatocellular carcinoma (HCC)3, PBC can lead to multiple extrahepatic manifestations, including extrahepatic malignancies (EMs)7, autoimmune diseases8, dyslipidemia, and other metabolic diseases9. The prevalence of EM in PBC patients ranged from 4.5%10 to 15.7%11. Although PBC patients have a high incidence of HCC12, the risk of EM is even higher than that of HCC13. The onset of EM in PBC patients does not influence the natural history of their liver disease7. Moreover, antimitochondrial antibodies (AMAs) are detected in over 90% of patients with PBC2,3. The high frequency of AMA underlines not only its autoimmune process but also that PBC can occur in association with other autoimmune disorders14. Consistently, up to 1/3 of PBC patients have a concomitant extrahepatic autoimmune disease15. Common extrahepatic autoimmune conditions include Sjögren syndrome (SS), autoimmune thyroid diseases, and systemic lupus erythematosus (SLE)8. Although extrahepatic autoimmune diseases might be associated with EM development in PBC7, whether extrahepatic autoimmune diseases affect the prognosis of PBC patients remains conflicting15,16. In addition, PBC patients are prone to hypercholesteremia2. However, PBC is not associated with an increased risk of myocardial infarction, stroke, transient ischemic attack (TIA)17, and subclinical atherosclerosis18. The harmlessness of hypercholesteremia in cardiovascular risks of PBC is probably due to lower amounts of visceral fat19, higher adiponectin concentrations20, and more lipoprotein X21 and lipoprotein A1 particles22, compared with the controls. However, the impacts of hypercholesteremia on cardiovascular events in PBC patients may vary with various disease stages, as less advanced PBC with moderate hypercholesterolemia has been linked to an increased cardiovascular risk23. Moreover, after accounting for hepatic and cancer-related deaths, there is unexplained excess mortality associated with PBC24. Even for asymptomatic PBC patients, the overall survival was shorter than that predicted for an age- and gender-matched control population25.
Especially in Asia, the extrahepatic complications and associated mortality in PBC patients remain undetermined, and the situation is even more complicated when considering the UDCA suboptimal responders who are at high risk for serious complications5. Accordingly, we conducted a 30-year hospital-based cohort study to elucidate the extrahepatic manifestations and mortality as well as the associated factors of PBC in an Asian country, Taiwan.
Methods
Patients
Patients older than 18 years of age with a diagnosis of PBC were consecutively recruited at a Taiwan tertiary referral center between July 1988 and June 2018. The diagnosis of PBC was based on the following criteria: (1) an AMA positivity higher than 1:40, (2) abnormal alkaline phosphatase (Alk-P) levels [≥1.5 X upper limit of normal (ULN)] and/or a compatible liver histology, and (3) an AMA negative variant: abnormal Alk-P levels, and a liver histology compatible with PBC. The subjects with autoimmune hepatitis, human immunodeficiency virus, hepatitis B infection, hepatitis C infection, hemochromatosis, active alcohol consumption, primary cancer and recipients of solid organ transplants, and those with a follow-up <1 year or poor compliance to UDCA therapy with were excluded.
Study design
As a retrospective study, the clinical details of each patient were included anonymously with a composite code number in a database that contained detailed information including baseline demographic data and symptoms, physical findings and laboratory results at presentation and at the time of diagnosis and referral, as well as data collected at subsequent 3-month routine clinical visits, and emergency and hospital admissions. Moreover, for each patient, the following were recorded: the dates of complications and treatment, data from the last follow-up, dates of liver transplantation and death, and the cause of death. Biochemistry tests including AMA, antinuclear antibody (ANA), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, albumin, Alk-p, γ-glutamyl transpeptidase (γ-GT), alpha-fetoprotein (AFP) and total cholesterol (TC) were conducted on the fasting serum of the enrolled participants. Cardiac injury or function tests, including creatine kinase and B-type natriuretic peptide, and electrocardiograms were performed as indicated. The aforementioned profiles were measured using routine automated techniques at the clinical pathology or research laboratories of the hospital. Liver ultrasound was performed every 6 months. All enrolled PBC patients were treated with UDCA at a standard dose of 13–15 mg/kg/day. Patients whose Alk-P levels > 1.67 × ULN after 1 year of treatment were defined as suboptimal responders26. The diagnosis of liver cirrhosis was made by earlier histologic findings or ultrasonographic findings compatible with cirrhosis and supplemented with esophageal or gastric varices, splenomegaly and/or thrombocytopenia. The incidence and risk factors of extrahepatic manifestations, including diabetes, hypertension, hyperuricemia, dyslipidemia, acute coronary syndrome (ACS), cerebrovascular accident (CVA), autoimmune diseases [SS, SLE, systemic sclerosis, rheumatoid arthritis (RA), autoimmune thyroid disease and psoriasis], osteoporosis, extrahepatic cancer and mortality, were surveyed. Diabetes was defined as a fasting plasma glucose level ≥126 mg/dl, plasma glucose ≥200 mg/dl two hours after a 75 g oral glucose load as in a glucose tolerance test, symptoms of high blood sugar and casual plasma glucose ≥200 mg/dl, glycated hemoglobin ≥6.5% in the diabetes control and complications trial27, or the associated diagnosis of diabetes [International Classification of Diseases, Ninth Revision (ICD-9) code 250] with anti-diabetic agents noted based on chart review. Hypertension was defined according to three criteria: systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg or the use of medication to control hypertension28 or the associated diagnosis of hypertension (ICD-9: 401.9). Dyslipidemia was defined by serum concentrations of TC ≥ 200 mg/dL, low-density lipoprotein-cholesterol levels ≥130 mg/dL, triglycerides ≥150 mg/dL29, the associated diagnosis of dyslipidemia (ICD-9:272.0~272.4) or the usage of lipid-lowering agents based on chart review. Hyperuricemia was defined by serum uric acid levels >7 mg/dL, the presence of monosodium urate crystals by aspiration and examination of synovial fluid30, the presence of an associated diagnosis (ICD-9: 274), or the use of associated medications, including colchicine and allopurinol, based on chart review. ACS was defined as the presence of unstable angina, non-ST elevation myocardial infarction, ST elevation myocardial infarction31, or the presence of an associated diagnosis (ICD-9: 411.1, 411.81, 410) based on chart review. The CVAs were defined as TIA, ischemic stroke and hemorrhagic stroke identified using the chart review (ICD-9: 430~437) and confirmed by a review of medical records/registries32. SLE was diagnosed based on the American College of rheumatology criteria33 or the presence of an associated diagnosis (ICD-9: 710.0) based on chart review. SS was diagnosed based on European-USA consensus criteria34 or the presence of an associated diagnosis (ICD 9: 710.2) based on chart review. RA was diagnosed based on the revised American College of Rheumatology/European League Against Rheumatism collaborative initiative criteria for RA35 or the presence of an associated diagnosis (ICD-9: 714.0 or 714.3) on chart review. Psoriasis was defined as the presence of an associated diagnosis (ICD-9: 696.0, 696.1,696.8) based on chart review. Osteoporosis was defined as the presence of an associated diagnosis (ICD-9: 733.00) from chart review. Systemic sclerosis was diagnosed as described previously36. The primary cancers were diagnosed based on pathology, confirmed by the specialists of each primary cancer, and the diagnosis and stage of each cancer were registered with the National Cancer Registration.
Statistical analyses
All statistical analyses were performed using the Statistical Package for Social Science (SPSS package version 21, SPSS Inc., Chicago, IL, USA) software. Continuous variables were analyzed using Student’s t-test, and categorical variables were analyzed using a chi-square test or Fisher’s exact test as appropriate. Nonparametric tests were applied where indicated. The collinearity of variables was determined by linear regression tests. Kaplan-Meier or univariate Cox regression analyses were used to assess the relationship between various variables and patient events. Multivariate Cox regression models were used to assess the relationship between various dependent and independent variables by adjusting for all the independent variables with a p value < 0.05 in the univariate analyses. Receiver operating characteristic curve (ROC) analyses were performed to evaluate if independent variables were significant predictors of the dependent variables. Youden index was assessed to identify the optimum cut-off values of the independent variables from the coordinate points of the ROC curves37. Binary logistic regression analysis was performed to assess the variables that accounted for the emergence of events. Statistical significance was defined at the 5% level based on two-tailed tests of the null hypothesis.
Institutional review board
The study was conducted in accordance with good clinical practice and all applicable regulations, including the Declaration of Helsinki and local regulatory requirements and was approved by the ethics committee of Chang-Gung Memorial Hospital. Informed consent was obtained from the participants.
Results
Baseline characteristics
A total of 150 consecutive patients with PBC were enrolled in the current study. The baseline characteristics are listed in Table 1. Of 150 patients, 128 (85.3%) were female, with a female-to-male ratio of 5.82:1 and a mean and median age of 53.75 and 53.00 years, respectively. Additionally, 34 (22.8%) patients had liver cirrhosis, 5 (3.4%) had varices, 10 (6.8%) had hypertension, 7 (4.8%) had dyslipidemia, 6 (4.1%) had diabetes, 3 (2.0%) had hyperuricemia and 7 (4.6%, 2 with SS, 2 with autoimmune thyroiditis, 1 with systemic sclerosis, 1 with both SS and autoimmune thyroiditis and 1 with both SS and systemic sclerosis) had autoimmune diseases. The female patients had higher AST/ALT ratios (p = 0.008) and cholesterol levels (p = 0.029) than the male patients with comparable ages (p = 0.629) and cirrhosis (p = 0.401) rates.
UDCA response
The UDCA responders accounted for 70.6% of all the enrolled PBC patients and for 28.6% and 87.8% in those with and without baseline cirrhosis, respectively. That is, those with baseline cirrhosis had a lower UDCA response rate (p = 0.003). However, higher UDCA response rates were noted in those with than those without baseline autoimmune diseases (85.7% vs. 40%, p = 0.023). The UDCA response rates were indifferent among those with and without other baseline extrahepatic events, including hypertension (p = 0.206), dyslipidemia (p = 0.363), diabetes (p = 0.609) and hyperuricemia (p = 0.674).
Cumulative incidence of various events
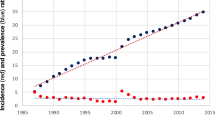
With a follow-up period of up to 30 years, the cumulative incidences of various incident events were as follows (Table 2): all-cause mortality and liver transplantation (i.e., mortality for all causes and number of liver transplantations): 46.7% (16.2% for 5 years, 24.9% for 10 years, 32.9% for 15 years and 41.3% for 20 years) (Fig. 1A); extrahepatic mortality: 24.5% (3.1% for 5 years; 6.9% for 10 years; 9.4% for 15 years and 11.9% for 20 years) (Fig. 1B); EM: 8.1%; hypertension: 46.2%; dyslipidemia: 44.1%; diabetes: 30.6%; hyperuricemia: 11.2%; ACS: 3.1%; CVA: 8.9% (Fig. 2); autoimmune diseases: 16% (SS: 12.1%; SLE: 9.2%; RA:1.6%; autoimmune thyroid disease: 3.4%); and osteoporosis: 4.4% for 3 and 5 years and 20.9% for 30 years.
Ten PBC patients died of extrahepatic causes, including 8 from sepsis subsequent to pneumonia (2 had baseline liver cirrhosis) or urinary tract infection, 1 from SLE and 1 from an uncertain cause. None of the 10 patients had HCC. Six PBC patients had incidents of EMs, including 3 colon cancers, 1 lung cancer, 1 cervical cancer and 1 brain cancer.
Independent baseline factors of various incident extrahepatic events
The independent factors for various incident extrahepatic events are as follows: (1) age (95% confidence interval (CI) of hazard ratio (HR):1.019–1.152; cut-off: 53 years, HR:6.499, 95% CI HR:1.323–31.935) and AFP (95% CI HR:1.002–1.058) with extrahepatic mortality (Supplementary Table 1); (2) age (95% CI HR: 1.022–1.225; cut-off: 57 years, HR: 5.948, 95% CI HR: 1.122–31.532) with incident EMs (Supplementary Table 2); (3) age (95% CI HR:1.012–1.083; cut-off: 53 years, HR: 3.124, 95% CI HR: 1.362–7.165) and hyperuricemia (95% HR: 1.701–37.06) with incident hypertension (Supplementary Table 3); (4) hypertension (95% CI HR: 1.417–24.19) with incident diabetes (Supplementary Table 4); (5) male gender (95% CI HR: 1.146–142.6) and hyperuricemia (95% CI HR: 1.14–355.4) with incident CVA (Supplementary Table 5 and Fig. 2); (6) UDCA response (95% CI HR: 1.049–24.33) with incident autoimmune disease (Supplementary Table 9 and Fig. 3); and (7) age (95% CI HR: 1.024–1.13; cut-off: 51 years; HR: 14.884, 95% CI HR:1.916–115.61) with incident osteoporosis (Supplementary Table 10). No independent factors could be identified for incident dyslipidemia, hyperuricemia and ACS (Supplementary Tables 6–8).
Discussion
The key results of the current study are: (1) The female-to-male ratio was 5.82:1 for the 150 Taiwanese PBC patients. The females had higher AST/ALT ratios than the males. (2) The 30-year cumulative incidences of the various extrahepatic events ranged from 3.1% to 46.7%. Sepsis accounted for most of the extrahepatic mortality. (3) The baseline factors for various incident events are: age and AFP for extrahepatic mortality; age for EMs; age and hyperuricemia for hypertension; hypertension for diabetes; male gender and hyperuricemia for CVA; UDCA response for autoimmune disease; and age for osteoporosis.
The female-to-male ratio of 5.82:1 in the current study was less than 9–10:1, a ratio classically reported for PBC5,38,39. However, several population-based studies suggest an increasing male prevalence for PBC8,40,41,42,43,44, and the opposite trend was observed for the sample size and female-to-male ratio44. Thus, the percentage of males with PBC in most studies might be underestimated and highlights the importance of surveying the gender impact on the prognosis of PBC. The fact that the females had higher baseline AST/ALT ratios than the males suggests more advanced hepatic fibrosis45 despite comparable cirrhosis rate. While the higher cholesterol levels in the females might be subsequent to PBC-related liver disease severity or non-PBC-specific gender-dimorphic lipid metabolism5. The hypercholesteremia in female PBC patients did not alter their prognosis, as incident CVA was the only investigated extrahepatic event affected by gender, and male patients were the dominant victims.
Notably, although extrahepatic mortality (24.5%) accounts for more than 1/2 of all-cause mortality (46.7%) within a 30-year follow-up, it accounts for only 1/5 (3.1% vs. 16.2%), 1/4 (6.9% vs. 24.9%), 1/3 (9.4% vs. 32.9%), and less than 1/2 (11.9 vs. 41.3%) of the all-cause mortality within a 5-year, 10-year, 15-year and 20-year follow-up, respectively. That is, most 20-year mortalities were hepatic; however, the longer the follow-up, the higher the extrahepatic mortality-to-all-cause mortality ratio. In contrast to studies conducted in western countries24,25, the extrahepatic mortality (24.5%) of the PBC patients was not higher than that (25.71%46) of the general population in Taiwan. Both baseline age and AFP levels were associated with extrahepatic mortality, which was mainly caused by sepsis. Age-related remodeling of the immune system, termed immunosenescence, leads to infections that occur frequently in the elderly47. In addition to be a marker for HCC48, elevated AFP levels indicate hepatic regeneration subsequent to hepatic injury49 and induce immune escapes50. Both old age and high AFP levels thus might signify impaired immunity in the cases died of extrahepatic cause. Moreover, of the 8 patients who died of sepsis, 2 had baseline cirrhosis, an infection-susceptible disease51. Collectively, PBC is a potential immunocompromised disease that might threaten patients’ lives with the risk of sepsis.
The cumulative incidence (8.1%) of EM was within the reported range (4.5–15.7%)10,11. PBC increases the risk of bladder and breast cancers as found in a study conducted in Europe52. However, in the current study, colon cancer was the major EM, and none of the patients developed bladder or breast cancer. Whether any ethnic or environmental difference accounts for the discrepancy demands further investigation.
In a study conducted in the UK, the rates of ACS and CVA in PBC patients were comparable with controls17. However, in the current study, with the exception of dyslipidemia (7.6% for 5 years) and osteoporosis (4.4% for 3 years), the PBC-associated phenomena2, all cumulative incidences of metabolic diseases, including hypertension (8.2% for 5 years), diabetes (8.8% for 5 years), hyperuricemia (3.4% for 10 years), ACS (0% for 5 years), CVA (1% for 5 years), were lower than the general population. In Taiwanese adults, the 5-year cumulative incidence of hypertension, hyperglycemia, dyslipidemia53 and ACS54 was 24.2%, 20.04%, 7.3% and 1.5%, respectively; the 10-year cumulative incidences of hyperuricemia ranged from 11.5% to 18.1%55; the 3.3-year cumulative incidences of CVA ranged from 5.64% to 10.23%56; and the 3-year cumulative incidences of osteoporosis ranged from 2.1% to 3.3%57. In addition, although the incidence of autoimmune diseases in PBC patients was higher than in the general population of Taiwan (5-year cumulative incidence: 7% vs. 2.5–5%)58, it is much lower than in European PBC patients (30-year cumulative incidence: 16% vs. 61.2%)59, and different ethnicities might account for this difference.
Baseline age was also associated with incident EM, hypertension and osteoporosis. This finding was consistent with the notions that cancer and hypertension occur more frequently in the elderly due to immunosenescence47, aging leads to functional and mechanical changes in arteries60, and age is a major determinant of osteoporosis61. Based on the cut-off values of the baseline age for predicting osteoporosis (51 years), extrahepatic mortality (53 years), hypertension (53 years), and EM (57 years), PBC patients ≥51 years demand special attention for these complications. In addition, consistent with a previous study62, baseline hypertension was associated with incident diabetes. Although more male than female Chinese PBC patients had cardiac involvement63, we did not find any gender-dimorphism in the ACS risk. Instead, male gender and baseline hyperuricemia were positive factors for incident CVA. Consistently, previous studies showed that stroke incidence was higher in men than in women64, and gout is associated with stroke risk65.
In contrast to a previous report7, we did not find any connection between extrahepatic autoimmune diseases and EM. Paradoxically, a favorable UDCA response was associated with incident autoimmune diseases. Some UDCA inadequate responders died too early to acquire autoimmune diseases, which might account for this finding. However, a higher UDCA response rate was also noted in those with than those without baseline autoimmune diseases. Furthermore, most UDCA responders acquired autoimmune diseases within the first 5 years of follow-up, while the UDCA inadequate responder acquired autoimmune diseases with a time lag of 15 years approximately (Fig. 3). If patients prone to developing autoimmune diseases are primed to be better responders to UDCA remains elusive, further investigation is required.
The strength of the study is the long follow-up with a comprehensive survey of extrahepatic events, while the retrospective nature is the main limitation to elucidate the associated biases including selection bias.
Taken together, in this Taiwanese PBC cohort, a lower female-to-male ratio than expected was noted, and most extrahepatic complications were less frequent than the general population. Gender dimorphism was only evident in incident CVA. The longer the follow-up, the higher the extrahepatic-to-all-cause mortality ratio. Most extrahepatic mortality resulted from sepsis. PBC patients ≥51 years warrant special attention for osteoporosis, extrahepatic mortality, EM and hypertension. The positive link between favorable UDCA response and incident autoimmune diseases warrants further investigation.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Beuers, U. et al. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. Hepatology. 62, 1620–2 (2015).
Carey, E. J., Ali, A. H. & Lindor, K. D. Primary biliary cirrhosis. Lancet. 386, 1565–75 (2015).
Abbas, G., Jorgensen, R. A. & Lindor, K. D. Fatigue in primary biliary cirrhosis. Nat Rev Gastroenterol Hepatol. 7, 313–9 (2010).
Samur, S. et al. Long-term clinical impact and cost-effectiveness of obeticholic acid for the treatment of primary biliary cholangitis. Hepatology. 65, 920–928 (2017).
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 67, 145–172 (2017).
Hirschfield, G. M. et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 148, 751–61 (2015).
Floreani, A. et al. Extrahepatic malignancies in primary biliary cirrhosis: a comparative study at two European centers. Clin Rev Allergy Immunol. 48, 254–62 (2015).
Fan, X. et al. Underestimated Male Prevalence of Primary Biliary Cholangitis in China: Results of a 16-yr cohort study involving 769 patients. Sci Rep. 7, 6560 (2017).
Burman, B. E., Jhaveri, M. A. & Kowdley, K. V. An Update on the Treatment and Follow-up of Patients with Primary Biliary Cholangitis. Clin Liver Dis. 21, 709–723 (2017).
Floreani, A. et al. Hepatic and extrahepatic malignancies in primary biliary cirrhosis. Hepatology. 29, 1425–8 (1999).
Ngu, J. H. et al. Mortality and the risk of malignancy in autoimmune liver diseases: a population-based study in Canterbury, New Zealand. Hepatology. 55, 522–9 (2012).
Trivedi, P. J. et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicentre international study. Gut. 65, 321–9 (2016).
Deutsch, M. et al. Risk of hepatocellular carcinoma and extrahepatic malignancies in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 20, 5–9 (2008).
Berg, P. A. The role of the innate immune recognition system in the pathogenesis of primary biliary cirrhosis: a conceptual view. Liver Int. 31, 920–31 (2011).
Floreani, A., Franceschet, I. & Cazzagon, N. Primary biliary cirrhosis: overlaps with other autoimmune disorders. Semin Liver Dis. 34, 352–60 (2014).
Floreani, A. et al. Thyroid Dysfunction in Primary Biliary Cholangitis: A Comparative Study at Two European Centers. Am J Gastroenterol. 112, 114–119 (2017).
Solaymani-Dodaran, M. et al. Risk of cardiovascular and cerebrovascular events in primary biliary cirrhosis: a population-based cohort study. Am J Gastroenterol. 103, 2784–8 (2008).
Allocca, M. et al. Hypercholesterolaemia is not associated with early atherosclerotic lesions in primary biliary cirrhosis. Gut. 55, 1795–800 (2006).
Alempijevic, T. et al. Assessment of metabolic syndrome in patients with primary biliary cirrhosis. Wien Klin Wochenschr. 124, 251–5 (2012).
Floreani, A. et al. Plasma adiponectin levels in primary biliary cirrhosis: a novel perspective for link between hypercholesterolemia and protection against atherosclerosis. Am J Gastroenterol. 103, 1959–65 (2008).
Chang, P. Y. et al. Lipoprotein-X reduces LDL atherogenicity in primary biliary cirrhosis by preventing LDL oxidation. J Lipid Res. 45, 2116–22 (2004).
O’Kane, M. J. et al. Abnormalities of serum apo A1 containing lipoprotein particles in patients with primary biliary cirrhosis. Atherosclerosis. 131, 203–10 (1997).
Longo, M. et al. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut. 51, 265–9 (2002).
Newton, J. L. et al. Reduced heart rate variability and baroreflex sensitivity in primary biliary cirrhosis. Liver Int. 26, 197–202 (2006).
Mahl, T. C., Shockcor, W. & Boyer, J. L. Primary biliary cirrhosis: survival of a large cohort of symptomatic and asymptomatic patients followed for 24 years. J Hepatol 20, 707–713 (1994).
Hirschfield, G. M. et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 67, 1568–1594 (2018).
American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 33(Supplement 1), S62–S69 (2010).
Treff, C., Benseñor, I. M. & Lotufo, P. A. Leisure-time and commuting physical activity and high blood pressure: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J Hum Hypertens. 31, 278–283 (2017).
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106, 3143–421 (2002).
Harris, M. D., Siegel, L. B. & Alloway, J. A. Gout and hyperuricemia. Am Fam Physician. 59, 925–34 (1999).
Kumar, A. & Cannon, C. P. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc. 84, 917–38 (2009).
Ungprasert, P., Wijarnpreecha, K. & Thongprayoon, C. Risk of cerebrovascular accident in patients with primary biliary cirrhosis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 28, 90–4 (2016).
Hochberg, M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40, 1725 (1997).
Vitali, C. et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 61, 554–8 (2002).
Aletaha, D. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–81 (2010).
van den Hoogen, F. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 65(47), 2737 (2013).
Youden, W. J. Index for rating diagnostic tests. Cancer. 3, 32–5 (1950).
Zhang, H. et al. Geoepidemiology, Genetic and Environmental Risk Factors for PBC. Dig Dis. 33(Suppl 2), 94–101 (2015).
Boonstra, K. et al. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 56, 1181–8 (2012).
Lleo, A. et al. Evolving Trends in Female to Male Incidence and Male Mortality of Primary Biliary Cholangitis. Sci Rep. 6(25906), 25–27 (2016).
Zöller, B. et al. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 379, 244–9 (2012).
Myers, R. P. et al. Epidemiology and natural history of primary biliary cirrhosis in a Canadian health region: a population-based study. Hepatology 50, 1884–1892 (2009).
Lu, M. et al. Increasing Prevalence of Primary Biliary Cholangitis and Reduced Mortality With Treatment. Clin Gastroenterol Hepatol. 16, 1342–1350.e1 (2018).
Tanaka, A. et al. Autoimmune liver diseases in the Asia-Pacific region: Proceedings of APASL symposium on AIH and PBC 2016. Hepatol Int. 10, 909–915 (2016).
Nyblom, H. et al. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 26, 840–5 (2006).
Lo, W. C. et al. Adult mortality of diseases and injuries attributable to selectedmetabolic, lifestyle, environmental, and infectious risk factorsin Taiwan: a comparative risk assessment. Popul Health Metr. 15, 17 (2017).
Bueno, V., Sant’Anna, O. A. & Lord, J. M. Ageing and myeloid-derived suppressor cells: possible involvement in immunosenescence and age-related disease. Age (Dordr). 36, 9729 (2014).
Tzartzeva, K. et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 154, 1706–1718 (2018).
Liaw, Y. F. et al. Alpha-fetoprotein changes in the course of chronic hepatitis: relation to bridging hepatic necrosis and hepatocellular carcinoma. Liver. 6, 133–7 (1986).
Wang, X. & Wang, Q. Alpha-Fetoprotein and Hepatocellular Carcinoma Immunity. Can J Gastroenterol Hepatol. 2018, 9049252 (2018).
Bunchorntavakul, C., Chamroonkul, N. & Chavalitdhamrong, D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J Hepatol 8, 307–21 (2016).
Boonstra, K. et al. Increased cancer risk in a large population-based cohort of patients with primary biliary cirrhosis: follow-up for up to 36 years. Hepatol Int. 8, 266–74 (2014).
Bai, C. H. et al. Incidence of hypertension, hyperglycemia and hyperlipidemia: The results from a national survey. American Public Health Association 139 annual meeting and exposition, Monday, October 31, (2011).
Lin, J. N. et al. Increased Risk of Acute Coronary Syndrome in Patients With Diverticular Disease: A Nationwide Population-Based Study. Medicine (Baltimore). 94, e2020 (2015).
Kuo, D. et al. Hyperuricemia and incident cardiovascular disease and noncardiac vascular events in patients with rheumatoid arthritis. Int J Rheumatol. 2014, 523897 (2014).
Lai, H. C. et al. Atrial fibrillation, liver disease, antithrombotics and risk of cerebrovascular events: A population-based cohort study. Int J Cardiol. 223, 829–837 (2016).
Kok, V. C. et al. Gout as a risk factor for osteoporosis: epidemiologic evidence from a population-based longitudinal study involving 108,060 individuals. Osteoporos Int. 29, 973–985 (2018).
Liu, Y. C. et al. Systemic lupus erythematosus and thyroid disease - Experience in a single medical center in Taiwan. J Microbiol Immunol Infect, https://doi.org/10.1016/j.jmii.2016.11.008. (2017).
Floreani, A. et al. Extrahepatic autoimmune conditions associated with primary biliary cirrhosis. Clin Rev Allergy Immunol. 48, 192–7 (2015).
Harvey, A., Montezano, A. C. & Touyz, R. M. Vascular biology of ageing-Implications in hypertension. J Mol Cell Cardiol. 83, 112–21 (2015).
Boonen, S. et al. Osteoporosis and osteoporotic fracture occurrence and prevention in the elderly: a geriatric perspective. Best Pract Res Clin Endocrinol Metab. 22, 765–85 (2008).
Conen, D. et al. Blood pressure and risk of developing type 2 diabetes mellitus: the Women’s Health Study. Eur Heart J. 28, 2937–43 (2007).
Bian, S. et al. Cardiac involvement in patients with primary biliary cholangitis: A 14-year longitudinal survey-based study. PLoS One. 13, e0194397 (2018).
Petrea, R. E. et al. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 40, 1032–7 (2009).
Seminog, O. O. & Goldacre, M. J. Gout as a risk factor for myocardial infarction and stroke in England: evidence from record linkage studies. Rheumatology (Oxford). 52, 2251–9 (2013).
Acknowledgements
The authors thank Ms. Chia-Hui Tsai and Shuen-Shian Shiau from the Liver Research Center, Division of Gastroenterology, Department of Gastroenterology and Hepatology, Chang Gung Memorial Hospital, Taoyuan, Taiwan, for her assistance with data mining, and Dr. Jur-Shan Cheng from Clinical Informatics and Medical Statistics Research Center, College of Medicine, Chang Gung University for her assistance in statistical analyses. This study was supported by grants from the Chang Gung Medical Research Program (CMRPG3F0472, CRRPG3F0013, CMRPG3I0411 and CMRPG3H1911) and the National Science Council, Taiwan (105-2629-B-182-001- and 106-2314-B-182-041-MY2).
Author information
Authors and Affiliations
Contributions
C.Y.L. statistical analysis, data collection and interpretation, and manuscript writing. Y.T.C. data collection and manuscript writing. M.L.C. and R.N.C. study design and implementation, manuscript drafting, and critical revision of the manuscript for important intellectual content. All authors approved the final version of the article, including the authorship list.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, CY., Cheng, YT., Chang, ML. et al. The extrahepatic events of Asian patients with primary biliary cholangitis: A 30-year cohort study. Sci Rep 9, 7577 (2019). https://doi.org/10.1038/s41598-019-44081-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44081-x
This article is cited by
-
CT and MR imaging of primary biliary cholangitis: a pictorial review
Insights into Imaging (2023)
-
Acute coronary syndrome after liver transplantation in a young primary biliary cholangitis recipient with dyslipidemia: a case report
Surgical Case Reports (2022)
-
APASL clinical practice guidance: the diagnosis and management of patients with primary biliary cholangitis
Hepatology International (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.