Abstract
Tea is popular health beverage consumed by millions of people worldwide. Drought is among the acute abiotic stress severely affecting tea cultivation, globally. In current study, transcriptome sequencing of four diverse tea genotypes with inherent contrasting genetic response to drought (tolerant & sensitive) generated more than 140 million reads. De novo and reference-based assembly and functional annotation of 67,093 transcripts with multifarious public protein databases yielded 54,484 (78.2%) transcripts with significant enrichment of GO and KEGG drought responsive pathways in tolerant genotypes. Comparative DGE and qRT analysis revealed key role of ABA dependent & independent pathways, potassium & ABC membrane transporters (AtABCG22, AtABCG11, AtABCC5 & AtABCC4) and antioxidant defence system against oxidative stress in tolerant genotypes, while seems to be failed in sensitive genotypes. Additionally, highly expressed UPL3HECT E3 ligases and RING E3 ligases possibly enhance drought tolerance by actively regulating functional modification of stress related genes. Further, ascertainment of, 80803 high quality putative SNPs with functional validation of key non-synonymous SNPs suggested their implications for developing high-throughput genotyping platform in tea. Futuristically, functionally relevant genomic resources can be potentially utilized for gene discovery, genetic engineering and marker-assisted genetic improvement for better yield and quality in tea under drought conditions.
Similar content being viewed by others
Introduction
Drought is an environmental condition which negatively affects growth, yield and considered as primary cause of crop loss worldwide1. Countries like India are facing consecutive drought conditions since last decades and causing significant losses of farm economy and worsening the food security. Tea [Camellia sinensis (L.) O. Kuntze], a perennial evergreen woody plant belongs to family Theaceae is highly cross pollinated and heterozygous plantation crop2. It is consumed by millions of people worldwide due to its numerous health benefits, wherein, India contribute significantly by being the second largest producer of tea in the world3. Nevertheless, tea cultivation is significantly constrained due to the impact of climate change and prolonged rainless periods leading to drought conditions, and reducing tea production by 14–33% with mortality of up to 6–19%4. Therefore, tremendous focus of breeders is now on identification and development of high yielding quality tea varieties better adapted to drought conditions5. In general, plants are adapted to overcome drought stress via avoidance, escape, tolerance and recovery, vis-à-vis involvement of several regulatory and functional genes, metabolic and photosynthesis-associated pathways6,7.
Drought-induced transitions have been studied earlier at biochemical, physiological and molecular level8,9,10,11,12. Biochemical and physiological parameters revealed high water status and photosynthesis rate are key factors involved in osmotic adjustments in tolerant genotypes8. Furthermore, transcriptomic studies indicated the putative role of ABA, ethylene and jasmonic acid biosynthesis and signaling, protein kinases and TFs in drought tolerance and recovery9,10,11,12. Nonetheless, being a multi-genic complex trait, wide range of additional genes are expected to be involved in drought tolerance, wherein, recently reported draft genome sequence information of tea (CSA) might provide better opportunities to elucidate drought stress mechanisms in tea13. Considering enormous gene pool with abundance of vigorous and high level of genetic diversity, traditional tea cultivars in India exhibits inherent genetic variations among wide range of desirable traits (quality & yield) including tolerance to drought stress14. However, due to out-breeding and long gestation periods, tea require next generation breeding strategies to improve drought tolerance via deeper understanding of key regulators and their variants for precision introgressions to have better yield and quality under drought conditions. Therefore, efforts are needed to elucidate global transcriptomic dynamics of multiple tea genotypes having contrasting response to drought stress (tolerant & sensitive) to critically discern key molecular players, which were partly discussed in previous studies in tea.
Current study deals with comprehensive transcriptome sequencing of four tea genotypes exhibiting tolerant (TV 17&TRI2024) and sensitive (TV 03&C 6017) response to drought stress. de novo analysis and functional annotations with several databases identified array of genes, regulators and pathways involved in drought stress. In addition to previous study, enrichment analysis of DEGs elucidated the role of novel regulators and genes involved in ABA biosynthesis and transport, antioxidant enzymes, secondary metabolites against oxidative stresses, membrane transporters, ions & osmolytes providing adaptation/tolerance under drought stress in tolerant genotypes. Furthermore, ubiquitin-based regulation of stress hormone signaling and non-proteolytic function during drought stress commencing drought tolerance is also discussed. Additionally, ascertainment and polymorphic potential evaluation of trait specific high quality single nucleotide variations suggest their futuristic implications to expedite the molecular breeding efforts for combining drought tolerance in high yielding quality genotypes of tea.
Results
Transcriptome sequencing and assembly
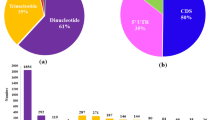
To gain deeper understanding of global gene(s) dynamics, comprehensive transcriptome sequencing of four traditional tea genotypes, two each of exhibiting inherent tolerant [TV 17 (DT_G1) & TRI 2024 (DT_G2)] & sensitive [TV 03 (DS_G1) & C 6017 (DS_G2)] genetic response to drought stress were sequenced using Illumina Genome Analyser-IIx (Fig. 1). Quality filtering of 140.2 million raw reads with NGS QC Tool kit generated 123.6 million high quality reads. de novo assembly using Trinity yielded 67,093 non-redundant (NR) transcripts with average length of 1,086 bp and N50 of 1501 bp. Further, CD hit clustering of transcripts with 90% sequence similarity retrieved 54,508 NR transcripts (Supplementary Table S1). Reference-based mapping of high quality reads to CSA genome13 detected overall mapping rate of 74.45% to 1,73,311 contigs of draft genome. The raw reads of tea samples were submitted to National Centre for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the bioproject PRJNA450985 and PRJNA520786.
Functional annotation and classification
Sequence alignments of 67,093NR transcripts with multifarious public protein databases annotated 55,367 (82.5%) transcripts with NCBI’s Nr (52,484; 78.20%), TAIR (47,962; 71.40%), GO annotations (45,080; 66.3%), Swiss-Prot (41,475; 61.08%), TF database (27,204; 40.54%) and KEGG database (13,454; 20.05%). Meanwhile, transcripts in GO annotation were categorised into Cellular Component (28%), Biological Processes (50%) and Molecular Functions (22%) (Supplementary Fig. S1a). Comparison to KEGG database revealed Enzymes (ko: 01000; 1631 genes), Peptidases (ko: 01002; 120 genes) from metabolism category, Chromosome (ko: 030362; 76 genes), Membrane trafficking (ko: 04131; 239 genes), Spliceosome (ko: 03041; 197 genes) and Ubiquitin system (ko: 04121; 196 genes) from genetic information processing category, and Exosome (ko: 04147; 168 genes) and GTP-binding proteins (ko: 04031; 24 genes) from signaling and cellular processes were among the significantly represented pathways (Supplementary Fig. S1b). Considering, key regulators of plant response against drought stress, 27,204 transcripts encoding 58 TF families with significant abundance of bHLH (2802), NAC (1919), MYB-related (1840), ERF (1581), C2H2 (1289), WRKY (1187), C3H (1137), B3 (1086), FAR1 (995), MYB (958) and bZIP (932) were successfully annotated9 (Supplementary Fig. S1c; Table S3).
Differential gene expression (DEGs) analysis
High quality reads were mapped to de novo and reference-genome assembled transcripts to quantify drought responsive differential gene expressions (DEGs)13. De novo DGEs analysis using control for each treatment in tolerant and sensitive genotypes identified 7473 (DT_G1_C vs DT_G1_T), 4876 (DT_G2_C vs DT_G2_T), 5392 (DS_G1_C vs DS_G1_T), 3220 (DS_G2_C vs DS_G2_T) differentially expressed transcripts. Further, comparison of DEGs in tolerant and sensitive genotypes resulted into 6750 (DS_G1_T vs DT_G1_T), 6997 (DS_G2_T vs DT_G1_T), 6637 (DT_G2_T vs DS_G1_T) and 6766 (DS_G2_T vs DT_G2_T) transcripts. Overall, 3361 and 2250 transcripts were commonly up-regulated in tolerant and sensitive genotypes, respectively. Of these, uniquely up-regulated transcripts were 1849 (DT_G1_T), 1563 (DT_G2_T), 2186 (DS_G1_T) and 2313 (DS_G2_T) (Supplementary Table S4). Whereas, reference genome-based pair-wise comparative DGE analysis resulted in 2056 (DS_G1_T vs DT_G1_T), 3634 (DS_G2_T vs DT_G1_T), 1807 (DT_G2_T vs DS_G1_T), 3267 (DS_G2_T vs DT_G2_T) differentially expressed transcripts (Supplementary Table S5). The significantly less DEGs in reference based analysis possibly due to incomplete & incorrect annotations and exon level difference in expression as also reported in Arabidopsis15. Therefore, DEGs obtained in de novo assembly were utilized in downstream analysis. Expression based clustering to identify key transcripts with similar drought response expression pattern resulted into 5 Sub-clusters (Sub-cluster I: 74 transcripts, Sub-cluster II: 152 transcripts, Sub-cluster III: 87 transcripts, Sub-cluster IV: 74 transcripts and Sub-cluster V: 119 transcripts). Among these, sub-cluster II and III were highly up-regulated in tolerant genotypes, wherein, transcripts encoding E3 ubiquitin ligase involved in plant growth via ubiquitination dependant regulation of protein stability were abundant, significantly. Additionally, up-regulated transcription factors (NAC domain-containing protein 100, bZIP11 and WRKY4), ABA transporters (Protein NRT1/PTR family), Glutathione S-Transferase, Chaperone Protein dnaJ, Laccase-15, LEA protein Dc3 and other stress responsive genes possibly promotes drought tolerance via ABA mediated signalling, ROS scavenging and maintaining proteins functional conformation in tolerant genotypes16,17. Sub-cluster I represents up-regulated transcripts encoding Thaumatin like proteins, Chla-b binding protein, Histone proteins, Phospholipases D beta and Magnesium-chelatase subunit ChlH in DT_G2. Nevertheless, presence of up-regulated expression of few drought responsive genes (14-3-3-like proteins, senescence/dehydration-associated proteins) in sub-cluster IV and V possibly improves stress tolerance under minor drought stress in sensitive genotypes (Supplementary Fig. S3b).
GO and KEGG enrichment analysis
GO and KEGG enrichment analysis was carried out to critically discern the drought responsive key pathways enriched in tolerant and sensitive genotypes. Among the enriched cellular processes, CUL 4 RING ubiquitin ligase complex (GO: 0009911) and mitochondrial inner membrane (GO: 0005743) were enriched in tolerant and sensitive genotypes, respectively. Nevertheless, intracellular organelle (GO: 0043229) and chloroplast thylakoid membrane (GO: 0009535) were enriched irrespective to tolerant and sensitive genotypes. In biological processes, developmental processes (GO: 0032502), response to stimulus (GO: 0009507, GO: 0048583), regulation of ABA mediated signaling pathway (GO: 0005575) and response to salt (GO: 0003674) were highly enriched in tolerant genotypes. Surprisingly, cellular metabolic processes (GO: 00044237) and cellular amino acid biosynthetic processes (GO: 0009733) were only enriched in drought tolerant quality genotype (DT_G1_T). Moreover, ATP binding (GO: 0005524), calcium-transporting ATPase (GO: 0005388) via hydrolase activity (GO: 0004091), ubiquitin-protein ligase activity (GO: 0016567) via ligase activity (GO: 0019786), protein kinase activity (GO: 0016740) and oxido-reductase activity (GO: 000018) were highly enriched molecular functions in case of tolerant genotypes. Whereas, cation trans-membrane transporter activity (GO: 0209) and structural constituent of ribosome (GO: 0003735) were the prominently enriched molecular functions in sensitive genotypes (Supplementary Figs S4–S7). Furthermore, tree map representation of KEGG pathways revealed maximal mapping of transcripts in genetic information processing (2328 transcripts), followed by metabolism (2188 transcripts) and signaling & cellular processes (435 transcripts; Supplementary Fig. S1b). Additionally, higher enrichment of flavonoid pathway, amino acid bio-synthesis and porphyrin & chlorophyll metabolism in tolerant genotypes suggests their key relevance to integration of drought stress and quality related traits in tea (Supplementary Fig. S8–S10).
Dynamics of key pathways involved in drought tolerance
Comprehensive transcriptome analysis of tolerant and sensitive genotypes indicated the role of key pathways such as ABA-dependent and independent pathway, metabolic pathway & antioxidant defense enzymes, membrane transporters and ubiquitinization are crucial for drought tolerance in tea (Fig. 2).
ABA-dependent and independent pathway
ABA regulates various abiotic stress responses in plants, therefore genes involved in ABA metabolism and their specific transporters, receptors and signalling intermediates were scrutinized during the study. Generally, ABA biosynthesis starts in plastids via MEP pathway under regulatory control of Zeaxanthin epoxidase (ZEP) and 9-cis-epoxycarotenoid dioxygenase (NCED 5) along with Abscisic acid 8′-hydroxylase 2 (CYP707A2) involved in oxidative catabolism of ABA, interestingly these regulatory elements were up-regulated in tolerant genotypes. Furthermore, import of synthesized ABA to targeted organs for its perception via receptor complex to initiate downstream signalling cascade are crucial for activation of drought responsive genes. Up-regulated expression of ABA importers [NRT1/PTR FAMILY (NPF1.2)], ABA transporter (low affinity nitrate transporter) and ABA receptor [GPCR-type G protein 2 (plasma membrane perception) and PYL9 (intracellular perception)] in tolerant genotypes, suggests their precise role during drought stress tolerance18. Moreover, ABA-activated protein kinases and phosphatases, having integral involvement in ABA-receptor complex [SNF1-related protein kinase (KING1), Mitogen-activated protein kinase 3 (MPK3), Mitogen-activated protein kinase kinase kinase 1& 7 (MEKKK 1 & 7) and Protein phosphatase 2 C (PP2C)] were also up-regulated in tolerant genotypes. This receptor complex downstream activates ABA-dependent transcription factors such as, bZIP, ABSCISIC ACID-INSENSITIVE 5 (ABF2), Homeobox-leucine zipper protein (ATHB21), bHLH and WRKY TFs regulating drought stress-responsive genes, were also up-regulated in tolerant genotypes. Alternatively, regulation of various drought stress inducible genes in ABA independent manner including dehydration-responsive element-binding protein (DREB1A, 2 A, 2 C & 2D), NAC80, 100 and B3 domain-containing protein were also highly up-regulated in tolerant genotypes. Additionally, up-regulated expression of few histone modifying enzymes (HD1 and HDA15) in tolerant genotypes also indicate their epigenetic impact on stress-responsive genes (Fig. 3; Supplementary Table S6b).
Metabolic pathways and antioxidant defence
Most of genes involved in primary (Glycolysis, Citrate cycle, Pentose Phosphate Pathway, Calvin Cycle and Photosynthesis) and secondary (Flavonoid, Theanine and Caffeine) metabolic pathways were up-regulated in tolerant genotypes (Fig. 4a; Supplementary Table S6b). Further, expression analysis revealed that genes involved in flavonoid pathway (PAL2, C4H, 4CLL2, CSH, CHS2, CFI, LAR, ANR, Anthocyanidin reductase and Isoflavone reductase); theanine biosynthetic pathway (GLT, GS-NADH, GDH and ADC2) and caffeine biosynthesis pathway (AMPDA and SAM1) were also found up-regulated in tolerant genotypes (Fig. 4b). Additionally, higher KEGG enrichment of flavonoid, amino acid biosynthesis and porphyrin & chloroplast metabolism in tolerant genotypes also complements the expression data (Supplementary Fig. S8–S10; Table S6).
Schematic representation of Primary, Secondary and Antioxidant defence signalling during drought response in tea. (a) Primary metabolic pathways (Photosynthesis, Glycolysis and TCA cycle); (b) Secondary metabolic pathways (Flavonoid, Theanine and Caffeine biosynthetic pathway); (c) ROS detoxification. Boxes in green represent transcripts up-regulated in tolerant while red represents transcripts up-regulated in sensitive genotypes. (PAL: Phenylalanine ammonia lyase, C4H: Cinnamate 4-hydroxylase, 4CL: 4-coumarate CoA ligase, CHS: Chalcone synthase, CHI: Chalcone isomerase, F3′H: Flavonoid 3′-hydroxylase, FNS: Flavone synthase II, F3′5′H: Flavonoid 3′,5′-hydroxylase, F3H: Flavanone 3-hydroxylase, FLS: Flavonol synthase, DFR: Dihydroxyflavonol 4-reductase, LCR: Leucoanthocyanidin reductase, ANS: Anthocyanidin synthase, ANR: Anthocyanidin reductase, GS: Glutamine synthetase, GOGAT: Glutamate synthase, GDH: Glutamate dehydrogenase, ADC: Arginine decarboxylase, GMPS: GMP Synthase, RBK: Ribokinase, ASL: Adenylosuccinatelyase, AMPDA: AMP deaminase, SAMS: S adenosylmethionine synthase).
Prolonged and severe drought conditions undoubtedly lead to oxidative stress which affects the primary metabolic activity of the cell. Up-regulated expression of superoxide dismutase irrespective to tolerant and sensitive genotypes suggest its involvement to maintain first line of defence in all genotypes, while downstream H2O2 scavenging enzymes, namely Glutathione S transferase, Catalase and Ascorbate peroxidase only expressed in tolerant genotypes were possibly involved to cope-up with drought stress in tolerant genotypes (Fig. 4c).
Membrane transport dynamics during stress adaptation
Despite important role in stress adaptation, plant membrane transport system was least discussed in tea. During this study, efforts were made to critically understand the role of various transporter families such as ABC, Ion transporters and Aquaporin channels involved in osmotic adjustments during drought stress (Supplementary Table S6b).
ABC Transporters: Expression data revealed active involvement of ABC protein superfamily during drought stress in tolerant and sensitive genotypes. Among these, members of ABCG subfamily namely AtABCG 1 (ABC transporter G family member 1, 7, 11, 15, 22 & 36) reportedly involved in stomatal function and ABCC subfamily (AtABC C4, 5, 9, 10 & 13) participates in the guard cell physiology/stomatal regulation and root development, exhibited higher expression in tolerant genotypes (Fig. 5a). Moreover, ABCB transporter proteins (AtABCB 1, 11, 19, 20, 28 & 29) involved in auxin mediated development and transport; and few additional transporters with unknown functions such as ABC Transporter I (AtABCI 17, 19 & 20), ABC Transporter D (AtABCD 1 & AtABCD 2), ABC Transporter A (ABCA 2 & ABCA 7) and ABC Transporter F (AtABCF 1 & AtABCF 4) were also highly up-regulated in tolerant genotypes.
Ion Transporters: Ion transporters not only help to maintain sustainable plant growth and productivity but also important for immediate drought response via rapid and fast adjustment of stomatal aperture to minimize transpirational water loss. Transcripts encoding potassium transporters (POT 1, 2 and HAK 25) and Zinc transporters (ZIP 5 and ZIP 6) were expressed in tolerant genotypes. Contrarily, higher expression of nitrate transporter (NAR2.1) reportedly involved in stomatal opening probably causing excess water loss in sensitive genotypes (Fig. 5b).
Amino acid transporters: Amino acid transporters provide drought tolerance in plants via osmotic adjustments to protect cells from dehydration by increasing cellular osmolarity19. Higher expression of Lysine histidine transporters (LHT1 and AATL1) in tolerant genotypes further support their involvement in controlling osmolarity to acquire tolerance during drought stress.
Aquaporins: Aquaporins are membrane channel proteins having role in plant growth, development and defense against drought stress20. Expression of transcripts encoding three major subfamilies viz; Plasma Membrane Intrinsic Proteins (PIPs) and Tonoplast Intrinsic Proteins (TIPs) of aquaporins revealed significant differential expression among tolerant and sensitive genotypes. Of the two PIP subgroups (PIP1, PIP2; isoform with unique localization and functions), PIP1-2, PIP2-5, PIP2-7 was up-regulated in tolerant genotypes, and among TIPS, TIP1-1, TIP2-1, TIP3-1 and TIP1-3 participates in water exchange were also up-regulated in tolerant genotypes.
DGEs of Ubiquitination complex during drought response
Key mechanism underlying functional modification during drought stress is still obscure in tea. Ubiquitination, a protein modification mechanism involved in cellular signalling begins with active participation of three important enzymes to facilitate ubiquitin-mediated degradation of target proteins, different stress responsive TFs and hormonal receptors. Among these, E1 Ubiquitin-activating and E2 Ubiquitin-conjugating enzymes (UBC 2, 5, 7, 16, 24, 25, 27 and 28) were expressed irrespective to tolerant and sensitive genotypes, while, E3s Ubiquitin ligase mainly UPL3 HECT E3 ligases, RING-E3 ligases and CHIP U-box E3 ligase involved in leaf senescence, ABA stress related signalling and protein turnover metabolism, respectively were up-regulated in tolerant genotype. Additionally, Cullin-RING ligases (CRLs) and Skp1-cullin-F-box (SCF) involved in plant hormone assisted drought stress adaptation were up-regulated irrespective to all genotypes, whereas, BTB (bric-a-brac-tramtrack-broad) complex, DNA damage-binding (DDB) and Anaphase-promoting complex (APC) were up-regulated only in tolerant genotypes. Furthermore, ubiquitin like modifiers (UBLs) such as, NEDD8, SUMO-UBC9, SUMO-SIZ1 and UFL1 was up-regulated in tolerant, however MMS1 SUMO conjugating enzyme was more active in sensitive genotypes.
Expression of TFs in response to drought stress
Among the ascertained transcription factor (TF) families, transcripts encoding bHLH, NAC, WRKY, B3, MYB and bZIP were upregulated more in tolerant genotypes, while, C2H2 domain up-regulated in sensitive genotypes. Among these, high expression of bHLH 13, 48 and NAC 2, 78 possibly providing drought tolerance via activating ABA signalling in tolerant genotypes21. Moreover, tolerant genotype specific up-regulated expression MYB family (Myb39, 44) and WRKY (WRKY 4, 19, 24, 33 & 75) might improve drought tolerance via stomata closureby increasing osmotic stress response. Additionally, higher expression of bZIP 19, 23 in tolerant genotypes was reported to be crucial in abiotic stress signalling17.
Real Time quantitative PCR expression analysis
To validate key drought responsive candidates, quantitative real time (qRT-PCR) expression pattern of random eight genes representing major pathways (ABA-dependent and independent pathway and Ubiquitination) were corresponded with RNA seq expression data (Fig. 6; Supplementary Table S7).
Discovery and validation of drought responsive putative single nucleotide polymorphism
Single nucleotide polymorphism (SNP) ascertained from trait specific transcriptome analysis have been successfully utilized for high-throughput genome mapping and discovery studies22. Using stringent pipeline, 80,803 high quality putative SNPs localized in CDS were predicted in 28,096 transcripts by mapping high quality filtered RNA-Seq reads to de novo and reference genome of tea. Of these, 28045 SNPs were non-synonymous, while 39679 SNPs categorized as synonymous. Among the non-synonymous SNPs, transversions (A/C: 1060, G/T: 32107, C/G: 1851, T/A: 1326) were more abundant than transitions (A/G: 7395 and C/T: 3224). Interestingly, SNPs ascertained in major drought specific pathways varied from 40 SNPs identified in ABA dependent and independent to 79 SNPs in primary and secondary metabolism, while 61 SNPs and 76 SNPs identified in transporters and ubiquitination encoding transcripts, respectively (Supplementary Fig. S11; Table S8). GO enrichment analyses of SNPs containing transcripts revealed enrichment of several drought responsive GO categories in contrasting genotypes. Among the GO enrichment in tolerant genotypes, biological processes category; regulation of biological processes (GO: 0050789), primary metabolic processes (GO: 0044238), developmental processes (GO: 0032502), response to stimulus (GO: 0050896) and response to abiotic stimulus (GO: 0009628) were highly enriched, while, metabolic processes (GO: 0008152) was the only category moderately enriched in sensitive genotypes. Interestingly, molecular function category, protein binding (GO: 0005517) were only enriched in tolerant genotypes, whereas, cell (GO: 00005623) and cell parts (GO: 0044464) of cellular components were found to be enriched irrespective to tolerant and sensitive genotypes (Supplementary Fig. S12–S14).
Allele specific functional validation of drought responsive SNPs
In total, 37 non-synonymous SNPs ascertained in key drought responsive pathway genes and localized to CDS region were utilized for amplification validation in 24 tea genotypes (Supplementary Table S9). Of these, 26 putative SNPs were successfully validated using allele specific PCR with four primer combinations wherein two allele specific inner primers were used to discriminate the single nucleotide change between different genotypes (Supplementary Fig. S15). Considering, derivation of validated SNPs in the transcripts encoding drought stress responsive genes such as, RAP2–4, PP2C, RAP2–12, ERF 118, WRKY 118, 4, NAC 17, NPF2.11, NPF 5.2, RAP2–3, ERF 18, MKK9 (ABA dependent and Independent pathway), Glutathione-S transferase (Antioxidant defence), ABCG1, 7, ABCB 9, ABCF 1 (Transporters), F3H, ASS, CS2, ACO, SDHAF4, IDH and RBCL (Primary & Secondary metabolism) and XERICO (Ubiquitination), might be having functional and adaptive significance against drought stress.
Discussion
Transcriptome analysis
Being polygenic complex trait, achieving genetic gain with single/few gene(s) under drought stress is difficult. Therefore, it is crucial to comprehensively understand the genetic control of drought tolerance in plants. In current study, high throughput transcriptome sequencing of multiple traditional genotypes with contrasting inherent genetic response to drought stress was performed to provide better perspective of the molecular machinery operating in drought stress in tea23. Combined approach using both reference-based and de novo transcriptome analysis provides better resolution of key candidates underlying in drought stress associated metabolic network24. Overall, high transcriptome read mapping (74.45%) obtained with CSA genome suggests high quality assembly of short reads in this study. Nevertheless, metrics used in genome assemblies are not suitable for transcriptome assembly due to abundance of chimerism in longer sequences, hence de novo assembly is preferred in global transcriptional analysis15. Therefore, considering multiple advantages including better opportunity of abundance of novel candidates, TRINITY assisted denovo assembly was utilized for downstream dissection of drought tolerance in the current study23,25.
Approximately 123 million high quality reads (67,093 transcripts with an average length: 1086 bp; N50: 1501 bp) were obtained here are comparable to the earlier studies in tea and other complex crops10,26. Overall functional annotation of 78.2% transcripts suggests that optimal annotation of the transcriptome data. Moreover, higher GO enrichment of important relevant biological processes; response to stimulus and regulation of abscisic acid mediated signaling pathway indicates activation of drought tolerance responsive genes in tolerant genotypes. Although, overall DEGs was found higher in sensitive as compare to tolerant genotypes, yet, key transcripts involved in drought tolerance were highly up-regulated in tolerant genotypes27,28,29.
Signal transduction via ABA-dependent and Independent Pathways
ABA plays critical role in drought stress and seems to work efficiently in tolerant genotypes. Drought stress signal is sensed by membrane receptors which downstream activate signalling cascade to generate secondary signal molecules viz., Ca2+, ROS and ABA. Higher expression of ABA biosynthesis and catabolism enzymes in tolerant genotypes indicates the fine tuning of synthesis and degradation of ABA during drought stress in tolerant genotypes30. Furthermore, both synthesis and transport of ABA to site of action is crucial for stress response and can be correlated extensively with stomatal closing. Up-regulation of NRT1/PTR FAMILY members possibly stipulate their role in stomatal closure under drought stress to minimize water loss in tolerant genotypes16. While, perception of ABA to regulate downstream expression of various ABA-responsive genes is important to promote drought stress tolerance. Higher expression of PYL9 receptor in tolerant genotypes provides putative evidences that these receptors in the presence of ABA following ABA core signalling to form receptor-complex. This complex possibly trigger phosphorylation and activation of SNF1-related protein kinases to phosphorylate bZIP, ABF2, MYB and WRKY transcription factors by regulating ABRE-dependent signalling cascade to improve drought stress in tolerant genotypes. Incase of ABA-independent pathway, dehydration induced DREB proteins and perhaps mediate transcriptional regulation of osmotic stress responsive genes. Higher expression of DREB2A subfamily in tolerant genotypes reveals that DREB subfamily could be one of the putative key candidates, which can be futuristically explored to enhance drought tolerance in tea18.
Detoxifying enzymes and secondary metabolites against oxidative stress
Drought stress enhances production of different oxidative free radicals (O2−, OH− and H2O2) consequently causes damage to photosynthetic machinery of cell. Antioxidant defence system is an adaptative strategy, wherein, superoxide dismutase causes dis-mutation of O2۰−radical to H2O2 and O2 which is further scavenged to water and oxygen by downstream enzymes viz., Catalase and Glutathione peroxidase. Higher expression of SOD in both tolerant and sensitive genotypes; and lower expression of downstream antioxidant defence enzymes in sensitive genotypes reveals that first line of defence is working efficiently irrespective to tolerant and sensitive genotypes however, downstream H2O2 scavenging enzymes could be the key factor for failure of drought tolerance in sensitive genotypes. Hence, up-regulated expression of SOD with active H2O2 scavenging enzymes may be a putative mechanism to handle oxidative stress during drought in tea31. Moreover, stress-induced higher expression of non-enzymatic antioxidants like flavonoids in tolerant genotypes suggests that active metabolites possibly involved to cope-up with oxidative damage and perhaps helped for maintaining tea quality during drought stress4.
Role of transporters in stress adaptation in tea
ABC transporter proteins, the largest known transporters of ABA and auxin involved in stomatal functioning were highly up-regulated in tolerant genotypes during drought stress. Higher expression of lipid transporter, AtABCG 11 indicates that cuticle covering in tolerant genotypes might protects plant from dehydration via controlling non-stomatal water loss32. Similarly, higher expression AtABCG 22 probably involved in stomatal closure via enhanced influx of ABA into guard cells of tolerant genotypes. Additionally, up-regulated transporter subfamily AtABCC 5 and AtABCC 4 suggests that stomata closure due to loss of turgor in guard cells via Cl− and K− ion efflux along with ABA accumulation in tolerant genotypes, but not in sensitive genotype hence may also be key molecular players involved in drought adaptation.
Controlled regulation of potassium transporters via ABA and auxin is key factor during osmotic adjustments. Higher expression of these genes reflects activation of potassium ion transport system leading to potassium homeostasis in guard cells and regulating stomatal aperture in tolerant genotypes33. Accumulation of copper and zinc ions in stressed plants via COPT 5, ZIP 5 and ZIP 6 transporters possibly involved in free radical removal and signalling in tolerant genotypes34,35. Interestingly, up-regulation of nitrate transporter (NAR2.1) in sensitive genotypes suggests accumulation of nitrate in guard cells inducing depolarization enhances stomatal opening, causing drought sensitivity36. Higher expression of LHT transporters and aquaporins stipulates water scarcity in tolerant genotypes is possibly managed via increasing cellular osmolarity during drought stress19,20.
Functional modification in response to drought stress
Ubiquitination, key mechanism necessary for various cellular signalling triggers drought stress response. Higher expression of UPL3 HECT E3 ligase might delayed leaf senescence via degradation of WRKY53 gene during drought tolerance37. Further, up-regulated expression of various RING E3 ligases such RGLG2, XERICO and SDIR1 possibly stipulate drought stress adaptation via controlling ABA signalling in tea38,39. Additionally, up-regulated expression of RMA1H1 RING E3 ligases regulates aquaporin trafficking to plasma membrane in tolerant genotypes possibly suggests ubiquitin mediated regulation of aquaporin level during drought stress40. Nevertheless, up-regulated expression of PUB23 (negative regulator of drought stress) might resulted in dehydration and failing drought tolerance in sensitive genotypes41. Higher expression of CRLs (BTB, DDB and APC) perhaps anticipates strong hormonal responses in tolerant genotypes. Further, drought response protein degradation caused due over-expression of KEG E3 ligases is perhaps maintained by up-regulated expression of SIZ1 via sumoylation in tolerant genotypes42. Thus, expression data reveals that ubiquitination system work effectively via complex regulatory hormone responses and downstream signal transduction cascade in tolerant genotypes of tea.
SNPs in drought stress responsive genes
To increase the resolution of genetic changes involved during drought tolerance, high throughput mining of candidate SNPs in transcriptome data and their utilization for genotyping and genome-wide association studies have been appropriately utilized in trait dissection43. Moreover, SNP ascertained from transcriptomic data had a higher success rate than the whole genome resequencing data44. Ascertainment of 80803 high quality putative SNPs from multiple genotypes with significant abundance in key drought responsive pathways [ABA dependent and independent (40 SNPs), Antioxidant defense (79 SNPs), transporters (61 SNPs) and ubiquitination (76 SNPs)] suggests higher success rate in marker validation and their implications in drought dissection in tea22. Furthermore, unique abundance of non-synonymous functional SNPs (CDS region) can affect the thermostability and activity of gene in contrasting genotypes (tolerant: 1178 SNPs and Sensitive: 334 SNPs) and experimental validation of 37 trait-specific SNPs suggests potential implications of ascertained SNPs in QTLs analysis of drought tolerance in tea22.
Conclusion
Tea quality and yield have been significantly constraint due to negative impact of drought stress. In the current study, global transcriptome analysis using multiple diverse genotypes with inherent contrasting response to drought (Tolerant & Sensitive) followed by de novo and reference guided genome assembly and annotation with multiple public databases have comprehensively enriched the drought responsive genomic resource in tea. Comparative transcriptional analysis successfully identified novel regulatory and putative functional candidates providing drought tolerance in tea via ABA dependent & independent stress signalling, membrane transporters (AtABCG22, AtABCG11, AtABCC5 & AtABCC4) and aquaporins (Figs 3, 5). Moreover, antioxidant defence enzymes against oxidative stress and post-translational modification of various drought responsive genes via ubiquitination and sumoylation can be additional potential candidates for enhancing drought tolerance in tea (Fig. 4c). Additionally, abundant genomic resources comprising potential key candidates and trait-specific putative SNPs can be futuristically utilized for combining drought tolerance in high yielding quality tea genotypes using genetic engineering and genome mapping studies.
Materials and Methods
Plant material and treatment
Four traditional genotypes of tea [C. Sinensis (L) O. Kuntze] exhibiting inherent tolerant [TV 17 (DT_G1), TRI 2024 (DT_G2)] and sensitive [TV 03 (DS_G1) and C 6017 (DS_G2)] response to drought stress used in this study were maintained at CSIR- Institute of Himalayan Bio-resource Technology Palampur, Himachal Pradesh (1,300 m Altitude, 32° 06′N, 76° 33′E). Shoot cuttings of tolerant and sensitive plants were collected during actively growing period (April, 2017; IST 10–11am) in de-ionised water and incubated in plant growth chamber at 25 ± 1 °C for 24 hrs. Thereafter, cuttings of tolerant and sensitive genotypes were transferred to half-strength Hoagland’s nutrient medium containing 10% polyethylene glycol 8000 (PEG 8000, Formally 6000, USB) for a period of 48 hours to induce uniform drought stress in relatively controlled manner alongwith control samples for each genotypes45. Four leaves and a bud tissues from all four genotypes was harvested after 48 h, immediately frozen in liquid nitrogen and stored at (−) 80 °C for RNA isolation.
RNA isolation, cDNA library preparation and sequencing
Total RNA isolation was carried out using iRIS protocol followed by quantity and quality determination using Nanodrop 2000 (Thermo Scientific Lithuania) and Agilent Bio-analyzer Chip RNA 7500 series II (Agilent Technologies USA)46. Equimolar concentration of total RNA from three technical replicates was pooled prior to cDNA library preparation for minimising biological biasness. 4 µg of RNA from each sample was used for construction of cDNA library using illumine TruSeq RNA Sample Prep Kit v2 LS (Illumina Inc., San Diego, CA). Prepared libraries were quantified using Bio-analyser Chip DNA 1000 Series II (Agilent Technologies USA). Diluted libraries of 10 pM concentration were loaded on to the flow cell for paired end (PE) sequencing of (2 × 72 bp) using Illumina Genome Analyser-IIx (Illumina, San Diego, CA).
De novo assembly and functional annotation
Base calling and de-multiplexing of raw data was performed using Illumina Casava 1.8.2 pipeline (http://support.illumina.com/) followed by Paired-End quality filtering using NGS QC Toolkit47. Trinity Software package ver2.3.2 was utilized for de novo transcriptome assembly with minimum cut-off length of 300 bp, further CD hit clustering tool was used to cluster the transcripts with 90% sequence similarity48,49. Homology based putative function prediction of assembled transcripts with various publically available databases through BLASTx similarity search was performed viz., NCBI Nr (Non-Redundant), The Arabidopsis Information Resource (TAIR 10), Swiss-Prot, KEGG automatic annotation server (http://www.kegg.jp/kegg/tool/annotate_sequence.html) and Plant Transcription Factor database (http://planttfdb.cbi.pku.edu.cn/) with an e-value of ≤ 1e −5.
Differential expression analysis, GO and KEGG pathway enrichment analysis
For identification of differentially expressed (DE) transcripts among tolerant and sensitive genotypes, sample specific filtered reads were mapped to both de novo assembled transcripts and reference genome using Bowtie2 ver2.2.4 and Tuxedo pipeline respectively50,51. In de novo analysis, transcript abundance was estimated and normalised to FPKM (Fragments per Kilobase of Transcript per Million Mapped reads) using RSEM tool ver1.2.3152. Differential transcripts expression was performed using edgeR package in drought tolerant control vs drought tolerant treated (DT_G1_C vs DT_G1_T and DT_G2_C vs DT_G2_T), drought sensitive control vs drought sensitive treated (DS_G1_C vs DS_G1_T and DS_G2_C vs DS_G2_T), drought tolerant control vs drought sensitive control (DT_G1 vs DS_G1, DT_G1 vs DS_G2, DT_G2 vs DS_G1 and DT_G2 vs DS_G2) and drought tolerant treated vs drought sensitive treated (DT_G1 vs DS_G1, DT_G1 vs DS_G2, DT_G2 vs DS_G1 and DT_G2 vs DS_G2)53. Transcripts with log2 FC >1, p-value ≤ 0.05 were considered for downstream analysis. Further, significant DE transcripts (FC ≥2; p-value < 1e-4) were extracted and clustered based on similar expression pattern. Moreover, reference based DE analysis was performed using TopHat ver2.1.0 to extract significantly DE transcripts with P-value < 0.05. Heat map-based representation of DE transcripts of various pathways was developed using MeV package v.4.9.0 (Multiple Experiment Viewer). Statistically significant DE transcripts were subjected to GO and KEGG enrichment analysis via agriGO tool and KEGG pathway database respectively54,55. Singular enrichment analysis (SEA) was performed to extract the GO enrichment of differentially expressed transcripts using AgriGO toolkit with fisher’s statistical test (Hochberg-FDR adjustment, Cut off <0.01) for optimal gene enrichment54.
Quantitative Real Time PCR (qRT-PCR) expression analysis
Gene expression inferences obtained in RNA-Seq data were validated using qRT-PCR. DNase I (Thermo Scientific) treatment was given to RNA samples to remove any contamination of DNA. From all four genotypes, 4 µg of RNA were used for cDNA synthesis using cDNA synthesis kit (Thermo Scientific, Revert H Minus) and 10X dilutions were used for qRT-PCR. Gene specific primers were designed using BatchPrimer3 (http://probes.pw.usda.gov/batchprimer3/) (Supplementary Table S7). Differential expression pattern of 8 genes from important pathways induced during drought stress among tolerant and sensitive genotypes were analyzed (StepOnePlus Real-Time PCR system, Applied Bio-system, USA) using 18 s as internal control with three technical replicates to calculate standard error. Equal concentration of cDNA in each reaction was established using internal control and relative expression of genes and fold change was calculated using 2−ΔΔCTprotocol56,57,58,59.
SNPs prediction from de novo and reference genome
Read specific single nucleotide variations were ascertained among all four genotypes utilizing KISSPLICE ver 2.4 with default parameters. BLAT ver. 3.6 was utilized to extract the positioned variants in both de novo and reference genome assembly; and their functional impact were predicted using kISSPLICE2REF TRANSCRIPTOME (K2RT)60.
Tetra Primer ARM PCR for SNPs validation
Tetra primer ARM PCR is fast and inexpensive method for genotyping and also distinguishes homozygote from heterozygote SNPs. In current study, 37 drought stress responsive transcripts containing non-synonymous SNPs were used for allele specific primer designing utilizing online programme PRIMER1: Primer design for tetra-primer ARMS-PCR61 (http://primer1.soton.ac.uk/primer1.html). Two outer primers (FOP and ROP) and two allele specific inner primers (FIP and RIP) were designed along with a mismatch base added to the 3′ end of inner primers and fragment size was kept between 100–450 bp. SNPs were validated in 24 diverse genotypes of tea (including four genotypes used for transcriptome study62 (Supplementary Table S9). Total genomic DNA was isolated using DNeasy Plant Mini Kit (Qiagen, Germany) according to manufacturer’s instructions. Quantity and quality of DNA was determined using NanoDrop 2000 OD260/OD280 (Thermo Scientific, Lithuania) and integrity using 0.8% agarose gel. PCR amplification was performed using 40 ng of genomic DNA and amplified PCR products were separated on 3% metaphore agarose gel, visualised on UV trans-illuminator (BioRad).
References
Fahad, S. et al. Crop Production under Drought and Heat Stress; Plant Responses and Management Options. Front. Plant Sci. 8, 1147 (2017).
Sharma, H. et al. Identification and cross-species transferability of 112 novel unigene-derived microsatellite markers in tea (Camellia sinensis). Am. J. Bot. 6, 133–138 (2011).
Sharma, R. K. et al. AFLP-based genetic diversity assessment of commercially important tea germplasm in India. Biochem. Genet. 48, 549–564 (2010).
Cheruiyot, E. K., Mumera, L. M., Ngetich, W. K., Hassanali, A. & Wachira, F. N. High fertilizer rates increase susceptibility of tea to water stress. J Plant Nutr. 33, 115–129 (2009).
Kamunya, S. M. et al. Genomic mapping and testing for quantitative trait loci in tea (Camellia sinensis (L.) O. Kuntze). Tree Genetics & Genomes. 6, 915–929 (2010).
de Carvalho, MariaHelenaCruz Drought stress and reactive oxygen species; Production, scavenging and signaling. Plant Signaling & Behavior. 3, 156–165 (2008).
Bartels, D. & Sunkar, R. Drought and salt tolerance in plants. Crit Rev Plant Sci. 24(1), 23–58 (2005).
Damayanthi, M. M. N., Mohotti, A. J. & Nissanka, S. P. Comparison of Tolerant Ability of Mature Field Grown Tea (Camellia sinensis L.) Cultivars Exposed to a Drought Stress in Passara Area. Tropical Agricultural Research. 22(1), 66–75 (2010).
Wang, W. et al. Transcriptomic Analysis Reveals the Molecular Mechanisms of Drought-Stress-Induced Decreases in Camellia sinensis Leaf Quality. Front. Plant Sci. 7, 385 (2016).
Dong, B. et al. Transcriptome analysis of the tea oil camellia (Camellia oleifera) reveals candidate drought stress genes. PLoS ONE. 12(7) (2017).
Maritim, T. et al. Transcriptome based identification of water-deficit stress responsive genes in the tea plant. Camellia sinensis. J Plant Biotechnol. 43, 302–310 (2016).
Liu, S. C. et al. Transcriptomic Analysis of Tea Plant Responding to Drought Stress and Recovery. PLoS ONE. 11(1) (2016).
Xia, E. H. et al. The Tea Tree Genome Provides Insights into Tea Flavor and Independent Evolution of Caffeine Biosynthesis. Molecular Plant. 1–12 (2017).
Seth, R. et al. Global Transcriptional Insights of Pollen-Pistil Interactions Commencing Self-Incompatibility and Fertilization in Tea [Camellia sinensis (L.) O. Kuntze]. Int. J. Mol. Sci. 20, 539, https://doi.org/10.3390/ijms20030539 (2019).
Wang, S. & Gribskov, M. Comprehensive evaluation of de novo transcriptome assembly programs and their effects on differential gene expression analysis. Bioinformatics 33(3), 327–333 (2017).
Kanno, Y. et al. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. PNAS. 109, 9653–9658 (2012).
Cheruiyot, E. K., Mumera, L. M., Ngetich, W. K., Hassanali, A. & Wachira, F. Polyphenols as potential indicators for drought tolerance in tea (Camellia sinensis L.). BiosciBiotechnolBiochem. 71(9), 2190–7 (2007).
Fujita, Y. et al. AREB1 Is a Transcription Activator of Novel ABRE-Dependent ABA Signaling That Enhances Drought Stress Tolerance in Arabidopsis. The Plant Cell. 17, 3470–3488 (2005).
Toka, I. et al. Mutations in the Hyperosmotic Stress-Responsive Mitochondrial BASIC AMINO ACID CARRIER2 Enhance Proline Accumulation in Arabidopsis. Plant Physiology. 152, 1851–1862 (2010).
Afzal, Z., Howton, T. C., Sun, Y. & M. Mukhtar, S. The Roles of Aquaporins in Plant Stress Responses. Journal of Development Biology. 4(1), 9 (2016).
Lu, P. L. et al. A novel drought-inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the expression of stress-responsive genes in Arabidopsis. Plant Mol Biol. 63, 289–305 (2006).
Rasheed, A. et al. Crop Breeding Chips and Genotyping Platforms: Progress Challenges and Perspectives. Mol. Plant. 10, 1047–064 (2017).
Garg, R. et al. Transcriptome analyses reveal genotype- and developmental stage specific molecular responses to drought and salinity stresses in chickpea. Sci. Rep. 6 (2016).
Kovi, M. R., Helga, A., Muath, A. & Odd, A. R. de novo and reference transcriptome assembly of transcripts expressed during flowering provide insight into seed setting in tetraploid red clover. Sci. Rep. 7, 44383 (2017).
Jayakodi, M. et al. Transcriptome profiling and comparative analysis of Panax ginseng adventitious roots. J Ginseng Res. 38 (2014).
Singh, G. et al. Molecular dissection of transcriptional reprogramming of steviol glycosides synthesis in leaf tissue during developmental phase transitions in Stevia rebaudiana Bert. Sci, Rep. 7, 1 (2017).
Muthusamy, M., Uma, S., Backiyarani, S., Saraswathi, M. S. & Chandrasekar, A. Transcriptomic Changes of Drought-Tolerant and Sensitive Banana Cultivars Exposed to Drought Stress. Front. Plant Sci. 7 (2016).
Yates, S. A. et al. De novo assembly of red clover transcriptome based on RNA Seq data provides insight into drought response, gene discovery and marker identification. BMC Genomics. 15, 453 (2014).
Fracasso, A., Trindade, L. M. & Amaducci, S. Drought stress tolerance strategies revealed by RNA-Seq in two sorghum genotypes with contrasting WUE. BMC Plant Biol 16, 115 (2016).
Finkelstein, R. Abscisic Acid Synthesis and Response. The Arabidopsis Book., https://doi.org/10.1199/tab.0058 (2013).
McKersie, B. D., Bowley, S. R. & Jones, K. S. Winter survival of transgenic alfalfa over expressing superoxide dismutase. Plant Physiology. 119, 839–848 (1999).
Luo, B., Xue, X. Y., Hu, W. L., Wang, L. J. & Chen, X. Y. Transporter gene of Arabidopsis thaliana, AtWBC11, is involved in cuticle development and prevention of organ fusion. Plant Cell Physiol. 48(12), 1790–1802 (2007).
Osakabe, Y. et al. Osmotic Stress Responses and Plant Growth Controlled by Potassium Transporters in Arabidopsis. The Plant Cell. 25, 609–624 (2013).
Vicente, S. O., SergiPuig, H. M., Dennis, J. T. & Lola, P. Identification of a copper transporter family in Arabidopsis thaliana. Plant Molecular Biology. 51, 577–587 (2003).
Wang, M., Yuan, M., Xu, Q. & Yu, J. The putative Arabidopsis zinc transporter ZTP29 is involved in the response to salt stress. Plant Mol Biol. 73, 467–479 (2010).
Guo, F. Q., Young, J. & Crawford, N. M. The Nitrate Transporter AtNRT1.1 (CHL1) Functions in Stomatal Opening and Contributes to Drought Susceptibility in Arabidopsis. The Plant Cell. 15, 107–117 (2003).
Miao, Y. & Zentgraf, U. A HECT E3 ubiquitin ligase negatively regulates Arabidopsis leaf senescence through degradation of the transcription factor WRKY53. The Plant Journal. 63, 179–188 (2010).
Wu, Q. et al. Ubiquitin Ligases RGLG1 and RGLG5 Regulate Abscisic Acid Signaling by Controlling the Turnover of Phosphatase PP2CA. Plant Cell Advance, https://doi.org/10.1105/tpc.16.00364 (2016).
Zhang, Y. Y. et al. Arabidopsis SDIR1 Enhances Drought Tolerance in Crop Plants. Biosci. Biotechnol. Biochem. 72(8), 2251–2254 (2008).
Lee, H. K. et al. Drought Stress-Induced Rma1H1, a RING Membrane-Anchor E3 Ubiquitin Ligase Homolog, Regulates Aquaporin Levels via Ubiquitination in Transgenic Arabidopsis Plants. The Plant Cell. 21, 622–641 (2009).
Cho, S. K., Ryu, M. Y., Song, C., Kwak, J. M. & Kim, W. T. Arabidopsis PUB22 and PUB23 Are homologous U-Box E3 ubiquitin ligases that play combinatory roles in response to drought Stress. The Plant Cell. 20(7), 1899–1914 (2008).
Catala, R. et al. The Arabidopsis E3 SUMO Ligase SIZ1 Regulates Plant Growth and Drought Responses. The Plant Cell. 19, 2952–2966 (2007).
Chopra, R., Burow, G., Burke, J. J., Gladman, N. & Xin, Z. Genome-wide association analysis of seedling traits in diverse Sorghum germplasm under thermal stress. BMC Plant Biology. 17, 103 (2017).
Hulse, K. et al. Development of a 63K SNP Array for Cotton and HighDensity Mapping of Intra-and Inter-Specific Populations of Gossypium spp. G3 (Bethesda) 5, 1187–1209 (2015).
Paul, A. et al. RNA-seq-mediated transcriptome analysis of activelygrowing and winter dormant shoots identifies non-deciduous habit of evergreen tree teaduring winters. Sci. Rep. 4, 5932 (2014).
Ghawana, S. et al. An RNA isolation system for plant tissues rich in secondary metabolites. BMC Research Notes. 4(1), 85 (2011).
Patel, R. K. & Jain, M. NGS QC toolkit: a tool for quality control of next generation sequencing data. PloS One 7, e30619 (2012).
Grabherr, M. G. et al. Trinity: reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nature biotechnology. 29(7), 644–652 (2011).
Fu, L., Niu, B., Zhu, Z., Wu, S. & Li, W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 28, 3150–3152 (2012).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nature Methods. 9, 357–359 (2012).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols. 1(7(3)), 562–78 (2012).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 4(12), 323 (2011).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatic. 26, 139–140 (2010).
Du, Z., Zhou, X., Ling, Y., Zhang, Z. & Su, Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 310 (2010).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000).
Bhandawat, A., Singh, G., Seth, R., Singh, P. & Sharma, R. K. Genome-Wide Transcriptional Profiling to Elucidate Key Candidates Involved in Bud Burst and Rattling Growth in a Subtropical Bamboo (Dendrocalamushamiltonii). Front. Plant Sci. 7, 2038 (2017).
Singh, P. et al. Spatial transcriptome analysis provides insights of key gene(s) involved in steroidal saponin biosynthesis in medicinally important herb Trillium govanianum. Scientific Reports 7, 45295 (2017).
Jayaswall, K. et al. Transcriptome Analysis Reveals Candidate Genes involved in BlisterBlightdefense in Tea (Camellia sinensis (L) Kuntze). Sci. Rep. 6, 30412 (2016).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25(4), 402–408 (2001).
Maestre, L. et al. SNP calling from RNA-seq data without a reference genome: identification, quantification, differential analysis and impact on the protein sequence. Nucleic Acids Res. 44, 148–148 (2016).
Collins, A. & Ke, X. Primer1: primer design web service for tetra-primer ARMS-PCR. Open Bioinformatics Journal. 6, 55–58 (2012).
Hayashi, K., Hashimoto, N., Daigen, M. & Ashikawa, I. Development of PCR-based SNP markers for rice blast resistance genes at the Piz locus. Theoretical and Applied Genetics. 108, 1212–1220 (2004).
Acknowledgements
The financial support was provided by Council of Scientific & Industrial Research, New Delhi concerning CSIR Projects-PLOMICS (BSC 301), FBR (MLP146), NTRF and Indo-Sri Lanka Joint Project, DST, Govt. of India. RP acknowledge CSIR New Delhi for Senior Research Fellowship. Mr. Mohit K. Swarnkar is acknowledged for assisting in Illumina GAIIx sequencing. This is IHBT communication No. 4309.
Author information
Authors and Affiliations
Contributions
R.K.S.: conceived and designed the study. R.P.: performed experiments. R.P., R.S., P.S. and G.S.: analysed data. R.P. and R.K.S.: wrote the manuscript. S.K.: helped in editing of manuscript. R.K.S.: approved the final version of the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parmar, R., Seth, R., Singh, P. et al. Transcriptional profiling of contrasting genotypes revealed key candidates and nucleotide variations for drought dissection in Camellia sinensis (L.) O. Kuntze. Sci Rep 9, 7487 (2019). https://doi.org/10.1038/s41598-019-43925-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43925-w
This article is cited by
-
Genome-wide transcriptional profiling and physiological investigation elucidating the molecular mechanism of multiple abiotic stress response in Stevia rebaudiana Bertoni
Scientific Reports (2023)
-
Transcriptome analysis of response strategy in Hemerocallis fulva under drought stress
Genes & Genomics (2023)
-
Genome-wide identification and characterization of functionally relevant microsatellite markers from transcription factor genes of Tea (Camellia sinensis (L.) O. Kuntze)
Scientific Reports (2022)
-
Underpinning the molecular programming attributing heat stress associated thermotolerance in tea (Camellia sinensis (L.) O. Kuntze)
Horticulture Research (2021)
-
Tolerance mechanisms in maize identified through phenotyping and transcriptome analysis in response to water deficit stress
Physiology and Molecular Biology of Plants (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.