Abstract
Refractory pheochromocytoma and paraganglioma (PPGL) have a poor prognosis and the treatment strategy remains to be established. This multi-institutional phase I study was performed to determine the safety, dose-limiting toxicity (DLT), and efficacy of [131I]-meta-iodobenzylguanidine (131I-mIBG) therapy for refractory PPGLs. Twenty patients with refractory PPGL were enrolled in this study. We administered fixed doses of 131I-mIBG to all patients, delivering a second and third course of 131I-mIBG to eight and three patients, respectively. During the 20 weeks after 131I-mIBG injection, the authors surveyed the adverse events in accordance with the Common Terminology Criteria for Adverse Events. All patients experienced adverse events and adverse reactions, but none experienced a grade 4 adverse event. Twelve weeks after 131I-mIBG injection, examinations for the evaluation of therapeutic effects was performed in accordance with the Response Evaluation Criteria in Solid Tumours (RECIST). The best overall response rates (based on RECIST categories) were 10% (complete response), 65% (stable disease), 15% (progressive disease), and 10% (not all evaluated). The efficacy and safety of 131I-mIBG therapy was shown in patients with refractory PPGL, and DLT was observed in neither single nor repeated 131I-mIBG therapy, indicating a tolerability for 131I-mIBG therapy.
Similar content being viewed by others
Introduction
Since the 1980s, patients with unresectable, metastatic, and relapsed pheochromocytoma and paraganglioma (refractory PPGL) have been widely treated with radioisotope [131I]meta-iodobenzylguanidine (131I-mIBG) radiotherapy to cure or control inoperable tumors1. Through active uptake via norepinephrine transporter or passive diffusion, the mIBG radioisotope, a guanethidine analog resembling norepinephrine, can enter chromaffin cells where it is stored in catecholamine-containing neurosecretory granules, leading to the anticancer effect of 131I-mIBG therapy on lesions2,3,4,5.
In combination with surgery, external radiotherapy in the form of 131I-mIBG therapy has been performed to eradicate tumors. PPGLs occur in 2–8 per million persons per year and refractory PPGLs are very rare, occurring in 10–30% of PPGL cases6,7,8. Although 131I-mIBG therapy has been in use for over 30 years, researchers have conducted only a few phase clinical trials in patients with refractory PPGL regarding its safety and efficacy. Two phase II trials of high-dose 131I-mIBG therapy requiring preparation of autologous hematopoietic stem cell rescue for the severe adverse effect of bone marrow suppression9,10 and one phase I trial of a high-specific activity of 131I-mIBG therapy11 exist. No other phase trial confirming the safety and efficacy of 131I-mIBG therapy has been conducted12. A wide range of response rates to 131I-mIBG therapy on imaging (0–83%) and in hormonal examinations (20–100%) in patients with refractory PPGL was reported in a meta-analysis of retrospective studies13.
This phase I trial in a multi-center setting was conducted to assess the safety, dose-limiting toxicity (DLT), and efficacy of 131I-mIBG therapy in patients with refractory PPGL.
Results
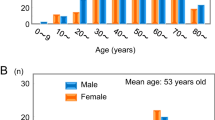
We enrolled 20 patients between February 2016 and July 2017 (Table 1). After the patients’ initial diagnoses of PPGLs refractory to therapy, 5.14 ± 4.90 years (range: 0.3–17.8 years) had passed. We described refractory PPGLs with severe local invasion at initial diagnosis (25%, 5/20), metastasis at initial diagnosis (25%, 5/20), local recurrence after surgical resection (20%, 4/20), and metastasis after surgical resection (65%, 13/20).
We administered 131I-mIBG therapy at doses of 7.4 and 5.55 GBq to patients at first treatment in this study. All patients were evaluated for response assessment. We performed a second and third course of 131I-mIBG therapy in eight (40%) and three (15%) patients, respectively. We treated two patients following our protocol (10%). However, the protocol was stopped in 18 patients (90%) as the attending physicians decided on discontinuation (12/20, 60%), based on PD in scintigraphic response (2/20, 10%), PD in best overall response based on Response Evaluation Criteria in Solid Tumors (RECIST) (1/20, 5%), PD in both scintigraphic response and overall response based on RECIST (1/20, 5%), and proposed discontinuation of therapy (not related to adverse events) (2/20, 10%).
Safety evaluation
We found no DLT in any patient. Although all patients had adverse events and adverse reactions, there was no death event within 6 months after enrollment. However, one patient died due to disease progression during the study period (5%).
The adverse events and reactions occurring at any grade in ≥50% of patients were in Table 2. The observed rate of adverse events and reactions did not differ between each course of 131I-mIBG therapy. We encountered no grade 4 adverse event in this study. We observed no unexpected changes in investigations, vital signs, and physical signs.
Response evaluation
The best overall response rate based on RECIST was 10% (2/20) in complete response (CR), 65% (13/20) in stable disease (SD), 15% (3/20) in progressive disease (PD), and 10% (2/20) in not evaluated (NE). The response rate [partial response (PR) + CR] was 10% [95% confidential interval (CI): 1.2–31.7%] (Table 3).
The scintigraphic response in the first course was 10% (2/20) in CR, 25% (5/20) in PR, 40% in SD (8/20), 20% in PD (4/20), and 5% in non-CR/non-PD (1/20). Response rate (PR + CR) was 35% (95% CI: 15.4–59.2%).
Eight patients received a second course of 131I-mIBG therapy. The scintigraphic response in the second course was 5% in CR (1/20), 10% in PR (2/20), 20% in SD (4/20), 5% in PD (1/20), and 60% in unknown (12/20). The response rate was 15% (95% CI: 3.2–37.9%).
Three patients received a third course of 131I-mIBG therapy. There were three patients with PR (15%) and 17 patients with unknown (85%) in the scintigraphic response. The response rate was 15% (95% CI: 3.2–37.9%).
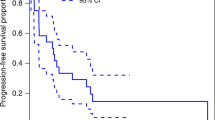
There was no death within 6 months of enrollment and overall survival (OS) rate was 100%. We did not determine the median OS statistically due to no death event. Four patients had progression events during the 6 months since enrollment and progression-free survival (PFS) at 6 months was 80.0%. In the follow-up period, five patients had disease progression and we did not determine median PFS statistically because we observed too few events.
Discussion
In this phase I multi-institutional clinical trial, along with the standardized treatment protocol, we evaluated the safety and efficacy of 131I-mIBG therapy for 20 patients with refractory PPGLs. We observed no DLT, defined as grade ≥ 4 hematological toxicity and grade ≥ 3 non-hematological toxicity, in our trial. There are no reports of prospective trials for the evaluation of adverse effects and reactions and our data agreed with previous reports of toxicities. Although selection bias might have occurred in retrospective cohort studies—especially for rare diseases—those studies revealed that the incidence of grade 3 or higher adverse reactions was quite low and grade 4 hematological toxicity had never occurred at the fixed dose of 7.4 GBq of 131I-mIBG14,15,16. Herein, we showed the tolerance without DLT of 131I-mIBG therapy.
The response rate (CR + PR) in our trial was 10.0% (95% CI: 1.2–31.7%) based on RECIST and 35% (95% CI: 15.4–59.2%) based on 123I-mIBG scintigraphy. These are comparable with those obtained in a review of 116 patients with malignant PPGLs14. The initial response rate (CR + PR) in the review of radiological abnormality and diagnostic 131I-mIBG scintigraphy was 30% of the patients. Based on both our data and previous reports, we consider that a response rate for 131I-mIBG therapy based on imaging modalities would be approximately 10–30%.
We were able to safely administer 7.4 GBq of 131I-mIBG therapy two or three times with no difference in the rate of adverse events compared with that of the first course of 131I-mIBG therapy (mean cumulative 131I-mIBG therapy dose: 11.4 ± 5.7 GBq). A previous review reported the repeated 131I-mIBG therapy with a mean single therapy dose of 5.8 GBq; the number of times the 131I-mIBG therapy was repeated ranged from 1 to 11 (mean: 3.3 ± 2.2 times), and the cumulative131I-mIBG therapy dose was 3.6–85.9 GBq (mean: 18.1 ± 13.0 GBq)14. The common total administered activity of repeated 131I-mIBG varied between 10 and 40 GBq17. In a Japanese multi-center observation study, 31% of patients received repeated 131I-mIBG therapy of individual doses ranging from 3.7 to 14.8 GBq without severe adverse effects15. Our trial and these previous reports support the claim that the adverse effects of repeated 131I-mIBG therapy are tolerable.
Systemic treatment regimens for refractory PPGLs have not yet been standardized18, and the optimal timing for starting 131I-mIBG therapy remains under investigation. We confirmed here the safety and favorable tolerance of 131I-mIBG therapy. There are some arguments for promoting the use of 131I-mIBG therapy at an earlier stage: (1) due to disease progression, including tumor cell dedifferentiation, loss of specific neurotransmitter transporters, and gene expression, mIBG does not accumulate in parts of PPGL lesions19,20,21,22,23,24; and (2) sometimes, refractory PPGLs rapidly progress within a few months or years after initial diagnosis. Of course we should consider that some patients could live for >10 years in a status of stabilization25,26 regardless of the 5-year survival rate of 40–50%19,27,28. However, due to the demonstrated safety and tolerance of 131I-mIBG therapy, our trial would support the early induction of 131I-mIBG therapy after the initial diagnosis of refractory PPGLs.
There are few limitations of this study. First, follow-up period was insufficient to monitor long-term side effects; patients need to be carefully followed for hypothyroidism and a second occurrence of malignancy. Second, we did not evaluate improvements in catecholamine values and clinical symptoms as endpoints. Third, the number of patients was small. This limitation was difficult to overcome, given the relatively few patients with refractory pheochromocytoma who met the inclusion criteria. Thus, repeated 131I-mIBG therapy needs to be investigated in a larger, multicentric prospective study.
Conclusions
We showed the safety and efficacy of 131I-mIBG therapy in patients with refractory PPGLs during 20 weeks after the injection, indicating that 131I-mIBG therapy has a tolerability. We observed no case of grade 4 toxicity in repeated 131I-mIBG therapy. In addition, repeated 131I-mIBG therapy could be performed without severe adverse events in patients with refractory PPGLs.
Methods
Study outline
Following the previously published study protocol12, we enrolled 20 patients with refractory PPGLs in this study. We screened the patients in accordance with both inclusion and exclusion criteria and gave the registered patients fixed doses of 5.55 GBq or 7.40 GBq of 131I-mIBG. We surveyed the occurrence of adverse events during the 20 weeks after 131I-mIBG injection and reported all severe adverse events in detail. We performed both examinations and imaging diagnosis 12 weeks after 131I-mIBG injection to evaluate the therapeutic effect. We administered the next course on finding neither severe adverse reactions nor progression of the disease during the previous course.
Ethical considerations and registration
We conducted this study in accordance with the International Committee for Harmonization Good Clinical Practice (ICH—GCP) guideline and the Declaration of Helsinki. The institutional review boards of all participating institutions (Kanazawa University ethics committee, Gunma University ethics committee, Kagoshima University ethics committee, and Hokkaido University ethics committee) approved the study protocol. All patients provided informed consent before registration. This study was registered with UMIN Clinical Trials Registry (UMIN000018497) and the date of registration was July 30, 2015.
Collaborative institutions
Currently, four institutions have the facilities to perform 131I-mIBG therapy in Japan in patients with refractory PPGL. This study was conducted at all these institutions—Kanazawa University Hospital, Gunma University Hospital, Hokkaido University Hospital, and Kagoshima University Hospital. Each of these institutions had at least one physician of nuclear medicine certified by the Japanese Society of Nuclear Medicine.
Endpoint
In this study, our primary endpoint was DLT. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. We defined DLT as follows: grade ≥ 4, hematological toxicity; grade ≥ 3, non-hematological toxicity except for grade 3 nausea, vomiting, anorexia, and hypertension. We evaluated DLT during the first 12 weeks after the first 131I-mIBG injection.
Our secondary endpoints were response rates according to RECIST 1.129 and scintigraphic evaluation of 123I-mIBG, OS, PFS, and adverse event/reaction.
Response evaluation
We performed primary response assessment at 20 weeks after enrollment. We graded the overall best responses based on computed tomography (CT) and 123I-mIBG scintigraphy.
We graded the CT response using the RECIST for measurable soft tissue disease and the sum of longest diameters (sLD) measured at study entry. For target lesions, we defined CR as the disappearance of all target lesions and reduction in the short axis of any pathological lymph node to <10 mm. We defined PR as a decrease of ≥30% in the sLD, SD as change that did not meet the definition of PR or PD, and PD as an increase of at least 20%, with the sum also demonstrating an absolute increase of at least 5 mm in the sLD or at a new site. For non-target lesions, we defined CR as the disappearance of all target lesions, with all lymph nodes of a non-pathological size with a short axis of <10 mm and the normalization of serum and urine catecholamine values. We interpreted residual radiographic abnormality as representing either fibrosis or scarring. We defined non-CR/non-PD as the persistence of one or more non-target lesions and serum and urine catecholamine values greater than the upper limit of the normal value. We defined PD as the unequivocal progression of existing non-target lesions. When, for any reason, examination was not performed, we defined the patient with the target or non-target lesions as NE. We performed comprehensive evaluation based on RECIST (Table 4). After the 131I-mIBG therapy, we evaluated the best overall response criteria based on RECIST (Table 5).
We evaluated the scintigraphic response for target and non-target lesions, with target lesions meeting the following criteria: 1) a lesion must be confirmed clearly on 123I-mIBG whole body scintigraphy; (2) a lesion must be confirmed clearly on 123I-mIBG single photon emission tomography (SPECT); (3) a lesion must be observed on CT; and (4) a background region must be set on the SPECT image. We also recorded other non-target lesions. We measured the increased rate of mean count (IRmean) and maximum count (IRmax) on target lesions using the following method: IR = (count in target region − count in background region)/count in background lesion. We defined background regions as follows: (1) liver—if a target lesion was in the liver and liver uptake was normal; (2) ipsilateral area—if a lesion was not in the midline and ipsilateral uptake was normal; (3) thigh—if a lesion was not in the thigh and abnormal uptake was -not observed; and (4) intracranial cavity—if a lesion was not in the intracranial cavity and abnormal uptake was not observed. We calculated the reduction rates (RRs) of IRmax and IRmean (RRmax and RRmean) using the following method:
For target lesions, we defined CR as the disappearance of all target lesions, where RRmax and RRmean were >90%. We defined PR as a decrease of ≥30% in RRmax and RRmean, where RRmax and RRmean were at least >90% in one lesion. We defined SD as change that did not meet the definition of PR or PD and PD as an increase of at least 30% in RRmax or RRmean in one target lesion. For non-target lesions, we defined CR as the disappearance of all non-target lesions and catecholamine values within normal limits. We defined PD as the significant progression of non-target lesions, including relapse. We defined non-CR/non-PD as change that did not meet the definition of CR or PD. When, for any reason, an examination was not performed, we defined the patient with the target or non-target lesions as NE. We performed comprehensive evaluation based on 123I-mIBG scintigraphy after 131I-mIBG therapy (Table 6).
Eligibility criteria
Prior to enrollment in the study, patients must fulfill all of the following criteria: confirmed diagnosis of refractory PPGLs; no prior history of surgical treatment or radical external irradiation; aged ≥ 20 years; having an Eastern Cooperative Oncology Group Performance Status of 0–2 or a Karnofsky Performance Scale of ≥80%; independence in feeding, excretion, and sleeping; written informed consent; and having adequate bone marrow, liver, renal, and respiratory function, as shown below.
-
(i)
white blood cell ≥ 3,000/mm3
-
(ii)
hemoglobin ≥ 9.0 g/dL
-
(iii)
platelets ≥ 100,000/mm3 without G-CSF
-
(iv)
estimated glomerular filtration rate ≥ 30 mL/min/1.73 m2
-
(v)
aspartate transaminase < 100 IU/L
-
(vi)
alanine transaminase < 100 IU/L
-
(vii)
lactate dehydrogenase < 400 IU/L
-
(viii)
New York Heart Association Functional Classification class I or below
-
(ix)
HbA1c < 8.0%
-
(x)
oxygen saturation ≥ 96% at room air
In this study, we defined refractory PPGL as follows: PPGLs with severe local invasion at initial diagnosis; PPGLs with metastasis at initial diagnosis; PPGLs with local recurrence after surgical resection; and malignant PPGLs with metastasis after surgical resection8.
Exclusion criteria
Patients were excluded for any of the following reasons: having had malignancies of other histologies apart from thyroid medullary carcinoma with multiple endocrine neoplasia type 2, angioblastoma of the retina with von Hippel–Lindau disease, and neurofibroma with neurofibromatosis type 1 within the preceding 5 years; having a history of tumor deterioration, CTCAE grade ≥ 2 non-hematologic toxicity, or grade ≥ 3 hematologic toxicity while undergoing 131I-mIBG therapy; having any CTCAE grade ≥ 2 toxicity; having a verified hepatitis B or C virus antibody or human immunodeficiency virus antibody positivity; having any other infections currently being treated; having a history of episodes of severe symptoms due to uncontrollable increase of catecholamines, severe arrhythmia, or asystole; having a diagnosis of uncontrollable symptomatic arrhythmia, thyroid dysfunction (hyperthyroidism or hypothyroidism), respiratory disease, or pleural effusion or ascites; having a diagnosis of coronary artery disease, amiodarone-treated arrhythmia, severe valvular disease of the heart, aortic disease, bleeding disorder, or psychosis; being a pregnant or lactating woman or a woman planning to become pregnant; having a diagnosis for any diseases currently treated with adrenal corticosteroids or immunosuppressants; no ability to stay in an isolated room for radiation control; having episodes of allergic reaction to potassium iodide; or having any symptomatic lesions currently treated with palliative external irradiation.
Patient registration
We sent a patient registration form to the independent data center of an academic research organization at Kanazawa University Hospital. We ran patient registration between February 1, 2016 and July 31, 2017. We continued making observations until October 31, 2017.
Treatment
We planned a treatment protocol in accordance with the Japanese draft guidelines for 131I-mIBG therapy by the Drafting Committee for Guidelines of Radiotherapy with 131I-mIBG, Committee for Nuclear Oncology and Immunology, Japanese Society of Nuclear Medicine, and referred to the procedure guidelines for 131I-mIBG therapy by the European Association of Nuclear Medicine30,31.
After admitting the patients to an isolated radiation treatment room, we administered 7.4 GBq of 131I-mIBG (ATC Code: V10X A02, National Centre for Nuclear Research Radioisotope Centre POLATOM, Poland) injection over 1 h at day 0. Because the maximum permitted amount of radioisotope agents in Hokkaido University Hospital is 5.55 GBq, one patient received 5.55 GBq of 131I-mIBG. Before and after injection, we noted blood pressure, heart rate, and the presence of any symptoms. We discharged the patients from the radiation treatment room when they had satisfied the release criteria set out by Japanese regulations.
Prescribed, recommended, or acceptable supportive treatments
Oral administration of potassium iodide was prescribed for the protection of the thyroid gland and 5-hydroxytryptamine (serotonin) receptor antagonist was recommended to avoid vomiting, whereas bisphosphonates and denosumab were acceptable for coadministration.
Second or third 131I-mIBG therapy
For patients who had not experienced severe adverse reactions or progression of disease in 20 weeks after the first course, we administered the second and third course of 131I-mIBG therapy every 24 weeks until the end of study period.
Follow-up schedule
The study period extended from the date of enrollment to 20 weeks after the 131I-mIBG injections. We collected the results of physical and blood examinations at enrollment every day from day 0 to day 4 and 2, 4, 6, 8, 12, 16, and 20 weeks after the 131I-mIBG injection to evaluate its safety. We evaluated its efficacy by making comparisons between baseline and 12 weeks after 131I-mIBG injection. We performed physiological examinations, electrocardiography, cardiac ultrasonography, blood examination (common and catecholamine), urinary examination, CT, and 123I-mIBG scintigraphy on the day of enrollment and 12 weeks after 131I-mIBG injection.
Sample size
Our target sample size was a total of 20 patients, based on the precision of a one-sided 90% CI estimate of the DLT rate. More specifically, the upper confidence limit using the exact method in 15 evaluable patients and 2 observed DLTs (13%) would rule out a null rate of 33%. It is commonly considered acceptable in chemotherapy using a cytotoxic agent that DLT can occur in one-third or less of patients. Therefore, the incidence of the DLT would be allowed if it occurred in two or fewer patients under 131I-mIBG therapy. Because of the limited use of radioactive drugs, each of our institute could only perform 131I-mIBG therapy on a limited number of patients. In considering this problem, we determined the feasible number of treated patients to be 15. We determined the total number of patients for registration allowing for a drop-out rate of approximately 20%.
Statistical analysis
We included all treated patients in the population analyzed for the primary endpoint. We summarized the DLT using the occurrence rate and calculated the CI of the DLT rate using an exact method based on binomial distribution.
Our secondary endpoints included PFS, OS, and toxicity. We used the date of the 131I-mIBG injection as the starting point for the Kaplan–Meier estimation of PFS and OS. For PFS, we defined an event as PD or death from any cause. For a simple summary of the outcome by group, we based the PFS and OS rates on Kaplan–Meier estimates. All reported P values are two-sided.
Change history
19 January 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-05239-2
References
Wieland, D. M., Swanson, D. P., Brown, L. E. & Beierwaltes, W. H. Imaging the adrenal medulla with an I-131-labeled antiadrenergic agent. J Nucl Med 20, 155–158 (1979).
Bomanji, J. et al. Uptake of iodine-123 MIBG by pheochromocytomas, paragangliomas, and neuroblastomas: a histopathological comparison. J Nucl Med 28, 973–978 (1987).
Vaidyanathan, G. Meta-iodobenzylguanidine and analogues: chemistry and biology. Q J Nucl Med Mol Imaging 52, 351–368 (2008).
Taieb, D. et al. EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging 39, 1977–1995, https://doi.org/10.1007/s00259-012-2215-8 (2012).
Castellani, M. R., Chiti, A., Seregni, E. & Bombardieri, E. Role of 131I-metaiodobenzylguanidine (MIBG) in the treatment of neuroendocrine tumours. Experience of the National Cancer Institute of Milan. Q J Nucl Med 44, 77–87 (2000).
Lenders, J. W., Eisenhofer, G., Mannelli, M. & Pacak, K. Phaeochromocytoma. Lancet 366, 665–675, https://doi.org/10.1016/S0140-6736(05)67139-5 (2005).
Kimura, N. et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer 21, 405–414, https://doi.org/10.1530/ERC-13-0494 (2014).
Lam, A. K. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr Pathol 28, 213–227, https://doi.org/10.1007/s12022-017-9484-5 (2017).
Fitzgerald, P. A. et al. Malignant pheochromocytomas and paragangliomas: a phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG). Ann N Y Acad Sci 1073, 465–490, https://doi.org/10.1196/annals.1353.050 (2006).
Gonias, S. et al. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol 27, 4162–4168, https://doi.org/10.1200/JCO.2008.21.3496 (2009).
Noto, R. B. et al. Phase 1 Study of High-Specific-Activity I-131 MIBG for Metastatic and/or Recurrent Pheochromocytoma or Paraganglioma. J Clin Endocrinol Metab 103, 213–220, https://doi.org/10.1210/jc.2017-02030 (2018).
Inaki, A. et al. A phase I clinical trial for [(131)I]meta-iodobenzylguanidine therapy in patients with refractory pheochromocytoma and paraganglioma: a study protocol. J Med Invest 64, 205–209, https://doi.org/10.2152/jmi.64.205 (2017).
van Hulsteijn, L. T., Niemeijer, N. D., Dekkers, O. M. & Corssmit, E. P. (131)I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf) 80, 487–501, https://doi.org/10.1111/cen.12341 (2014).
Loh, K. C., Fitzgerald, P. A., Matthay, K. K., Yeo, P. P. & Price, D. C. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. J Endocrinol Invest 20, 648–658 (1997).
Yoshinaga, K. et al. Effects and safety of (1)(3)(1)I-metaiodobenzylguanidine (MIBG) radiotherapy in malignant neuroendocrine tumors: results from a multicenter observational registry. Endocr J 61, 1171–1180, https://doi.org/10.1507/endocrj.EJ14-0211 (2014).
Gedik, G. K., Hoefnagel, C. A., Bais, E. & Olmos, R. A. 131I-MIBG therapy in metastatic phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging 35, 725–733, https://doi.org/10.1007/s00259-007-0652-6 (2008).
Ezziddin, S. et al. Repeated Radionuclide therapy in metastatic paraganglioma leading to the highest reported cumulative activity of 131I-MIBG. Radiat Oncol 7, 8, https://doi.org/10.1186/1748-717X-7-8 (2012).
Baudin, E. et al. Therapy of endocrine disease: treatment of malignant pheochromocytoma and paraganglioma. Eur J Endocrinol 171, R111–122, https://doi.org/10.1530/EJE-14-0113 (2014).
Eisenhofer, G. et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer 11, 423–436 (2004).
Fiebrich, H. B. et al. 6-[F-18]Fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to conventional imaging with (123)I-metaiodobenzylguanidine scintigraphy, computer tomography, and magnetic resonance imaging in localizing tumors causing catecholamine excess. J Clin Endocrinol Metab 94, 3922–3930, https://doi.org/10.1210/jc.2009-1054 (2009).
Rufini, V., Treglia, G., Perotti, G. & Giordano, A. The evolution in the use of MIBG scintigraphy in pheochromocytomas and paragangliomas. Hormones (Athens) 12, 58–68 (2013).
Safford, S. D. et al. Iodine -131 metaiodobenzylguanidine is an effective treatment for malignant pheochromocytoma and paraganglioma. Surgery 134, 956–962; discussion 962–953, https://doi.org/10.1016/S0039 (2003).
Tan, T. H., Hussein, Z., Saad, F. F. & Shuaib, I. L. Diagnostic Performance of (68)Ga-DOTATATE PET/CT, (18)F-FDG PET/CT and (131)I-MIBG Scintigraphy in Mapping Metastatic Pheochromocytoma and Paraganglioma. Nucl Med Mol Imaging 49, 143–151, https://doi.org/10.1007/s13139-015-0331-7 (2015).
Fonte, J. S. et al. False-negative 123I-MIBG SPECT is most commonly found in SDHB-related pheochromocytoma or paraganglioma with high frequency to develop metastatic disease. Endocr Relat Cancer 19, 83–93, https://doi.org/10.1530/ERC-11-0243 (2012).
Sisson, J. C., Shulkin, B. L. & Esfandiari, N. H. Courses of malignant pheochromocytoma: implications for therapy. Ann N Y Acad Sci 1073, 505–511, https://doi.org/10.1196/annals.1353.053 (2006).
Wakabayashi, H. et al. Prognostic values of initial responses to low-dose (131)I-MIBG therapy in patients with malignant pheochromocytoma and paraganglioma. Ann Nucl Med 27, 839–846, https://doi.org/10.1007/s12149-013-0755-z (2013).
Averbuch, S. D. et al. Malignant pheochromocytoma: effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine. Ann Intern Med 109, 267–273 (1988).
Hescot, S. et al. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 98, 4006–4012, https://doi.org/10.1210/jc.2013-1907 (2013).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45, 228–247, https://doi.org/10.1016/j.ejca.2008.10.026 (2009).
Giammarile, F. et al. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur J Nucl Med Mol Imaging 35, 1039–1047, https://doi.org/10.1007/s00259-008-0715-3 (2008).
Kinuya, S. et al. Draft guidelines regarding appropriate use of I - MIBG radiotherapy for neuroendocrine tumors: Guideline Drafting Committee for Radiotherapy with I - MIBG, Commit- tee for Nuclear Oncology and Immunology, The Japanese Society of Nuclear Medicine. Ann Nucl Med., https://doi.org/10.1007/s12149-015-0960-z (2015).
Acknowledgements
The current study has been supported by a grant from the Japan Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Contributions
H.W., A.I., T.H., M.J., T.S. and S.K. contributed to the recruitment and initiation of patients into the clinical trial, and collected data. K.Y., T.M. and Y.I. contributed to the experimental design and data analysis. All authors contributed to the drafting of the manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this article contained an error in the 95% confidence intervals calculated for the second and third courses of 131I-mIBG. Modifications have been made to the Results section. Full information regarding the corrections made can be found in the correction for this Article.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wakabayashi, H., Inaki, A., Yoshimura, K. et al. A phase I clinical trial for [131I]meta-iodobenzylguanidine therapy in patients with refractory pheochromocytoma and paraganglioma. Sci Rep 9, 7625 (2019). https://doi.org/10.1038/s41598-019-43880-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43880-6
This article is cited by
-
Prognostic factors for refractory pheochromocytoma and paraganglioma after 131I-metaiodobenzylguanidine therapy
Annals of Nuclear Medicine (2022)
-
An open-label, single-arm, multi-center, phase II clinical trial of single-dose [131I]meta-iodobenzylguanidine therapy for patients with refractory pheochromocytoma and paraganglioma
Annals of Nuclear Medicine (2022)
-
Model systems in SDHx-related pheochromocytoma/paraganglioma
Cancer and Metastasis Reviews (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.