Abstract
Adhesions are a very common complication in the abdominal surgery. Animal studies and human trials have evaluated strategies designed to reduce and prevent postsurgical adhesions but few have an evidence base that justifies routine use. A strategy to prevent adhesions effectively remains an urgent need. We studied a reproducible model of intra-peritoneal adhesion formation in rats using laparotomy with several peritoneal sutures to produce the adhesions. Here we show that entraining endogenous stem cells into injury sites using the combined effect of AMD3100 and low-dose FK-506 (AF) can reduce the adhesion score significantly and abolish peritoneal adhesions in 45% of animals in a rat model of severe postsurgical intra-abdominal adhesions, compared with saline controls. Searching for mechanisms, we found AF treatment dramatically increased SDF-1 expressing cells, HGF expressing Ym1+ M2 macrophages and CD133+ stem cells in the injury sites of peritoneal surface at day 5 post-operation. Our results demonstrate that medically induced recruitment of autologous stem cells using AF significantly reduced postsurgical intra-abdominal adhesions. These findings suggest a novel effective therapeutic approach to preventing adhesions in patients.
Similar content being viewed by others
Introduction
Formation of peritoneal adhesions which are a normal response to injury of the peritoneal surfaces aimed at repairing the damage following surgery that violates the peritoneum has become frequent1. These adhesions commonly cause problems and require additional procedures1,2,3,4,5. Up to 20% of these patients will experience symptoms ranging from pain to intestinal obstruction2. Approximately 10% of bowel obstructions caused by adhesions require reoperation known as adhesiolysis1, and the presence of severe adhesions at reoperation leads to increasing surgical difficulties and longer surgical times1,3,4. Pelvic peritoneal adhesions are responsible for approximately 10 percent of cases of female infertility6. Although attempts at amelioration have been adopted, the number of adhesiolysis procedures has been growing over the past decade5. The need for this is not only unfortunate for patients but increases the cost of health care.
Peritoneal injury produces reaction aimed at healing the damaged peritoneal surfaces which results in the formation of adhesions (fibrotic scars) between two damaged peritoneal surfaces. In the last few decades attempts to stop this have included improved surgical techniques, optimized laparoscopy conditions, anti-inflammatory pharmacotherapies targeted at the inflammatory response and/or fibrin deposition, and creating a material interposition for prevention of peritoneal apposition. Sodium hyaluronate/carboxymethylcellulose (Seprafilm), oxidized regenerated celluose (Intercreed) and 4% icodextrin solution (Adept) are approved and generally viewed as best practice to prevent adhesions7,8,9,10,11. Nevertheless, these have been only marginally helpful and a strategy to prevent adhesions effectively remains an urgent need.
We here report a new solution. This is from a novel entrainment of bone marrow precursor cells using a combination of AMD3100 (plerixafor) and FK506 (tacrolimus) that was found by chance to enable permanent liver allograft protection from rejection and survival in rats with just one week of treatment12. Further, treatment for a week every month for three months provided the same protection for rat and swine renal allografts13,14. This protection against rejection involved the presence of recipient stem cells found in the graft (allograft chimerism) causing local immunosuppression. We also showed that this medical mobilization of undifferentiated bone marrow cells produced a 25% reduction in healing time of skin wounds15. Importantly, this allowed reduced scars and provided for hair follicle growth not seen in the damaged area in controls. In the organ transplantation models, we incidentally observed that there were fewer abdominal peritoneal adhesions in small animals and no adhesion formation in large animals treated with AF combination drugs, even 3–4 years after pig kidney transplantation, however animals treated with saline or single drug formed severe intra-abdominal adhesions. In experiments with 85% partial hepatectomy it was noted that adhesions were minimized in the test group receiving combination therapy16. Based on these observations, we hypothesized that medical conscription and recruitment of undifferentiated bone marrow cells by this drug combination may promote regeneration of damaged peritoneal surfaces following surgery, and therefore prevents the formation of peritoneal adhesions. Here we test this hypothesis objectively by testing it in severe peritoneal scarring in rats. We show that this treatment was able to reduce the adhesion score significantly and abolish peritoneal adhesions in 45% of animals.
Results
Creating suture knots on the parietal peritoneum recapitulates postsurgical intra-abdominal adhesion in rats
All animals that underwent surgery survived, no wound complications or infections occurred and all animals completed the study protocol. The surgical procedure reliably produced abdominal adhesions in control animals 14 days post-operation. Based on the adhesion grade and assessment criteria (Fig. 1), a majority of animals (10/12) in the control group with this surgical procedure developed severe intra-abdominal adhesions.
Adhesion grade and assessment in a rat model of postsurgical intra-abdominal adhesion. Abdominal adhesions were induced by creating four 1–0 Prolene suture knots on the parietal peritoneum in a linear distribution. Each suture encapsulated approximately 2 cm of the parietal peritoneum. The diameter of the tied suture was approximately 5 mm. (A) Scoring criteria of adhesion grades and points. (B) Representative images of the intra-abdominal adhesion formation.
AF combination therapy, but not single drug prevents postsurgical abdominal adhesions in rats
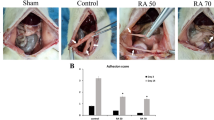
On post-operative day 14, 83.3% animals (10/12) that received saline control treatment subcutaneously developed severe intra-abdominal adhesions (adhesion scores: >4), while 16.7% (2/12) showed moderate intra-abdominal adhesions (adhesion scores: =3–4). Animals that received AMD3100 sc displayed severe (75%; 6/8) or moderate (25%; 2/8) intra-abdominal adhesions. Similarly, seven out of eight animals (87.5%) that received sc FK506 developed severe intra-abdominal adhesions, only one animal showed mild intra-abdominal adhesion (adhesion scores: =2–3). In contrast, animals that received a combination of AMD3100 and FK506 (AF) therapy sc displayed significantly less intra-abdominal adhesion formation (Fig. 2A): about 45% animals (5/11) showed few intra-abdominal adhesions, 18% animals with mild intra-abdominal adhesions (2/11) (adhesion scores: <2), 18% (2/11) animals developed moderate intra-abdominal adhesions and only 18% animals had severe intra-abdominal adhesions (Fig. 2B). The intra-abdominal adhesion scores were further analyzed quantitatively in animals treated with saline, single drug or AF combination. The adhesion scores remained the same in animals receiving AMD3100 (4.38 ± 0.74, n = 8) or FK506 (4.15 ± 0.52, n = 8) alone compared to control animals treated with saline (4.35 ± 0.52, n = 12). However, the adhesion scores were significantly decreased in dual drug treated animals (2.77 ± 1.42, n = 11) compared to saline or single drug treated animals (Fig. 2C).
Analysis for postsurgical adhesion grades for each group. (A) Representative images of the intra-abdominal adhesion formation from six animals in each treatment group on day 14 post operation. (B) Percentage of rats with few, mild, moderate or severe postsurgical intra-abdominal adhesions in different treatment groups. (C) Quantitative analysis of the intra-abdominal adhesion scores in animals treated with saline, single drug or AF combination. The adhesion scores remained the same in animals receiving AMD3100 (4.38 ± 0.74, n = 8) or FK506 (4.15 ± 0.52, n = 8) alone treatment compared to control animals treated with saline (4.35 ± 0.52, n = 12). The adhesion scores were significantly decreased in dual drug treated animals (2.77 ± 1.42, n = 11) compared to animals treated with saline or single drug. The data are shown as mean ± standard deviation (SD). *P < 0.05, **P < 0.01.
Entrainment of CD133 cells into the injury of the peritoneal surface parallels elevated SDF-1 and HGF following therapy
We have reported a synergy between AMD3100 and low-dose FK506 for entrainment of bone marrow-derived CD133 stem cells into damaged tissue12,13,14,15,16. To find whether this treatment recruits undifferentiated bone marrow cells into the injury sites of the peritoneal surface after creating suture knots on the parietal peritoneum, immunohistochemistry staining for CD133, SDF-1 and HGF was done. Figure 3 demonstrates that few CD133 positive cells were evident in intra-abdominal adhesion tissues from saline control or single drug treated animals on day 5 following surgery. In contrast, numerous CD133 positive cells were found in intra-abdominal adhesion tissues from AF combination treated animals on day 5 after surgery.
Histological analysis of the intra-abdominal adhesion tissues. Hematoxylinand eosin (HE) staining and a representative result in intra-abdominal adhesion tissues of immunohistochemical staining for CD133, SDF-1 and HGF. Few CD133, SDF-1 or HGF positive cells were recognized in adhesion tissues from saline control or single drug treated animals on day 5 after surgery. In contrast, numerous CD133, SDF-1 and HGF positive cells were recognized in adhesion tissues from AF combination treated animals on day 5 after surgery. Images were taken at 400× magnification. Scale bar = 100 um.
SDF1 (stromal cell-derived factor-1) can entrain undifferentiated stem cells into injured tissues17,18 by its attraction to the CXCR4 receptor on mobilized stem cells. Few SDF-1 positive cells were recognized histologically in animals treated with saline or single drug on day 5 after surgery. However, SDF-1 positive cells were strongly increased in treated animals (Fig. 3). Importantly, the higher number of SDF-1 positive cells in intra-abdominal adhesion tissues of AF treated animals paralleled the increase in CD133 stem cells.
HGF is crucial in regeneration, cellular growth and motility, and tissue formation. It has been reported that HGF prevents postoperative peritoneal adhesions19, probably through stimulating the regeneration of peritoneal mesothelial cells20, inhibition of collagen deposition and its fibrinolytic capacity21. No HGF expression was found in adhesions from control animals treated with saline or AMD3100, but a few HGF positive cells were present in animals receiving low-dose FK506 alone on postoperative day 5. The number of HGF positive cells was dramatically increased in animals treated with AF combination therapy on postoperative day 5. Thus, entrainment of CD133 cells in the injury sites on the peritoneal surface was associated with increased HGF expression. The increased HGF expression may be important in preventing adhesions.
AF combination therapy increases HGF-producing M2 macrophages in the injury sites of peritoneal surface
To determine whether AF combination therapy promotes immunomodulatory M2-polarization22 of infiltrating macrophages in the injury sites of the peritoneal surface, immunofluorescent staining for the known M2 macrophage marker Ym1/Chi3l3 was performed. Few Ym1 positive cells were recognized in intra-abdominal adhesion tissues from saline control or AMD3100 treated animals, while Ym1 positive cells were slightly increased in low-dose FK506 treated animals on day 5 following surgery. In contrast, Ym1 positive cells were dramatically increased in adhesion tissues from animals with AF combination therapy (Fig. 4). Interestingly, a majority of Ym1 positive cells also expressed HGF in combination-treated animals but not those treated with FK506 alone. These results suggest that low-dose FK506 alone may promote M2 polarization of infiltrating macrophages, while the combination resulted in synergistic facilitation of M2 polarization and entrainment of M2 macrophages. Production of HGF by infiltrating M2 macrophages may not only promote healing of the injured peritoneal surface but also contribute to freedom from peritoneal scarring.
Immunofluorescence double staining for HGF (green) and Ym1 (red) in the intra-abdominal adhesion tissues. Tissue sections were counter-stained with DAPI (blue). HGF and Ym1 (a marker of M2 macrophages) positive cells increased on day 5 after surgery in adhesion tissues from AF treated animals, and double positive cells were recognized. Images were taken at 400× magnification.
Discussion
These findings show the importance of endogenous bone marrow-derived stem cells in preventing postsurgical adhesions. In control animals treated with stem cell mobilizer AMD3100 or low dose FK506 alone, few stem cells appeared in the peritoneal injury and this injury resulted in formation of severe intra-abdominal adhesions following surgery. AF combination therapy dramatically increased CD133 and HGF expressing M2 macrophages in the injured peritoneum. We have shown that this was associated with attenuation of intra-abdominal adhesions.
The peritoneum lines the abdomen as a protective film of mesothelium that reduces friction between the viscera23. The peritoneum is easily damaged due to the loose association between the mesothelial cells24. Peritoneal injury results in tissue ischemia25,26,27, which sets in motion the reparative hypoxia/coagulation cascade. But this increased oxidative stress leads to mesothelial cell damage and intra-abdominal adhesion formation28. In this study, we have established a postsurgical intra-abdominal adhesion model in rats by creating four 1–0 Prolene suture knots on the parietal peritoneum in a linear distribution. The suture knots caused local tissue ischemia that is essential for triggering adhesion formation28. Indeed, most control animals (83%) developed severe intra-abdominal adhesions on postoperative day 14 (Fig. 2B). These results indicate that this suture knots-induced adhesion technique is a reliable model of severe postsurgical intra-abdominal adhesions that is suitable for testing therapeutic interventions.
Because adhesions occur at the injured surface lining of the peritoneum, a rapid rate of remesothelialization and restriction of inflammation likely are important factors that limit postoperative adhesion development. The healing time of small and large peritoneal wounds is the same29 suggesting that healing is not a local event at the margins of the injury and that stem cells may be involved. Mesothelial stem cells can differentiate into mesothelial cells which can derive from differentiation of adult stem cells in adjacent muscle and contribute to healing29,30. Intraperitoneal delivery of autologous stem cells from muscle prevents abdominal adhesions29. Interestingly, intra-abdominal application of mesenchymal stem cells (MSCs) showed promising results for preventing postsurgical intra-abdominal adhesions31,32,33. This provides a precedent for prevention of abdominal adhesions by other adult stem cells. Because the in vitro preparation of autogenous stem/progenitor cells takes time and raises questions of quality, quantity and effectiveness, this stem cell therapy has limited practical application in the treatment of postsurgical abdominal adhesions. For this reason, the mobilization and recruitment of autologous stem cells that we have described is a new paradigm for a simple way to provide stem cells to injured sites of the peritoneum.
The current studies are based on our previous findings with allograft tolerance12,13,14,15,16. The mechanism of action of the combination treatment is that it mobilizes bone marrow stem cells into the circulation via blocking the CXCR4 binding with SDF-1, but also promotes accumulation of the stem cells into the injured organ/tissues as the AMD3100 CXCR4 blocking effect wanes (Fig. 5). In our experiments, neither agent alone increased stem cells in the injured sites of peritoneum on post-operation day 5, although Ym1 positive M2 macrophages were slightly increased in low-dose FK506 treated animals. Consequently, single drug treatment did not reduce postsurgical abdominal adhesion formation. In contrast, the AF combination treatment dramatically increased CD133 cells and M2 macrophages in the injured sites of peritoneum and subsequently prevented/attenuated postsurgical intra-abdominal adhesion formation. Bone marrow-derived stem cells including MSCs have two unique properties: the ability to differentiate into the tissue type requiring regeneration; and their immunomodulatory properties that reduce inflammation and fibrosis through cytokine/growth factor release. Thus, concentrated CD133 stem cells in the injured peritoneal areas may not only promote remesothelialization, but also reduce the inflammatory response.
Schematic representation of therapeutic mechanism of AF combination treatment in preventing postsurgical intra-abdominal adhesion. AMD3100 and low-dose FJ506 reacted synergistically in mobilization and recruitment of bone marrow-derived CD133 stem cells. Low-dose FK506 promotes M2 polarization of infiltrating macrophages and induces HGF production. AF combination therapy may prevent/attenuate postsurgical intra-abdominal adhesion formation via targeting two important factors – remesothelialization and inflammation through recruiting CD133 stem cells and HGF producing M2 macrophages. Rapid remesothelialization following injury may result in adhesion free healing.
Postsurgical adhesion formation is due to the peritoneal injury plus the associated inflammation. The inflammatory cells are predominantly macrophages at 24 hours following injury34. M2 macrophages secret anti-inflammatory cytokines (IL-4, IL-10) which inhibit production of pro-inflammatory cytokines (e.g. IL-1, 6 and TNF). It was found that increased M2 macrophages in sites of polyglycolic acid injury correlated with fewer peritoneal adhesions35. By using immunofluorescence staining for Ym1 (Chi3l3), a marker of M2 macrophages, we found that AF combination therapy dramatically increased Ym1 positive M2 macrophages in the injured peritoneum. Interestingly, a majority of Ym1 positive cells co-stained with hepatocyte growth factor (HGF) which is important in the action of M2 macrophages in intestinal healing through the secretion of HGF36. HGF decreases adhesion formation by inhibiting IFN-γ and PAI-137 and induces mesothelial cell proliferation38. Thus, HGF-producing M2 macrophages induced by AF combination therapy may reduce adhesion formation through regulating the inflammatory response and promoting wound repair/regeneration.
In our experiments, low-dose FK506 increased Ym1 positive M2 macrophages in the injured peritoneum. Interestingly, low-dose FK506 had a synergistic effect with AMD3100 in increasing M2 macrophages in the injured sites, similar to the report from Bai et al.39. FK506 at low dose activates the bone morphogenetic protein (BMP) signaling pathway40 through FKBP12 ligands41 and BMP4 expression induces macrophage polarization towards M242. In addition, MSCs can induce M2-like macrophages in vitro that can suppress T cell and NK cell immune response and induce regulatory T cells43,44. CD133 is also a marker of circulating MSCs45. A high concentration of CD133 stem cells in the injured peritoneal sites may create an environment conducive to M2 macrophages.
Evidence from bone marrow transplant recipients suggests that the bone marrow progenitor cells might regenerate mesothelium46. Although mobilized bone marrow stem cells may generate mesothelial cells and promote remesothelialization, we chose to direct our focus to attenuation of intra-abdominal adhesion formation. Unlike reports of the effect of delivering processed adult stem cells into the peritoneal cavity in ameliorating intra-abdominal adhesion, this study expands on that to demonstrate that pharmacological mobilization and entrainment of the stem cells can reduce/attenuate postsurgical abdominal adhesion formation by stem cells accumulated directly from the circulation through release from the bone marrow. Our AF combination therapy may prevent/attenuate postsurgical intra-abdominal adhesion formation via targeting two important factors – remesothelialization and inflammation through recruiting CD133 stem cells and HGF producing M2 macrophages.
This effect of warding off adhesive peritoneal healing is not incompatible with the increase in speed and completeness of healing of all three intestinal layers in colonic anastomoses that we also found with this treatment (Chen et al. unpublished data). In both cases this combination treatment optimized the ability of the stem cells to do the same thing: return the damaged tissue to normal. That required normal mesothelium for the peritoneal surface and normal bowel in the case of the experiments with intestinal anastomoses (and no adhesions to the suture line). This predisposition to return inflamed or damaged tissue to normal is likely a generally applicable feature of this combination therapy.
Materials and Methods
Animals
Fifty-five Lewis rats including fourteen males and forty-two females were purchased from Hilltop Lab Animals Inc. (Scottdale, PA, USA) and used at 12 to 15 weeks of ages with body weight between 250 to 350 grams. Animals were maintained in a pathogen-free facility of Johns Hopkins University School of Medicine and all animal experiments were performed in accordance with the United States National Institutes of Health (NIH) guidance12,13,15. All animal protocols were reviewed and approved by the Johns Hopkins University Animal Care and Use Committee.
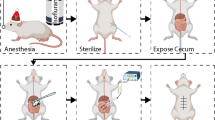
A model of postsurgical intra-abdominal adhesions
Animals were anesthetized with the inhalation of Isoflurane (Baxter, IL, USA) and the abdominal skin was prepared and disinfected by povidone-iodine before the procedure12,13. A 3 cm vertical midline incision was made to access the abdominal cavity. Abdominal adhesions were induced by creating four 1–0 Prolene (Ethicon, Blue Monofilament) suture knots on the anterior parietal peritoneum in a linear distribution. Each suture encapsulated approximately 2 cm of the parietal peritoneum. The diameter of the tied suture was approximately 5 mm. After the creation of the sutures, the peritoneum was closed in two layers by interrupted 3–0 silk sutures. All of the surgical procedures were performed by the same surgeon.
Experimental design
Fifty-five animals with surgical procedures were allocated into four experimental groups: (1) Control group (n = 16): animals received the same volume of saline (2 ml/kg, subcutaneous injection) immediately after surgery and every other day for 10 days; (2) AMD3100 treatment group (n = 12): animals received AMD3100 (1 mg/kg, subcutaneous injection) immediately after surgery and every other day for 10 days; (3) FK506 treatment group (n = 12): animals received FK506 (0.1 mg/kg, subcutaneous injection) immediately after surgery and every other day for 10 days; and (4) AF treatment group (n = 15): animals received AF combination therapy (AMD3100 1 mg/kg and FK506 0.1 mg/kg, subcutaneous injection) immediately after surgery and every other day for 10 days. Sixteen animals (n = 4/group) were sacrificed for collecting tissue samples on postoperative day 5, and the other animals were sacrificed on postoperative day 14 for evaluation of intra-abdominal peritoneal adhesions.
Adhesion grade and assessment
On postoperative day 14 rats were euthanized and a U-shape incision to avoid adhesion sites was used for determination of abdominal adhesions. Adhesion sites were photographed and the difficulty to lyse the adhesion was described by a surgeon without knowing the treatment. The severity of intra-abdominal adhesions was evaluated and scored blindly on the pictures by five individual surgeons who were not involved in surgery and treatment to control for bias. For detailed assessment, the scoring criteria of Zühlke et al.47 was modified by including evaluation of the difficulty to lyse the adhesion which is described in the classification of Mazuji et al.48 (Fig. 1A). The adhesion scores (mean ± SD) were calculated for each animal to minimize inter-observer variability. Those scores were then used to calculate mean ± SD adhesion scores for each treatment group.
Histological analysis
The parietal peritoneum and surrounding adhesion tissues were explanted and fixed in 2% PFA. Cut sections of 6 µm were prepared from PFA-fixed paraffin-embedded tissues for CD133, SDF-1 or HGF staining or frozen tissue for double staining of HGF and Ym1, a marker of M2 macrophages. Each representative section was stained with hematoxylin-eosin, and immunohistochemical stains were performed with the avidin-biotin-peroxidase complex method12,13,49, using VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA). Antigen retrieval of paraffin section was achieved by a microwave, using the antigen retrieval solution (Dako, Carpinteria, CA). Non-specific serum blocking was performed by incubation with 5% goat serum for 30 min. Tissue sections were then incubated with primary antibodies at 4 °C overnight, followed by incubation with secondary antibodies at room temperature for 45 minutes. Tissue sections were then incubated with AB complex (VECTASTAIN ABC kit; Vector, Burlingame, CA) for 30 min according to the manufacture instruction to amplify signals, and reacted with DAB (SIGMAFAST 3,3′-Diamino-benzidine tablets, SIGMA Life Science, St Louis, MO, USA). Counterstaining was performed by using Haematoxylin for 20 seconds. The following antibodies were used: biotin conjugated anti-CD133 antibody (1:100; abcam 19898), anti-HGF antibody (1:200; abcam 837060), anti-SDF-1 antibody (1:200; abcam 25117), and biotinylated goat anti-rabbit IgG (1:20; Cell signaling). Double staining of HGF and Ym1 was also performed by immunofluorescent stains using frozen sections. HGF staining was carried out using anti-rabbit HGF antibody (1:200; abcam 837060) and FITC-conjugated donkey anti-rabbit IgG antibody (1:200; Jackson immunoreserch laboratories, West Grove, PA lot 712-095-153). After HGF staining, the sections were incubated with phycoerythrin (PE) conjugated anti-Ym1 antibody (1:200; abcam 211621) for 1 hour at room temperature. Cell nuclei were stained blue with DAPI. Tissue sections were analyzed by fluorescent microscopy.
Statistical analysis
Quantitative data are expressed as mean ± standard deviation (SD). Adhesion scores were assessed using the one-way ANOVA test followed by Bonferroni-Holm posthoc tests for comparisons between four groups. Statistical analysis was performed using SPSS 13.0 software. P < 0.05 was considered significant.
References
Okabayashi, K. et al. Adhesions after abdominal surgery: a systematic review of the incidence, distribution and severity. Surgery Today 44(3), 405–420 (2014).
Schnüriger, B. et al. Prevention of postoperative peritoneal adhesions: a review of the literature. Am J Surg. 201, 111–121 (2011).
Miller, G., Boman, J., Shrier, I. & Gordon, P. H. Natural history of patients with adhesive small bowel obstruction. Br J Surg. 87, 1240–1247 (2000).
en Broek, R. P. et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. BMJ. 347, f5588 (2013).
DeFrances, C. J., Lucas, C. A., Buie, V. C. & Golosinskiy, A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 30, 1–20 (2008).
Awonuga, A. O., Fletcher, N. M., Saed, G. M. & Diamond, M. P. Postoperative adhesion development following cesarean and open intra-abdominal gynecological operations: a review. Reprod Sci. 18(12), 1166–85 (2011).
Hemadeh, O., Chilukuri, S., Bonet, V., Hussein, S. & Chaudry, I. H. Prevention of peritoneal adhesions by administration of sodium carboxymethyl cellulose and oral vitamin E. Surgery. 114, 907–910 (1993).
Leach, R. E., Burns, J. W., Dawe, E. J., SmithBarbour, M. D. & Diamond, M. P. Reduction of postsurgical adhesion formation in the rabbit uterine horn model with use of hyaluronate/carboxymethylcellulose gel. Fertil Steril. 69, 415–418 (1998).
Hellebrekers, B. W., Trimbos-Kemper, G. C., van Blitterswijk, C. A., Bakkum, E. A. & Trimbos, J. B. Effects of five different barrier materials on postsurgical adhesion formation in the rat. Hum Reprod. 15, 1358–1363 (2000).
Dinarvand, P. et al. Function of poly (lactic-co-glycolic acid) nanofiber in reduction of adhesion bands. J Surg Res. 172, e1–9 (2012).
FDA. USAdept Adhesion Reduction Solution (4% Icodextrin)-P050011. In United States FDA (2006).
Okabayashi, T. et al. Mobilization of host stem cells enables long-term liver transplant acceptance in a strongly rejecting rat strain combination. Am J Transplant. 11, 2046–2056 (2011).
Hu, X. et al. Chimeric allografts induced by short-term treatment with stem cell-mobilizing agents result in long-term kidney transplant survival without immunosuppression: A study in rats. Am J Transplant. 16(7), 2055–2065 (2016).
Cameron, A. M. et al. Chimeric allografts induced by short-term treatment with stem cell-mobilizing agents result in long-term kidney transplant survival without immunosuppression: II, study in miniature swine. Am J Transplant. 16(7), 2066–2076 (2016).
Lin, Q. et al. Pharmacological mobilization of endogenous stem cells significantly promotes skin regeneration after full-thickness excision: the synergistic activity of AMD3100 and tacrolimus. J Invest Dermatol. 134(9), 2458–2468 (2014).
Zhai, R. et al. Pharmacological mobilization of endogenous bone marrow stem cells promotes liver regeneration after extensive liver resection in rats. Sci Rep. 8(1), 3587 (2018).
Liekens, S., Schols, D. & Hatse, S. CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell mobilization. Curr Pharm Des. 16(35), 3903–3920 (2010).
Kavanagh, D. P. & Kalia, N. Hematopoietic stem cell homing to injured tissues. Stem Cell Rev. 7(3), 672–682 (2011).
Liu, H. J. et al. Adenoviral-mediated gene expression of hepatocyte growth factor prevents postoperative peritoneal adhesion in a rat model. Surgery. 140, 441–447 (2006).
Naiki, Y., Matsuo, K., Matsuoka, T. & Maeda, Y. Possible role of hepatocyte growth factor in regeneration of human peritoneal mesothelial cells. Int J Artif Organs. 28(2), 141–149 (2005).
Yang, J., Dai, C. & Liu, Y. Systemic administration of naked plasmid encoding hepatocyte growth factor ameliorates chronic renal fibrosis in mice. Gene Ther. 8, 1470–1479 (2001).
Mills, C. D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 32(6), 463–488 (2012).
diZerega, G. S. The cause and prevention of postsurgical adhesions: a contemporary update. Prog Clin Biol Res. 381, 1–18 (1993).
Mutsaers, S. E. & Wilkosz, S. Structure and function of mesothelial cells. Cancer Treat Res. 134, 1–19 (2007).
Liakakos, T., Thomakos, N., Fine, P. M., Dervenis, C. & Young, R. L. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg 4, 260–273 (2001).
Hellebrekers, B. W. J. & Kooistra, T. Pathogenesis of postoperative adhesion formation. Br J Surg 98, 1503–1516 (2011).
Fortin, C. N., Saed, G. M. & Diamond, M. P. Predisposing factors to post-operative adhesion development. Human Reproduction Update. 21(4), 536–551 (2015).
Reed, K. L. et al. A neurokinin-1 receptor antagonist that reduces intra-abdominal adhesion formation decreases oxidative stress in the peritoneum. Am J Physiol Gastrointest Liver Physiol. 293(3), G544–G551 (2007).
Lucas, P. A. Stem cells for mesothelial repair: an understudied modality. Int J Artif Organs. 30(6), 550–556 (2007).
Mutsaers, S. E., Prêle, C. M., Pengelly, S. & Herrick, S. E. Mesothelial cells and peritoneal homeostasis. Fertil Steril. 106(5), 1018–1024 (2016).
Lucas, P. A., Warejcka, D. J., Zhang, L. M., Newman, W. H. & Young, H. E. Effect of rat mesenchymal stem cells on development of abdominal adhesions after surgery. J Surg Res. 62, 229–232 (1996).
Wang, N. et al. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS One. 7, e43768 (2012).
Wang, N. et al. Novel mechanism for mesenchymal stem cells in attenuating peritoneal adhesion: accumulating in the lung and secreting tumor necrosis factor α-stimulating gene-6. Stem Cell Res Ther. 3, 51 (2012).
Maciver, A. H., McCall, M. & James Shapiro, A. M. Intra-abdominal adhesions: cellular mechanisms and strategies for prevention. Int J Surg. 9(8), 589–94 (2011).
Matoba, M. et al. Prevention of Polyglycolic Acid-Induced Peritoneal Adhesions Using Alginate in a Rat Model. Biomed Res Int. 2015, 403–413 (2015).
D’Angelo, F. et al. Macrophages promote epithelial repair through hepatocyte growth factor secretion. Clin Exp Immunol. 174(1), 60–72 (2013).
Ohashi, K. et al. Interferon γ and plasminogen activator inhibitor 1 regulate adhesion formation after partial hepatectomy. Br J Surg. 101(4), 398–407 (2014).
Warn, R. et al. HGF/SF induces mesothelial cell migration and proliferation by autocrine and paracrine pathways. Exp Cell Res. 267, 258–266 (2001).
Bai, L. et al. Low- but not high-dose FK506 treatment confers atheroprotection due to alternative macrophage activation and unaffected cholesterol levels. Thromb Haemost. 104(1), 143–150 (2010).
Spiekerkoetter, E. et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 123(8), 3600–3613 (2013).
Peiffer, B. J. et al. Activation of BMP signaling by FKBP12 ligands synergizes with inhibition of CXCR4 to accelerate wound healing. Cell Chem Biol., https://doi.org/10.1016/j.chembiol.2019.01.011 (2019).
Martínez, V. G. et al. BMP4 Induces M2 Macrophage Polarization and Favors Tumor Progression in Bladder Cancer. Clin Cancer Res. 23(23), 7388–7399 (2017).
Anderson, P. et al. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 62(8), 1131–1141 (2013).
Chiossone, L. et al. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells. 34(7), 1909–1921 (2016).
Tondreau, T. et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 23(8), 1105–12 (2005).
Chen, K. S. et al. Potential role of bone marrow-derived cells in the turnover of mesothelium. Ren Fail. 32, 1081–1087 (2010).
Zü¨hlke, H. V., Lorenz, E. M. P., Straub, E. M. & Savvas, V. Pathophysiologieund klassifikation von adhäsionen. [Pathophysiology and classification of adhesions] [Article in German]. Langenbecks Arch Chir SupplII Verh Dtsch Ges Chir. 345, 1009–1016 (1990).
Mazuji, M. K., Kalambaheti, K. & Pawar, B. Prevention of adhesionswith polyvinylpyrrolidone. Arch Surg. 89, 1011–1015 (1964).
Tachibana, S. et al. Interleukin-6 is required for cell cycle arrest and activation of DNA repair enzymes after partial hepatectomy in mice. Cell Biosci. 4(1), 6 (2014).
Acknowledgements
This work was supported by a start-up fund from surgical department at Johns Hopkins.
Author information
Authors and Affiliations
Contributions
K.I. and Z.S. designed the study. K.I., A.R.A. and L.Q. performed experiments. K.I., M.C., W.W., K.K., A.T., J.B., A.M.C. and Z.S. analyzed data. Z.S. supervised the study and Z.S. wrote the manuscript. J.B. edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iwasaki, K., Ahmadi, A.R., Qi, L. et al. Pharmacological Mobilization and Recruitment of Stem Cells in Rats Stops Abdominal Adhesions After Laparotomy. Sci Rep 9, 7149 (2019). https://doi.org/10.1038/s41598-019-43734-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43734-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.