Abstract
Despite the highest global burden of malaria, information on bionomics and insecticide resistance status of malaria vectors is grossly lacking in the densely populated Sahelo-Sudanian region of Nigeria. To support evidence-based vector control we characterised transmission and resistance profiles of Anopheles coluzzii populations from three sites in northern Nigeria. High sporozoite infection (~19.51%) was found in the An. coluzzii populations. A high pyrethroid resistance was observed with only 1% mortality against deltamethrin, a high LD50 (96.57 µg/ml), and a high LT50 (170.27 min, resistance ratio of ~51 compared with the fully susceptible Ngoussou colony). Moderate carbamate resistance was observed. Synergist bioassays significantly recovered deltamethrin susceptibility implicating CYP450s (mortality = 85%, χ2 = 134.04, p < 0.0001) and esterases (mortality = 56%, χ2 = 47.31, p < 0.0001). Reduced bed net efficacy was also observed, with mortalities on exposure to the roof of PermaNet3.0 (PBO + deltamethrin) more than 22 times compared to the side panel (deltamethrin). TaqMan genotyping revealed a high frequency of 1014F kdr mutation (82%) with significant difference in genotype distribution associated with permethrin resistance [OR = 4.69 (CI:1.53–14.35, χ2 = 8.22 p = 0.004]. Sequencing of exons 18–21 of the VGSC led to detection of two additional nonsynonymous mutations, Ile10148Asn and Ser1156Gly. These findings highlight the threats posed by the highly resistant An. coluzzii to malaria control in Nigeria.
Similar content being viewed by others
Introduction
The WHO has set the ambitious goal to reduce global burden of malaria by preventing 90% of malaria-related cases and mortalities by 20301. However, this gradual process is already facing serious setbacks, especially in the WHO African Region which accounts for ~91% of all malaria-related deaths2. The recent rebound in malaria transmission with observed increase in cases, as seen in 2016 and 20172,3 is a warning of the risk posed to control and elimination efforts, and suggests that primary regions of interests for pre-elimination needs further attention. Sahelo-Sudanian regions such as northern Nigeria, with high seasonal malaria transmission4 are important for monitoring to provide sufficient evidences to support elimination. Nigeria, with the highest burden of malaria, alone account for 27% of global malaria burden; with still increased case incidence between 2010 to 20172,3. Indeed, the Nigerian National Malaria Control Programme (NMCP) Strategic Plan 2014–2020 reported that malaria was responsible for an estimated 20% of under-5 mortality5. Elimination efforts will have no chance of succeeding if significant progress is not made in key control areas (like Nigeria) bearing the highest burden of malaria. Unfortunately, control efforts in Nigeria are relatively poor and ill-guided, notably for vector control. Various factors contribute to the failure to make progress in Nigeria. These include a disproportionately low reports of cases through surveillance system (only 8%) and lower coverage of usage for Long-Lasting Insecticidal Nets (LLINs) (60% vs >80% reported for some West African countries, e.g. Senegal, Ghana and Mali)2,6. Also, the core malaria intervention tools (e.g. LLINs) are deployed across different eco-epidemiological regions, especially in northern Nigeria without prior knowledge of the composition of the indigenous malaria vector species, let alone their contribution to malaria transmission, their behaviour and insecticide susceptibility status. This lack of information is preventing implementation of evidence-based control strategies. The paucity of information on dominant vector species (DVS) is most extreme in the Sahelo-Sudanian region of the country. With the exception of few focal studies7,8 reliable information on the DVS from northern Nigeria is grossly lacking for decades following the comprehensive study conducted in Garki9. This is in stark contrast to the situation in southern part of Nigeria (especially the south-west), where significant progress on malaria vector bionomics has been achieved10,11,12,13.
Over-reliance on the pyrethroids (the only class of insecticides approved by WHO for impregnation of LLINs [http://www.who.int/whopes/en/]) alone has exposed malaria vectors to intense selection with pyrethroid resistance fast becoming the norm across Africa14. In the absence of new insecticide to substitute for the pyrethroids it becomes necessary to ensure that the current insecticides used for LLINs remain effective for as long as possible. However, this could only be achieved through management of insecticide resistance and innovative vector control approaches. For example, (i) tailoring control programs to the vector species involved in transmission15; (ii) monitoring insecticide resistance in the major malaria vectors to track changes over time and between areas2; (iii) establishing molecular markers of insecticide resistance to detect and track its spread to inform control programs16 and (iv) evaluating the efficacy of approved major vector control tools.
Prior to this study, in Sahelo-Sudanian regions of Nigeria, contribution of the major malaria vectors to transmission of malaria parasite has not been established comprehensively; though the Sahelo-Sudanian regions (with a high seasonal transmission) offer excellent targets for the malaria elimination through seasonal vector control and chemoprevention4. To help boost chances of malaria control in Sudan/Sahel regions and contribute to its elimination agenda, this study provided a comprehensive characterisation of a major malaria vector from three separate localities spanning Sudan and Sahelian regions of northern Nigeria, including species composition, their role in malaria transmission, insecticide resistance profile and the underlying molecular mechanisms driving the resistance in the field.
Methods
Study sites and mosquito collection
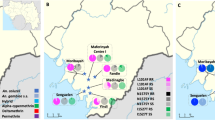
Blood fed female Anopheles mosquitoes resting indoor were collected using Improved Prokopack battery-operated aspirators (John. W. Hock, Florida, USA), from randomly selected houses, in the early morning hours (5:30 am–6:30) in three separate localities in northern Nigeria (Fig. 1): (i) Sahelo-Sudanian Savannah of Hadiyau village (12°21′38″N, 9°59′15″E) in Auyo Local Government, Jigawa State, where rice irrigation is practiced year round; (ii) Ladanai, a locality within the Sudan Savannah of Kano City (11°58′17″N, 8°35′9″E), characterised by a large Anopheles breeding site, in running stream used for industrial production of cement blocks; and (iii) Batagarawa town, Batagarawa Local Government in the Sahel Savannah of Katsina State (12°54′17″N, 7°37′11″E). Collections were conducted at Ladanai and Batagarawa for 3 days each, in the first week of the month of August in 2017, coinciding with peak malaria transmission season4. For Hadiyau, indoor collection was done for 6 days in the second week of August. The blood fed females were maintained on 10% sugar at 25 °C ± 2 and 70–75% relative humidity for 6 days, until fully gravid. They were then transferred into 1.5 ml tubes individually and forced to lay eggs, as described previously17. All F0 parents were identified as belonging to An. gambiae complex using morphological keys18 or as Culex species. After SINE200-PCR confirmation of the F0 parents as An. coluzzii19, DNA from subset of Hadiyau parents were transported to the Liverpool School of Tropical Medicine (LSTM), UK, under the DEFRA license (PATH/125/2012,) for downstream molecular analyses. Egg batches were transferred into paper cups for hatching in insectary at Bayero University Kano, Nigeria. Hatched eggs were pooled into larvae bowls and supplemented with TetraminTM baby fish food. 2- to 4-days old F1 females that emerged were randomly mixed in cages and used for subsequent bioassay experiments.
To establish indoor and outdoor resting densities of the Anopheles, infection with Plasmodium and source of blood meal, pyrethrum spray collection (PSC) and human landing catches (HLCs) were also conducted during the collection at Hadiyau. Procedures followed protocols as advised by the WHO20. PSC was conducted for 1 day in 13 randomly selected houses in early morning hours when occupants in houses were up (6:30 am–7:00 am). Female residents in the selected houses were trained on how to do collection and provided with the materials required. HLC was conducted by 2 adult male participants. Clearance for HLC was obtained from Ethical Sub-committee, Operational Research Advisory Committee, Ministry of Health, Kano, with reference number MOH/off/797/TI/402. Partial collection was done between 7:00 pm–11:00 pm, for 2 days. Collectors were aware of the risk associated with the HLC and were provided with appropriate prophylactic regimen of doxycycline. Mosquitoes were sorted according to morphological keys as either belonging to An. gambiae complex18 or as Culex species, and stored individually in PCR strips supplemented with silica gel.
Species Identification to molecular level
Following morphological identification, genomic DNA was extracted, respectively from 864, 344, and 301 F0 female Anopheles from Hadiyau, Ladanai and Batagarawa, which laid eggs successfully. DNA extraction was performed following the protocol of LIVAK21. Species identification to the molecular level was carried out using the SINE200 PCR19. For all An. gambiae s.l. collected from PSC and HLC PCR identification was also carried out as explained above after morphological identifications.
Estimation of entomological and parasitological parameters of transmission
Indoor resting densities and outdoor host-seeking behaviours
The Anopheles indoor resting density (IRD) was calculated from the number of Anopheles caught using the PSC relative to the number of houses sampled, following the procedures outlined by WHO20,22. For the outdoor feeding behaviour man landing frequency (MLF) was calculated from the number of Anopheles captured while landing on the 2 volunteers in the 2 days of collection22.
Human blood index and biting rate
To establish anthrophophilic index, blood-fed female An. gambiae s.l. collected from Hadiyau, using PSC were dissected <48 hours after collection to separate heads and thoraces from the abdomens. 143 blood fed females were dissected, heads/thoraces and abdomens prepared for DNA extraction as outlined in WHO guidelines20. DNA was extracted from these head/thoraces and abdomens using the DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany) according to manufacturer’s protocol. SINE200 PCR protocol was utilised first to establish the species identity. The human blood index was established from the proportion of blood fed Anopheles that have fed on humans relative to the total number of blood fed female Anopheles caught, following a cocktail PCR of Kent and Norris et al.23. Human biting rate was also estimated from the number of human blood fed mosquitoes relative to the total number of occupants in the houses.
Estimation of sporozoites infection rate and entomological inoculation rate
93 blood fed female An. coluzzii collected indoor using PSC, and 37 females which laid eggs (collected with aspirators) were used to detect sporozoite with a TaqMan genotyping approach previously described24. Real-time PCR MX 3005 (Agilent, Santa Clara, USA) was used for the amplification. 1 μl of gDNA extracted from each female head/thorax was used as a template for PCR, with an initial denaturation at 95 °C for 10 min, followed by 40 cycles each of 15 sec at 95 °C and 1 min at 60 °C. Primers described by Bass24 were used together with two probes labelled with fluorophores, FAM to detect Plasmodium falciparum, and HEX to detect combination of P. ovale, P. vivax and P. malariae. Positive controls (known FAM+ and OVM+) were used in addition to a negative control in which 1 μl of dH20 was added. To validate findings of the TaqMan assay a nested PCR of Snounou and colleagues25 was carried out using all the TaqMan-positive samples. Sporozoite rate was calculated as percentage of mosquitoes with sporozoites relative to the total number of the females examined20 and entomological inoculation rate was estimated from the sporozoite rate and human-biting rate, as previously described26.
WHO insecticide susceptibility bioassays
Insecticide susceptibility bioassays were performed following the WHO protocol27 with the insecticides from four major public health classes. At least four replicates of 20–25 adult F1 female Anopheles per tube were used for each insecticide, alongside 25 unexposed females (control). To confirm the efficacy of the papers, the fully susceptible An. coluzzii (Ngoussou strain)28 was tested first or simultaneously with the experimental populations. For Hadiyau, 11 insecticides were tested, including: (i) the type I pyrethroid: permethrin (0.75%); (ii) the type II pyrethroids: deltamethrin (0.05%), λ-cyhalothrin (0.05%), α-cypermethrin (0.05%) and cyfluthrin (0.15%); (iii) the organochlorine: DDT (4%); (iv) the carbamates: bendiocarb (0.1%) and propoxur (0.1%); and (v) the organophosphates: malathion (5%), pirimiphos-methyl (0.25%), chlorpyrifos-methyl (0.4%) and fenitrothion (1%, with 2 hr exposure29). For Ladanai populations, permethrin, deltamethrin, DDT, bendiocarb and malathion were tested; while for Batagarawa populations, permethrin, deltamethrin, DDT, bendiocarb and pirimiphos-methyl were tested. Availability of F1 females raised to adulthood successfully accounted for the differences seen in the number of insecticides tested for the different sites. In all the three populations knockdown rates were recorded for permethrin, deltamethrin and DDT during the exposure, in intervals of 15 min, 30 min, 45 min and 1 hr. After 1 hr exposure mosquitoes were transferred to holding tubes and supplied with 10% sugar. Mortality was scored 24 hr after exposure. Populations were considered susceptible to an insecticide where mortality was >98%, suspected to be resistant (moderately resistant) where mortality is between 90–98%, and resistant where mortality was found to be <90%27.
Synergist bioassays
To establish the potential insecticide detoxification enzyme systems responsible for resistance synergist bioassays were conducted with 2–5 days old F1 females from Hadiyau with 4% piperonyl butoxide (PBO: an inhibitor of CYP450s and carboxylesterases30), and with 0.25% S,S,S-tributylphosphorotrithioate (DEF: an inhibitor of carboxylesterases)31 against 0.05% deltamethrin. In addition, potential role of glutathione S-transferases (GSTs) in DDT resistance was also investigated by pre-exposure to 8% diethyl maleate (DEM). About 20–25 females were initially pre-exposed to a synergist for 1 hr and then transferred to tubes containing deltamethrin or DDT, for 1 hr27. Mosquitoes were treated as in conventional bioassays described above and mortalities scored after 24 hr. Two controls were set up: (i) 25 females exposed to only control paper with neither synergist, nor any insecticide; and (ii) 25 females exposed to PBO only.
Efficacy of insecticide-treated nets using Cone bioassays
The efficacy of PermaNet 3.0 (a PBO-deltamethrin combination bed net) supplied by Vestergaard (https://www.vestergaard.com/permanet-3-0) was tested with Hadiyau females using the WHO cone bioassay protocol32 with modification in the number of mosquitoes per replicate. Five different sections of the net, each measuring 30 × 30 cm were cut separately for both the roof (PBO + deltamethrin) and the side (deltamethrin only) of the PermNet 3.0. Five females were introduced into each of the 4 cones fixed onto the piece of net, making a total of 20 females per experiment. This was then replicated 5 times exposing the mosquitoes in each round for only 3 min. The females were then transferred to holding paper cups, supplied with sugar and allowed to rest for 24 hr before mortality was scored. Controls comprise 8 replicates each of 5 females in two pieces of untreated nets. In addition, 8 replicates each of 5 females of the lab susceptible Ngoussou colony were also used as positive control.
Estimation of resistance intensity with time-course and dose-response bioassays
To establish the strength of pyrethroid resistance with time, additional bioassays were performed with 0.05% deltamethrin, using the Hadiyau population. Deltamethrin was chosen due to its wide use in pyrethroid bed nets, and because the Hadiyau populations exhibited the highest resistance to it. Cohorts of 20–25 F1 females in 4 replicates were exposed in time-course bioassays for 60, 120, 180, 240 and 300 min to establish the time required to kill 50% of the females (LT50). Protocols were as described above in conventional bioassays, except for variation in time. The fully susceptible Ngoussou colony were also exposed to discriminating concentration of deltamethrin in time-course bioassays spanning 2.5, 5, 10, 15, 30, 45 and 60 min, and LT50 calculated. Resistance intensity was established by comparing the calculated LT50 from the Hadiyau populations to that of the Ngoussou.
The diagnostic dose which kills 50% (LD50) of the F1 females from Hadiyau was also established using the CDC bottle bioassays. This was conducted with ranges of deltamethrin concentration starting from 200 µg/ml, serially diluted 2-fold into 100, 50, 25, 12.5 (the discriminating dose in the conventional CDC protocol33), and 6.255 µg/ml, respectively. 4 replicates of 20–25 F1 females were used for each concentration. Mosquitoes were exposed for 1 hr, then transferred into holding cups and supplied with 10% sucrose. Mortality was recorded at 24 hr.
Polymorphism analysis of the voltage-gated sodium channel
Genotyping of L1014F and L1014S kdr mutations
To assess the frequency of the kdr mutations in the Hadiyau An. coluzzii, DNA from 31 blood fed F0 females, indoor-collected and which laid eggs successfully were randomly selected and screened with TaqMan real-time PCR assay (using Agilent Mx3005 qRT-PCR thermocycler). These females were genotyped for the presence of 1014F and 1014S kdr mutations following the protocols established by Bass and colleagues34,35. In a total volume of 10 µl comprise of 5 µl Sensimix (Bioline), 0.25 µl of 40x Probe Mix coupled to allelic-specific primers, 4.25 µl of dH20, and 1 µl of genomic DNA was added. Thermocycling conditions were initial 10 min at 95 °C, followed by 40 cycles each of 92 °C for 15 sec, and 60 °C for 1 min. Two probes labelled with fluorochromes FAM and HEX were utilised to detect the mutant alleles and the wild type susceptible alleles, respectively. Genotypes were scored from scatter plots of results produced by the Mx3005 v4.10 software. Three positive samples of known genotypes: (i) homozygote resistant for 1014F or 1014S kdr, (ii) heterozygote for 1014F or 1014S kdr, and (iii) susceptible L1014 were added as positive controls for each of the two experiments to determine the kdr-West or kdr-East, respectively. 1 µl of dH20 was added to the negative control well.
To assess the relation between the kdr genotypes and pyrethroids resistance phenotypes, 92 permethrin resistant individuals and 18 susceptible individuals were genotyped for the presence of 1014F and 1014S kdr mutations. Protocol was as explained above. However, with no mortalities obtained from conventional bioassays with deltamethrin and with only 7 individuals dead from exposure with permethrin, bioassays were repeated with permethrin to get a minimum of 16 susceptible females. Correlation between the L1014F kdr genotypes and permethrin resistance phenotype was assessed by estimating the odds ratio (OR) and the statistical significance based on Fisher exact probability test.
Sequencing of exons 18–21 of the voltage-gated sodium channel for novel kdr mutations
To detect potential mutations in the para-type sodium channel, a fragment of exons 18–21 (harbouring the 1014 kdr mutation in exon 20 of resistant populations) from the Hadiyau population and the Ngoussou strain were amplified and sequenced. Briefly, RNA was extracted from 5 batches each of 10 deltamethrin-resistant Hadiyau mosquitoes and from Ngoussou strain using the PicoPure RNA isolation Kit (Arcturus, Applied Biosystems, USA). cDNA was synthesized from extracted RNA using SuperScript III (Invitrogen, USA) with oligo-dT20 and RNAse H (New England Biolabs, USA). Amplification was carried out using the following primer pair COLZkdrEx18F: ATAATGTGGATAGATTCCCCG, and COLZkdrEx21R: CTTCGTCAATTCCTAGATCT. To 12.5 μl of the 2x AccuStartII PCR SuperMix (QuantaBio, Beverly, Massachusetts) containing optimised concentrations of MgCl2 and dNTP mixes, 0.2 μM each of the forward and reverse primer was added, followed by 1 μl cDNA. 10.5 μl of dH20 was added to obtain a total volume of 25 μl. Amplification was carried out using the following conditions: initial denaturation of one cycle at 94 °C for 3 min; followed by 35 cycles each of 94 °C for 30 sec (denaturation), 60 °C for 30 s (annealing), and extension at 72 °C for 1 min; and one cycle at 72 °C for 5 min (final elongation). PCR products (~750 bp) were cleaned individually with QIAquick® PCR Purification Kit (QIAGEN, Hilden, Germany) and cloned into pJET1.2/blunt according to manufacturer’s protocol (ThermoFisher Scientific, MA, USA). These were then used to transform the Escherichia coli DH5α, plasmids miniprepped with the QIAprep® Spin Miniprep Kit (QIAGEN, Hilden, Germany) and sequenced on both strands using the pJET1.2 sequencing primers.
Polymorphisms were detected through manual examination of sequence traces using BioEdit version 7.2.3.036 and CLC sequence viewer (QIAGEN, Hilden, Germany). Sequences have been deposited in GenBank for accession numbers.
Statistical analyses
R version 3.5.0 (https://cran.r-project.org/bin/windows/base/) was utilized for statistical analyses to calculate Odds Ratio (epiR package) for the 1014F kdr genotyping, and for the estimation of LT50 and LD50 probit analyses (glm with MASS package). Result of mortalities from synergist-insecticide exposure and temporal variation in pyrethroid resistance were compared with those obtained from exposure to synergists alone using a two-tailed Chi-Square test of independence as implemented in GraphPad Prism 7.02 (GraphPad Inc., La Jolla, CA, USA).
Results
Mosquito species distribution and composition
All the F0 female Anopheles collected indoor using aspirators and PSC, as well as outdoor from HLC were An. coluzzii. For all the 3 sites, 4,276 mosquitoes were caught indoor. These comprise 3,491 Anopheles: 2,661 blood fed females, 637 unfed and 193 males; and 785 Culex. Of the Culex, 568 were blood fed females, 127 unfed, and 90 were males (Supplementary Fig. S1b). 2,263 of all the An. coluzzii were collected from Hadiyau, with ~50% of them (1,736) blood-fed (Supplementary Fig. S1a). In contrast, 226 Culex mosquitoes were caught from Hadiyau, 131 blood-fed females and 52 unfed females (Supplementary Fig. S1b). For Ladanai, An. coluzzii constitutes 18% of the total mosquitoes caught from all 3 sites, with 508 blood-fed and 107 unfed (Supplementary Fig. S1a). Ladanai Culex constitutes 17% of the total Culex species caught from all sites, with 88 blood-fed females and 21 unfed (Supplementary Fig. S1b). The greatest proportion of Culex mosquitoes were caught at Batagarawa (424 in total), 349 of them blood-fed and 54 unfed (Supplementary Fig. S1b). 559 An. coluzzii were caught at Batagarawa, of which 417 were blood-fed and 114 were unfed.
A total of 225 mosquitoes were caught from PSC, 196 of them An. coluzzii (143 blood-fed females, 38 unfed, and 15 males), while 29 Culex were also recovered (18 blood-fed females, 7 unfed females and 4 males). Of the 113 female mosquitoes captured with the HLC 96 were An. coluzzii and 17 of them Culex.
Entomological and parasitological parameters of transmission
Indoor and outdoor resting densities
Indoor resting density of Hadiyau An. coluzzii was calculated from the 181 females collected in 8 houses as 22.625 female Anopheles per room. For outdoor, the 2 collectors caught 52 and 44 female Anopheles respectively, in two days. The man-landing frequency (MLF) of the An. coluzzii females was estimated as 24 (CI: 20–28).
Human blood index and biting rate (Anthropophilic index)
DNA extracted from 143 blood fed females collected using PSC was analysed for blood source. 139 of the abdomens contained human blood, 3 abdomens contained cow blood and only one of abdomen contained blood from a goat. The human blood index was found to be very high; 97.2% of the foraging female An. coluzzii have fed on humans. The human biting rate was calculated as ~2.05 (CI: 0.88–3.52) bites per person per night.
Estimation of sporozoites infection rate and entomological inoculation rate (EIR)
A total of 130 DNA-extracted heads/thoraces (93 from PSC-caught An. coluzzii, and 37 from females that have laid eggs) were used to establish infection with Plasmodium. 25 heads/thoraces were found to be infected with P. falciparum (F+ = 25), corresponding to a sporozoite rate of 19.51 (CI: 9.76–29.25). One additional sample tested positive for P. vivax/malariae/ovale. The EIR was calculated from the P. falciparum sporozoite rate and human biting rate as 0.39 ± 0.21 infective bites per person per night. Validation of the TaqMan results using nested PCR25 confirmed infection in 25 samples, including the non-falciparum sample which turn out to be P. vivax.
WHO insecticide susceptibility bioassays
2,084 F1 females were utilised for the conventional WHO bioassays: 1,136 from Hadiyau, 486 from Batagarawa, and 462 from Ladanai. Overall, knockdown was very low for pyrethroids and DDT, and increased with exposure time. However, mosquitoes were least knocked down by DDT, followed by deltamethrin, compared to permethrin (Supplementary Fig. S2). Hadiyau populations exhibited the lowest, but not significantly different knockdown with permethrin, with effect at 1 hr of exposure one-third the values obtained for the Batagarawa and Ladanai populations (χ2 = 2.59, df = 1, p = 0.10, compared to Batagarawa; and χ2 = 3.06, df = 1, p = 0.08 compared to Ladanai). An exception in the trend was observed with cyfluthrin tested against Hadiyau populations only (Supplementary Table S1). Exposure to this type II pyrethroid inflicted increased knockdown, reaching ~30% at 45 min, and ~40% at 1 hr. These differences were statistically significant compared to the other two type II pyrethroids, deltamethrin (χ2 = 31.8, df = 1, p = 1.73 × 10−8) and λ-cyhalothrin (χ2 = 30.1, df = 1, p = 0.001) tested with the Hadiyau population.
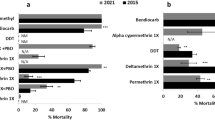
The fully susceptible Ngoussou strain exhibited 100% mortalities against all insecticides tested. Field An. coluzzii populations were highly resistant to type I and type II pyrethroids, except for cyfluthrin which was tested only with Hadiyau populations (Fig. 2a). For permethrin, lowest mortality was observed with Hadiyau populations, at 7% (95%, CI: 4–11), compared to 11% (CI: 7–15) and 12% (CI: 8–16) observed with Batagrawa and Ladanai populations. Lowest mortality was obtained with Hadiyau populations exposed to deltamethrin (1%, CI: 0–1), in contrast to 10% (CI: 8–13) and 18% (CI: 13–23) obtained with Batagarawa and Ladanai populations, respectively. Lowest resistance was also recorded in Hadiyau populations exposed to type II pyrethroids λ-cyhalothrin (1%, CI: 0–1) and α-cypermethrin (14%, CI: 9–19). Cyfluthrin exhibited the highest toxicity of all pyrethroids tested, with a mortality of 86% (CI: 78–93) in Hadiyau populations. For all the three sites, level of mortality from DDT exposure was comparable, on average less than 20%.
Resistance profiles of F1 An. coluzzii females. (a) Results of WHO bioassays with insecticides from different classes. Results are average of percentage mortalities from 4 replicates each ± SEM. (b) Effect of pre-exposure to synergists PBO and DEF against deltamethrin, and with DEM against DDT. Results are average of percentage mortalities from 4 replicates each ± SEM. Control 1: mosquitoes that were neither exposed to any synergist nor any insecticide; Control 2: mosquitoes exposed to PBO only. ***=statistically significant at p < 0.0001 from two tailed χ2 square test of independence, between results from synergists bioassay and conventional bioassays with deltamethrin. (c) Results of cone bioassays with PermaNet 3.0. Ngoussou (+control) exposed to the side panels of PermaNet 3.0 only. Results are average of percentage mortalities ± SEM.
In all the three sites mosquito populations were more sensitive to carbamate and organophosphate insecticides compared to pyrethroids and DDT. For example, in all three sites reduced mortalities were observed towards bendiocarb, ranging from 91% in Hadiyau (CI: 87–95) and Ladanai (CI: 84–98), to 93% in Batagarawa (CI: 88–98) (Fig. 2a). Hadiyau populations exhibited moderate mortalities to propoxur, another carbamate (86% mortality, CI: 75–96). Except for chlorpyrifos-methyl [moderate resistance in Hadiyau populations (90% mortality, CI 88–92)], full susceptibility was observed with the organophosphate insecticides, with mortalities in range of 98% (CI: 94–102) with fenitrothion for Hadiyau populations, to 99–100% for malathion and pirimiphos-methyl, in all the three respective populations tested (Fig. 2a).
Estimation of intensity of resistance
To establish levels of resistance WHO tube bioassays were conducted with deltamethrin varying exposure times from 60 min to 300 min. The LT50 of Hadiyau An. coluzzii populations was estimated as 170.27 min [95% CI: 159.59–180.96, Fiducial] (Supplementary Fig. S3a). The LT50 for the Ngoussou colony was estimated as 3.320 min [CI: 2.67–3.97] (Supplementary Fig. S3b). The resistance ratio between Hadiyau populations and the Ngoussou was calculated as 51.28.
Strength of pyrethroid resistance was also estimated by varying the concentrations of deltamethrin from 6.25 µg/ml through to 200 µg/ml in a CDC bottle bioassay (Supplementary Fig. S3c). LD50 was very high, estimated as 96.57 µg/ml [95% CI: 85.59–107.55, Fiducial].
Investigating the potential role of metabolic resistance using synergist bioassays
To investigate the possible role of metabolic enzyme systems in the observed resistance, bioassays were conducted with three synergists PBO and DEF against deltamethrin, as well as DEM against DDT. A very high recovery of susceptibility was obtained from Hadiyau populations from pre-exposure to PBO and DEF against deltamethrin, with mortalities on average 85 times and 56 times the values obtained in conventional bioassays without the synergists (Fig. 2b). Two-tailed test of independence indicated that the associations between the mortality and PBO pre-exposure are highly significant (χ2 = 134.104, df = 1, p < 0.0001). The same statistical significance was also obtained with pre-exposure to DEF (χ2 = 47.31, df = 1, p < 0.0001). These support the possible role of P450 monooxygenases and esterases in the observed deltamethrin resistance. No recovery of mortalities was seen on pre-exposure to DEM followed by DDT. No mortality was observed with control 1 (exposed to neither synergists nor insecticides) and control 2 (exposed to PBO only).
Determination of insecticide-treated bed nets efficacy
To evaluate the efficacy of conventional and combination long-lasting bed nets, cone bioassays were conducted. Initially, both the roof and side of the PermaNet 3.0 were used against the fully susceptible Ngoussou An. coluzzii, with 100% mortality after 24 hr. Hadiyau populations were then tested with the net. A low efficacy was observed with the side of the net (impregnated with deltamethrin only) with an average knockdown of only 3% ± 0.4, and average mortality of only 4% ± 0.88 in 24 hr (Fig. 2c). In contrast, exposure to the roof of the net (deltamethrin + PBO) resulted in significant recovery of susceptibility with average knockdown of 59% ± 4.8 and a 24 hr mortality of 87% ± 2.88. This mortality is on average 22-fold compared to the observation with the side of the net (χ2 = 133.9, df = 1, p < 0.0001).
Increased pyrethroid and DDT resistance Intensity in An. coluzzii populations
To determine the temporal changes in resistance levels in Hadiyau populations data published in 20147 (from collections done in 2009) and the results from this study were compared. Overall, increased pyrethroids and DDT resistance was observed. For example, in our study from 20147 we have established a percentage knockdown of about 30% respectively, for the type II pyrethroids λ-cyhalothrin and deltamethrin; and mortalities of 52% and 78%. In less than a decade the resistance has escalated with percentage knockdown of less than 5% in Hadiyau populations in 2017 and mortalities of about 1% for both λ-cyhalothrin (χ2 = 65.562, df = 1, p = 5.38 × 10−16) and deltamethrin (χ2 = 120.24, df = 1, p = 5.59 × 10−18 for deltamethrin) compared to 2009 populations. The DDT resistance has more than double, with mortalities of only 18% compared to 44.6% we have reported previously (χ2 = 15.586, df = 1, p = 0.0079). The only exception was the organophosphate malathion of which 99–100% mortalities were recorded between 2011–2017.
Polymorphism analysis of the voltage-gated sodium channel
Investigation of the role of 1014F and 1014S kdr mutations on pyrethroid resistance
To establish the frequency of kdr mutations in the field, 30 Hadiyau F0 females that laid eggs were randomly selected and genotyped for the L1014F and L104S kdr mutations. A high frequency of 1014F mutations were observed with 10 of the females (33.33%) scored as homozygote resistant (T/T), 15 as heterozygote (50% T/A), and only 5 (16.66%) females were susceptible (A/A) (Fig. 3a). The 1014F kdr genotype frequency was estimated as 0.83 in the wild populations (Fig. 3b). In contrast, no L1014S kdr mutation was observed in the field.
Polymorphism analyses of the Hadiyau An. coluzzii voltage-gated sodium channel. (a) Proportion of female An. coluzzii with 1014F kdr genotype; (b) the kdr frequency in female wild population. (c) Correlation between the L1014F kdr genotypes and permethrin resistance phenotype. The frequency of each genotype is plotted in each phenotype to indicate differences in survival between the genotypes (T/T: resistant kdr genotype; A/T: heterozygote; A/A: wild type susceptible). (d) Phylogenetic tree of the exons 18–21 fragment of Hadiyau populations vs Ngoussou cohort.
Additionally, 91 permethrin-resistant and 16 dead F1 females were successfully genotyped for 1014F and 1014S kdr mutations. As in the field high frequency of 1014F kdr mutation was obtained, with 29 females homozygote resistant (T/T), and 46 heterozygote (T/A) for the 1014F kdr mutation (Fig. 3c, Table 1). Only 16 of the resistant females were found to be homozygote susceptible (A/A). The 1014F kdr genotype frequency was established as ~0.82. The 1014S kdr mutation was not detected in these mosquitoes, as well. A significant correlation was observed between permethrin resistance and presence of 1014F mutation [Odds Ratio of 4.69 (95% CI: 1.53–14.35, χ2 = 8.219, p = 0.004)] (Table 1) when comparing the frequency of the resistance with susceptible allele in all alive and dead females. Similar association was observed in the RR females in relation to the SS females [OR of 4.83 (CI: 1.12–20.82, χ2 = 4.97, p = 0.026)] compared to the association observed between the latter (SS) and the heterozygote females (RS) [OR of 4.6 (CI: 1.31–16.12, χ2 = 6.31, p = 0.012)].
Sequencing of the Exons 18–21 fragments of the voltage-gated sodium channel for kdr mutations
A 747 bp fragment of the VGSC spanning exons 18–21 and encompassing the 1014 codon was amplified and sequenced. In addition to confirmation of the 1014F replacement (Fig. 4a,b) in the Hadiyau populations with respect to Ngoussou (TTA- > TTT), two additional non-synonymous mutations were discovered (Fig. 4c–f, Supplementary Fig. S4): (i) ATT in Ngoussou coding for isoleucine (Ile1048), replaced with AAT coding for asparagine (Asn1048) in Hadiyau populations; (ii) AGC in Ngoussou coding for Ser1156, replaced with GGC for Gly1156 in Hadiyau populations. However, the potential role of these mutations in pyrethroids resistance need further validation.
Discussion
The Sahel region characterised with high seasonal transmission offers excellent opportunity for control and elimination of malaria. Unfortunately, control efforts in these regions, especially Nigeria are very poor, partly due to lack of information on the bionomics and resistance profile of the dominant vector specie(s). To facilitate evidence-based control measures and resistance management we identified the major malaria vector in the Sahel/Sudan region of Nigeria, established its role in Plasmodium transmission, its resistance profile, and the possible underlying molecular mechanisms driving the resistance in the field.
The fact that An. coluzzii (the M molecular form of An. gambiae) was the only Anopheles species collected in the three separate sites in northern Nigeria, and within the rainy season (coinciding with the peak in malaria transmission) suggests that these species is the major malaria vector in this Sahelo-Sudanian region. Indeed, studies carried out decades ago have described An. gambiae s.l. as the major malaria vector in northern Nigeria37,38. Also, contrary to the previous observations that An. arabiensis tend to predominate in arid savannas whereas An. gambiae is the dominant species in humid forest zones39,40,41, the findings of An. coluzzii as the major malaria vector in two localities between 2009 and 2011 in northern Nigeria7 and the confirmation of its dominance in three separate sites in this study suggests that this malaria vector has probably adapted well in drier Sahel/Sudan region of Nigeria. In a study carried out less than 2 decades ago Awolola and colleagues42 have described An. gambiae s.s. (the S molecular form sibling species of An. gambiae) as the major malaria vector in the savannah.
Because information on malaria transmission indices in the Sudan/Sahel savannah of northern Nigeria is scanty it becomes necessary to compare our findings with studies carried 4–5 decades ago. The indoor resting density we have calculated for Hadiyau is higher in comparison to the findings of Garki project (IRD of 4.1–16)9. The human biting rate per person per night was however, lower than the range of man-biting rate reported in the Garki Project. The sporozoite rate we have founded was roughly 3 times higher than the rates White and Rosen (SR: 5.5% to 9.3%)43 have described in An. gambiae populations from Kaduna (a Sudan savannah of northern Nigeria). The Garki Project has described lower sporozoite rate of no more than 3% as well, and the entomological inoculation rate we have calculated was within the ranges described in the above project. Sporozoite rate of 11% has been described in An. gambiae s.l. populations from rainforest/swampy regions of south-eastern Nigeria44. Overall, the An. coluzzii populations from Hadiyau were highly endophilic and anthropophilic and pose a great risk as they have a very high infection rate.
Comparison of our findings with previous works suggests that insecticide resistance in northern Nigerian An. coluzzii has escalated in a few years. We have previously reported in 2014 An. coluzzii populations from two sites in the Sudan savanna of northern Nigeria exhibiting mortalities with pyrethroids in the range of between 52–78%7. Also, studies carried out in 2014 in northeast and south-west Nigeria8,12 has reported a high mortality with pyrethroids, and full susceptibility to carbamates and organophosphates. Our findings in this study suggest a dramatic rise in pyrethroid resistance in less than a decade; for all three sites mortalities for all pyrethroids, except for cyfluthrin were less than 20%, a four-fold decrease in mortality on average. In less than a decade these mortalities have become reduced to only 1% for the type II pyrethroids. Also, unlike previous studies, for the first time we have found resistance, though moderate to carbamate in the field populations from northern Nigeria, as well as possible resistance to the organophosphate, chlorpyrifos-methyl. The extremely high pyrethroid resistance observed in the Hadiyau populations is evident in its high LT50 with deltamethrin which is on average more than double the values described previously for the Tororo (Uganda) and Tiefora (Burkina Faso) resistant populations of An. gambiae, respectively with permethrin45. The LD50 we obtained with deltamethrin was also on the high side compared to data available from previous studies; it was on average three times the ranges described for the highly resistant VK7 An. gambiae populations from Burkina Faso46.
The high recovery of resistance when mosquitoes were pre-exposed to PBO and DEF indicates the potential role of P450 monoxygenases and carboxylesterases, respectively. Synergists are increasingly utilised for initial investigation of contribution of metabolic resistance in An. gambiae populations47,48. The results obtained with the synergists agrees with the outcome of the cone bioassay with low mortalities obtained with deltamethrin-impregnated side panels of the PermaNet 3.0 increasing 15-fold when compared with the top panel of the net. Differences in mortalities between the two sides of this LLIN has been recently reported in An. gambiae s.l.49 and An. funestus50. However, a single net utilised for the cone bioassays is one of the shortcomings of this study. Future work should assess this efficacy with several independent batches of nets.
The high frequency of 1014F kdr mutation observed in the Hadiyau population was similar to the frequency we have reported in An. coluzzii collected from same locality, in 20097. The absence of the 1014S kdr mutation in the Hadiyau population is however, consistent with the absence of An. arabiensis at Hadiyau, since this mutation was only found previously in a single female An. arabiensis in the same locality. In addition, the findings of two more mutations in the IIS6 domain indicates possible selection of additional knockdown phenotype that should be investigated thoroughly. However, future studies on these populations should also assess the additional role that other mutations such as N1575Y could play in this resistance profile. Indeed, as it has been shown that N1575Y mutation confers additional resistance to insecticides51, and that the N1575Y-L1014F double mutant channel was more resistant to pyrethroids than the L1014F mutant one52.
Conclusion
In less than a decade, pyrethroid resistance in An. coluzzii from northern Nigeria has escalated, posing a serious setback to the effort to reduce malaria burden by 90%, in line with WHO projection by the year 2030. Of considerable alarm also is the carbamate resistance on the rise in this field populations and the suspected resistance to one organophosphate, which would confound control measures-the indoor residual spraying. It is of utmost importance to continue surveillance of this resistance and its underlying mechanisms in these areas to inform the malaria control program on its progress. This will help in implementation of effective evidence-based control measures.
Data Availability
The nucleotide sequences generated in this study are available in the GenBank (accession number MK578253-578260).
References
WHO. Global Technical Strategy for Malaria 2016-2030. World Health Organisation. Geneva, Switzerland. ISBN 978 92 4 156499 1. (2015).
WHO. World Malaria Report. Report No. ISBN 978-92-4-156552-3, (Geneva, Switzerland, 2017).
WHO. World Malaria Report 2018 (2018).
Cairns, M. et al. Estimating the potential public health impact of seasonal malaria chemoprevention in African children. Nat Commun 3, 881, https://doi.org/10.1038/ncomms1879 (2012).
President’s Malaria Initiative. Nigeria: Malaria Operational Plan FY 2015. (USAID, USA, 2015).
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207 (2015).
Ibrahim, S. S., Manu, Y. A., Tukur, Z., Irving, H. & Wondji, C. S. High frequency of kdr L1014F is associated with pyrethroid resistance in Anopheles coluzzii in Sudan savannah of northern Nigeria. BMC infectious diseases 14, 441 (2014).
Umar, A. et al. Susceptibility test of female anopheles mosquitoes to ten insecticides for indoor residual spraying (IRS) baseline data collection in Northeastern Nigeria. Journal of Entomology and Nematology 6, 98–103 (2014).
Molineaux, L. & Gramiccia, G. The Garki Project: research on the epidemiology and control of malaria in the Sudan Savanna of West Africa (1980).
Oduola, A. O. et al. Evidence of carbamate resistance in urban populations of Anopheles gambiae s.s. mosquitoes resistant to DDT and deltamethrin insecticides in Lagos, South-Western Nigeria. Parasit Vectors 5, 116, https://doi.org/10.1186/1756-3305-5-116 (2012).
Djouaka, R. J. et al. Evidence of a multiple insecticide resistance in the malaria vector Anopheles funestus in South West Nigeria. Malaria journal 15, 565 (2016).
Okorie, P. N., Ademowo, O., Irving, H., Kelly‐Hope, L. A. & Wondji, C. S. Insecticide susceptibility of Anopheles coluzzii and Anopheles gambiae mosquitoes in Ibadan, Southwest Nigeria. Medical and veterinary entomology 29, 44–50 (2015).
Awolola, S. T. et al. Impact of PermaNet 3.0 on entomological indices in an area of pyrethroid resistant Anopheles gambiae in south-western Nigeria. Parasites & vectors 7, 236 (2014).
Hemingway, J. The way forward for vector control. Science 358, 998–999 (2017).
Shiff, C. Integrated approach to malaria control. Clinical microbiology reviews 15, 278–293 (2002).
Riveron, J. M. et al. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol 15, R27, https://doi.org/10.1186/gb-2014-15-2-r27 (2014).
Morgan, J. C., Irving, H., Okedi, L. M., Steven, A. & Wondji, C. S. Pyrethroid resistance in an Anopheles funestus population from Uganda. PLoS One 5, e11872, https://doi.org/10.1371/journal.pone.0011872 (2010).
Gillies, M. & Coetzee, M. A supplement to anophelinae of Africa south of Sahara (Afro-tropical region). Vol. 55 (1987).
Santolamazza, F. et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malaria Journal 7, 163 (2008).
Organization, W. H. Malaria entomology and vector control. Guide for participants. Geneva, Switzerland: World Health Organization (2013).
Livak, K. J. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 107, 611–634 (1984).
Organization, W. H. A toolkit for integrated vector management in sub-Saharan Africa (2016).
Kent, R. J., Thuma, P. E., Mharakurwa, S. & Norris, D. E. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am J Trop Med Hyg 76, 267–274 (2007).
Bass, C. et al. PCR-based detection of Plasmodium in Anopheles mosquitoes: a comparison of a new high-throughput assay with existing methods. Malaria Journal 7, 177 (2008).
Snounou, G. et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61, 315–320 (1993).
Beier, J. C. In Malaria Methods and Protocols 3–11 (Springer, 2002).
Organization, W. H. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes (2016).
Mitchell, S. N. et al. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proceedings of the National Academy of Sciences 109, 6147–6152 (2012).
WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. World Health Organization. Geneva Switzerland. ISBN 978 92 4 150515 4 (2013).
Feyereisen, R. Insect P450 inhibitors and insecticides: challenges and opportunities. Pest management science 71, 793–800 (2015).
Nwane, P. et al. Multiple insecticide resistance mechanisms in Anopheles gambiae sl populations from Cameroon, Central Africa. Parasites & vectors 6, 41 (2013).
Organization, W. H. Guidelines for laboratory and field-testing of long-lasting insecticidal nets (2013).
Brogdon, W. & Chan, A. Guidelines for evaluating insecticide resistance in vectors using the CDC bottle bioassay/methods in Anopheles research. 343 (2010).
Bass, C. et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J 6, 111, https://doi.org/10.1186/1475-2875-6-111 (2007).
Bass, C. et al. The vector population monitoring tool (VPMT): high-throughput DNA-based diagnostics for the monitoring of mosquito vector populations. Malaria research and treatment 2010 (2010).
Hall, T. A. BioEdit. a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic acids symposium series 95–98 (1999).
DAVIDSON, G. Insecticide resistance in Anopheles gambiae giles. Nature 178, 705 (1956).
Molineaux, L. & Gramiccia, G. The Garki Project. (World Health Organisation, 1980).
Lindsay, S., Parson, L. & Thomas, C. Mapping the range and relative abundance of the two principal African malaria vectors, Anopheles gambiae sensu stricto and An. arabiensis, using climate data. Proceedings of the Royal Society of London B: Biological Sciences 265, 847–854 (1998).
Coetzee, M., Craig, M. & Le Sueur, D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitology today 16, 74–77 (2000).
Onyabe, D. Y. & Conn, J. E. The distribution of two major malaria vectors, Anopheles gambiae and Anopheles arabiensis, in Nigeria. Mem Inst Oswaldo Cruz 96, 1081–1084 (2001).
Awolola, T. et al. Distribution of the molecular forms of Anopheles gambiae and pyrethroid knock down resistance gene in Nigeria. Acta tropica 95, 204–209 (2005).
White, G. & Rosen, P. Comparative studies on sibling species of the Anopheles gambiae Giles complex (Dipt., Culicidae). II. Ecology of species A and B in savanna around Kaduna, Nigeria, during transition from wet to dry season. Bulletin of Entomological Research 62, 613–625 (1973).
Ebenezer, A., Noutcha, A. E. M. & Okiwelu, S. N. Relationship of annual entomological inoculation rates to malaria transmission indices, Bayelsa State, Nigeria. Journal of vector borne diseases 53, 46 (2016).
Bagi, J. et al. When a discriminating dose assay is not enough: measuring the intensity of insecticide resistance in malaria vectors. Malaria journal 14, 210 (2015).
Toé, K. H. et al. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerging infectious diseases 20, 1691 (2014).
Chouaïbou, M., Zivanovic, G. B., Knox, T. B., Jamet, H. P. & Bonfoh, B. Synergist bioassays: A simple method for initial metabolic resistance investigation of field Anopheles gambiae sl populations. Acta tropica 130, 108–111 (2014).
Dadzie, S. K. et al. Evaluation of piperonyl butoxide in enhancing the efficacy of pyrethroid insecticides against resistant Anopheles gambiae sl in Ghana. Malaria journal 16, 342 (2017).
Riveron, J. M., Watsenga, F., Irving, H., Irish, S. R. & Wondji, C. S. High Plasmodium Infection Rate and Reduced Bed Net Efficacy in Multiple Insecticide-Resistant Malaria Vectors in Kinshasa, Democratic Republic of Congo. The Journal of infectious diseases 217, 320–328 (2017).
Riveron, J. M., Watsenga, F., Irving, H., Irish, S. R. & Wondji, C. S. High Plasmodium Infection Rate and Reduced Bed Net Efficacy in Multiple Insecticide-Resistant Malaria Vectors in Kinshasa, Democratic Republic of Congo. The Journal of infectious diseases 217, 320–328, https://doi.org/10.1093/infdis/jix570 (2018).
Jones, C. M. et al. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci USA 109, 6614–6619, https://doi.org/10.1073/pnas.1201475109 (2012).
Wang, L. et al. A mutation in the intracellular loop III/IV of mosquito sodium channel synergizes the effect of mutations in helix IIS6 on pyrethroid resistance. Molecular pharmacology 87, 421–429 (2015).
Acknowledgements
A Wellcome Trust International Training Fellowship in Public Health and Tropical Medicine (WT201918/Z/16/Z) to SSI supported this work.
Author information
Authors and Affiliations
Contributions
S.S.I. and C.S.W. conceived and designed this study. S.S.I., M.M.M., N.L. and F.I.S. carried out the field collection of mosquitoes. S.S.I., M.M.M., J.A.D., M.O.K. and W.T. conducted the bioassays. S.S.I., H.I. and M.M.M. carried out molecular analyses. S.S.I. carried out data analyses and wrote the manuscript with contribution from C.S.W. All authors read and approved the last version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, S.S., Mukhtar, M.M., Datti, J.A. et al. Temporal escalation of Pyrethroid Resistance in the major malaria vector Anopheles coluzzii from Sahelo-Sudanian Region of northern Nigeria. Sci Rep 9, 7395 (2019). https://doi.org/10.1038/s41598-019-43634-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43634-4
This article is cited by
-
Molecular drivers of insecticide resistance in the Sahelo-Sudanian populations of a major malaria vector Anopheles coluzzii
BMC Biology (2023)
-
Potentiation Effects of Ficus sycomorus Active Fraction Against Permethrin-Resistant Field-Population of Anopheles coluzzii (Diptera: Culicidae)
Neotropical Entomology (2021)
-
Resistance status of Anopheles gambiae (s.l.) to four commonly used insecticides for malaria vector control in South-East Nigeria
Parasites & Vectors (2020)
-
A combination of metabolic resistance and high frequency of the 1014F kdr mutation is driving pyrethroid resistance in Anopheles coluzzii population from Guinea savanna of Cameroon
Parasites & Vectors (2019)
-
High insecticide resistance in the major malaria vector Anopheles coluzzii in Chad Republic
Infectious Diseases of Poverty (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.