Abstract
CD26 displays variable levels between effector (TH17 ≫ TH1 > TH2 > Treg) and naïve/memory (memory > naïve) CD4+ T lymphocytes. Besides, IL-6/IL−6R is associated with TH17-differentiation and asthma severity. Allergic/atopic asthma (AA) is dominated by TH2 responses, while TH17 immunity might either modulate the TH2-dependent inflammation in AA or be an important mechanism boosting non-allergic asthma (NAA). Therefore, in this work we have compared the expression of CD26 and CD126 (IL-6Rα) in lymphocytes from different groups of donors: allergic (AA) and non-allergic (NAA) asthma, rhinitis, and healthy subjects. For this purpose, flow cytometry, haematological/biochemical, and in vitro proliferation assays were performed. Our results show a strong CD26-CD126 correlation and an over-representation of CD26− subsets with a highly-differentiated effector phenotype in AA (CD4+CD26−/low T cells) and NAA (CD4−CD26− γδ-T cells). In addition, we found that circulating levels of CD26 (sCD26) were reduced in both AA and NAA, while loss of CD126 expression on different leukocytes correlated with higher disease severity. Finally, selective inhibition of CD26-mRNA translation led to enhanced T cell proliferation in vitro. These findings support that CD26 down-modulation could play a role in facilitating the expansion of highly-differentiated effector T cell subsets in asthma.
Similar content being viewed by others
Introduction
Asthma is a heterogeneous disease with different phenotypes (e.g. allergic asthma/AA and non-allergic asthma/NAA) and endotypes that remain poorly understood1,2,3,4. The major endotype classification of asthma is based on the predominant T helper (TH)-type inflammation5. On the one hand, TH2high asthma is the most common endotype, characterised by an eosinophilic and TH2-driven inflammation and a central role for IgE1,6. On the other hand, TH2low asthma is more heterogeneous, including neutrophilic and paucygranulocytic inflammation and an involvement of TH1 and TH17 cells1,6. TH17 cells have been associated with asthma severity7,8. Indeed, IL-17 has been implicated in steroid resistance, airway remodeling, and the induction of neutrophilic inflammation9,10.
In 2012, Bengsch et al. highlighted the use of CD26 as a marker for human TH17 cells11. Moreover, they showed that the expression of CD26 amongst Teff cells is variable (TH17 ≫ TH1 > TH2)11, while our group described this molecule as a negative selection marker for regulatory T cells (Treg cells)12. CD26/Dipeptidyl peptidase 4 (DPP4) is a “moonlighting” protein: it is an enzyme that inactivates important soluble factors (e.g. cytokines like IL-3; chemokines like CCL11/Eotaxin or CCL5/RANTES; incretins) and also a protein with proteolytic-independent roles (e.g. co-stimulation)13,14,15. Furthermore, CD26high TH lymphocytes are considered memory16,17 or activated cells18, which explains the presence of CD26highCD4+ T cells in AA19,20. A soluble version of CD26 (sCD26) has been found in the bloodstream as a free or a vesicle-associated protein [http://www.exocarta.org]21. Our previous in vitro studies evidenced a positive correlation between soluble DPP4 activity (an indirect measurement of sCD26) and CD26 expression on CD4+ cells20. Immune cells also appear to be a source of sCD26 in vivo15. However, contrary to our expectations, allergic asthmatics displayed higher membrane expression of CD26 on CD4+ T cells, but decreased levels of sCD26. This finding can be explained through the expansion of a “triple low” (Tlow; CD25−CD127−CD26−/low) subpopulation of effector T cells (Teff)20.
IL-6 is an important cytokine in the differentiation of TH17 cells that acts via IL-6R (IL-6Rα/CD126 + gp130)22. In this sense, this cytokine down-modulates the TGF-β-driven expression of FoxP3 and up-regulates the levels of the transcription factor that controls the development of Th17 cells: RORγt23,24. IL-6 signalling is also essential for the generation of functionally active memory CD4+ T cells25. Like CD26, CD126 is also found in plasma as a soluble molecule: sIL-6Rα. This circulating protein binds to IL-6 and leads to the activation of CD126−gp130+ cells, a process known as trans-signalling26,27. Indeed, CD4+ T cells down-modulate IL-6R upon inflammatory activation, but these cells retain the IL-6 response capacity through the trans-signalling pathway28, a mechanism of paramount importance for the maintenance of inflammatory diseases such as asthma29,30,31,32.
Most research in asthma has been focused on the allergic phenotype and CD4+ T cells. However, CD4− lymphocyte subsets (e.g. CD8+ αβ-T cells or γδ-T lymphocytes) might also be relevant for this disease and its phenotypes/endotypes. Thus, γδ-T lymphocytes become activated by IL-6 trans-signalling33, and they are important inducers of allergic asthmatic responses34. Moreover, they are major initial producers of IL-1735. On the other hand, CD8+ T cells cooperate with CD4+ T cells to promote asthma and have been associated with poor lung fuction and airway obstruction36,37,38,39. Interestingly, the expression of both CD26 and CD126 defines diverse stages of differentiation of CD8+ T cells40,41, which might be modified in asthma41. Given this differential expression of CD26 and CD126 between different lymphocyte subsets, we postulate that both molecules might display coordinated expression levels and help distinguish different asthma phenotypes. Therefore, the aim of our study was to analyse the expression of CD26 and CD126 in CD4+ and CD4− lymphocytes from healthy subjects and patients with AA/NAA or rhinitis.
Herein, we report a high correlation between the expression of CD126 and CD26 in lymphocytes. These molecules help differentiate between lymphocytes with naïve (CD26intermediate or CD26int), central-memory (CD26high), or “terminally-differentiated” (TEMRA)/effector-memory (TEM) (CD26−/low) phenotypes. Moreover, allergic and non-allergic asthmatics display low circulating sCD26 levels, which is consistent with the expansion of CD26−/low TEM/TEMRA lymphocytes: CD4+CD26−/low T cells (previously named Tlow cells)20 in AA and CD4−CD26− γδ-T cells in NAA. Acceleration of the natural course of CD26 down-modulation on T lymphocytes by siRNA leads to higher in vitro proliferation rates, which suggests that CD26 molecules on T lymphocytes could be acting as a “brake mechanism” that prevents their proliferation and the acquisition of an effector phenotype. Finally, a decrease in the number of CD126 molecules on leukocytes correlates with higher asthma severity. Thus, our findings provide new advances in asthma immunophenotyping and on the role of CD26/CD126 in this disease.
Results
Characteristics of study subjects
We performed a case-control study including adult patients with different asthma phenotypes (AA; NAA), rhinitis and healthy controls (HC). The characteristics of the donors in this study are summarized in Table 1. Pulmonary function parameters (FEV1 and FEV1/FVC) were lower in both AA and NAA relative to patients with rhinitis (Table 1). Haematological count revealed an increment of eosinophil numbers in asthma patients (both AA and NAA) compared to HC (Table 1). Furthermore, AA displayed higher blood eosinophil counts than patients with rhinitis (Table 1). Levels of other leukocyte populations remained unchanged (Table 1).
As expected, AA subjects exhibited higher levels of IgE than other study groups (Table 1), and the same happened for rhinitis compared to NAA (Table 1). Moreover, our results evidenced a reduction of IgG1 in NAA relative to AA, while IgG4 was higher in AA vs. R (Table 1). We also analysed the erythrocyte sedimentation rate (ESR), which was elevated in NAA vs. remaining groups (Table 1).
CD26 and CD126 molecules display a highly correlated expression on lymphocytes, while the expansion of “triple low” (CD25−CD26−CD127−) CD4+ T cells explains the reduction of sCD26 in allergic asthma
T cells account for ~80% circulating lymphocytes and include CD4− and CD4+ cells. CD4+ lymphocytes (TH) were subdivided into “conventional” Teff (CD25lowCD127high), Treg (CD25highCD127low), and Tlow cells (CD25lowCD127low) (Fig. 1a). TH cells displayed the highest percentage of CD26+ cells within leukocytes (~85–90%), and this parameter evidenced a positive association with the percentage of CD126+ cells (Fig. 1b). Amongst TH lymphocytes, “conventional” Teff (CD26+/high) and especially Tlow (CD26−/low) cells exhibited the same high CD26-CD126 correlation, but Tregs appear to lose this association (Fig. 1c). Furthermore, we found a reinforced expression of CD26 (measured in Antibody Bound per Cell/ABC; see Material and Methods section) on CD4+ T cells from AA and NAA patients (Fig. 1d) compared to HC. Strikingly, CD126 showed an unaltered expression in CD4+ T cells from the different groups of donors (data not shown) despite the strong correlation between CD26 and CD126 commented above (Fig. 1b).
CD26 and CD126/IL-6Rα display a coordinated expression on effector CD4+ T cells, but only CD26/sCD26 levels are altered in asthma. (a) Gating strategy in flow cytometry assays to identify relevant CD4+ T cell subsets. Lymphocytes were gated based on the forward (FSC-Height) and side-scatter (SSC-Height) parameters (left dot plot). Then, TH lymphocytes were selected according to their high CD4 expression (middle dot plot). Finally, levels of CD25 and CD127 were used to identify three different TH subsets (right dot plot): “conventional” Teff cells (Teff), regulatory T cells (Treg), and “triple low” Teff cells (Tlow). (b,c) Correlation between the expression (% of positive cells) of CD26 and CD126 on CD4+ (red) or CD4− (blue) lymphocytes (b) or within different CD4+ subsets (c): “conventional” Teff (red), Treg (green), and Tlow cells (black) (data from all study subjects; N = 268). (d) Expression of CD26 (median ± IQR1-3) presented as number of antibody molecules bound per cell (ABC; see Material and Methods section) in healthy controls (HC; N = 32), rhinitis (R; N = 44), allergic asthma (AA; N = 100), and non-allergic asthma (NAA; N = 92). (e) Serum levels of sCD26 (ng/mL) measured by ELISA. (f) Percentage of Tlow cells within the CD4+ T cell gate. (d–f) Statistically significant differences between groups are indicated (Kruskal-Wallis test: P < 0.05).
The higher number of CD26 molecules on CD4+ T cells from AA and NAA patients compared to HC (Fig. 1d) was in line with the expected activated phenotype of these cells, but contrasted with the decreased levels of sCD26 in asthmatics (Fig. 1e). However, as most of sCD26 comes from T cells42, the expansion of CD26−/low T lymphocyte populations may explain the decreased levels of sCD26 in AA. Indeed, even though Teff and Treg proportions remained unchanged, we detected an expanded subpopulation of CD25−CD127−CD26−/low (Tlow) effector T cells in AA patients compared to HC (Fig. 1f). Moreover, contrary to CD4+ and “conventional” Teff cells (CD26int/high subsets) and despite the CD26−/low phenotype of these Tlow cells, the percentage of the last subset was negatively correlated with the percentage of CD26+ and CD126+ cells within the Tlow compartment (Supplementary Table S1). Interestingly, the percentage of CD127+ Treg cells was augmented in asthmatics compared to rhinitis and HC (Supplementary Fig. S1).
The CD4+CD26−/low T compartment from allergic asthmatics includes memory cells in an advanced stage of differentiation
CD26 and CD127 are useful markers for identifying effector/memory T cells11,16,17. Based on these molecules, CD4+ lymphocytes were segregated in CD26−/low, CD26int, and CD26high cells (Fig. 2a). As Fig. 2b illustrates, CD26int and CD26high populations showed elevated expression of co-stimulatory (CD27, CD28) and lymph node homing (CCR7) molecules, indicating an early differentiation stage. In addition, the CD26high subpopulation was mainly composed of CD45RA− cells (i.e. central-memory T cells/TCM; CD45RA−CCR7+CD27+CD28+), whereas most of naïve T lymphocytes (CD45RA+CCR7+CD27+CD28+) were included within the CD26int subset (Fig. 2b). In contrast, CD26−/low lymphocytes showed intermediate levels of CD45RA and a decreased expression of CD27, CD28, CCR7, and CD127 molecules (Fig. 2b), which agrees with an advanced differentiation stage (likely TEM or TEMRA, CD45RA+CCR7−CD27−CD28−). Moreover, down-modulation of CD27, CD28, and CCR7 was mainly observed in CD26−/low cells from patients with moderate-severe AA (Fig. 2c), the group of donors where Tlow cells (CD4+CD25−CD127−CD26−/low) were found expanded (Fig. 1f). Apart from Tlow cells, the CD26−/low compartment within the TH subset is also occupied by Treg lymphocytes (CD25highCD127low; Supplementary Fig. S2), as Bailey et al. have previously reported17. However, 57.5 ± 7.3% of Treg lymphocytes are also found within the CD26int subset (Supplementary Fig. S2). In summary, our results evidenced that: (a) most of naïve CD4+ T lymphocytes display intermediate expression levels of CD26; (b) The CD26high subpopulation is mainly composed of TCM cells; and (c) Tlow cells expanded in AA are likely effector T lymphocytes that have lost CD26 expression to become TEM or TEMRA cells (CD27−CD28−CCR7−CD45RA+/−).
CD26−/low CD4+ T cells contain effector lymphocytes with an advanced differentiation stage. (a) CD4+ T cells were identified by using the same strategy as in Fig. 1a. Then, CD127 and CD26 markers were used to delimitate three CD4+ lymphocyte subsets (right dot plot): CD26−/low (including CD127− and CD127‘+ cells), CD26int and CD26high. (b) Phenotyping of CD26−/low, CD26int, and CD26high subsets according to the surface expression of CD27, CD28, CCR7, CD45RA, and CD127. Data were obtained from 6 donors (3 healthy subjects and 3 moderate-severe allergic asthmatics) and expressed as % of positive cells for each marker (median ± IQR1-3). Kruskal-Wallis test followed by Dunn’s multiple comparison post-hoc analysis was used to assess significant changes between groups. *P < 0.05, **P < 0.01, ***P < 0.001. (c) Expression of each marker (Percentage of positive cells; median ± IQR1-3) in CD26−/low lymphocytes between healthy subjects (HC; N = 3) and moderate-severe allergic asthmatics (MSAA; N = 3). t-test was used to assess significant changes between HC and MSAA. *P < 0.05.
Non-allergic asthmatics display increased proportions of CD26−CD127− and CD126−CD127− subpopulations amongst CD4− lymphocytes compared to allergic patients
Next, we also decided to analyse the population of CD4− lymphocytes (Fig. 3a; left and middle dot plots). This cell compartment is more heterogeneous and includes γδ-T, B, NK, and NKT lymphocytes. Analogously to what happened with Tlow cells, the percentage of CD4− lymphocytes was negatively correlated with the percentage of CD26+/CD126+ cells in this subset (Supplementary Table S1). Moreover, along with the strong correlation between the expression levels of CD26 and CD126 on CD4− lymphocytes (Fig. 1b), we also detected a decreased proportion of CD4−CD26+ and CD4−CD126+ cells in NAA compared to AA and R patients (Fig. 3b). Additionally, both CD4−CD26+ and CD4−CD126+ cells had a negative correlation with age (rs = −0.482, P < 0.001; rs = −0.508, P < 0.001, respectively).
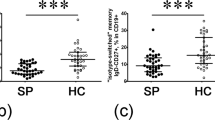
CD4− lymphocytes with a highly differentiated memory phenotype are expanded in non-allergic asthma. (a) CD4− lymphocytes were identified by flow cytometry (left and middle dot plots). Then CD127 and CD26 were used to identify five subsets within the CD4− lymphocyte compartment (right dot plot). (b) Percentage of CD4−CD26+ or CD4−CD126+ cells from healthy controls (HC; N = 32), rhinitis (R; N = 44), allergic asthma (AA; N = 100) and non-allergic asthma (NAA; N = 92). (c) Percentage (median ± IQR1-3) of CD26−CD127−, CD26intCD127+, and CD26highCD127+ cells amongst CD4− lymphocytes in HC, R, AA, and NAA. (d) Composition of CD4− lymphocyte subsets based on CD3 (T), CD8 (Tc), CD19 (B), and CD56 (NK, NKT) antigens (Data from 3 representative donors). (e) Phenotypic analysis of CD4− subsets based on CD45RA, CCR7, CD127, CD28, and CD27 markers (data from 3 representative donors). Statistically significant differences between groups are indicated (Kruskal-Wallis test: P < 0.05). In order to make the figure more understandable, sections (c,d and e) show only those CD4− subsets gated in (a) (right dot plot) where differences between groups were observed (CD26−CD127−, CD26intCD127+, and CD26highCD127+ cells).
To further characterize CD4− lymphocytes, these cells were subdivided in five subpopulations according to CD26 and CD127 levels: CD26int/highCD127−, CD26−CD127−, CD26−CD127+, CD26intCD127+, and CD26highCD127+ cells (Fig. 3a). As Fig. 3c shows, we only detected an increment of double negative cells (CD26−CD127−) in NAA compared to AA, which was accompanied by a reduction of both CD26intCD127+ and CD26highCD127+ lymphocytes. These two last CD4− subsets were rather homogeneous in terms of cell composition, exhibiting a high proportion (>80–90%) of cytotoxic T cells (Tc; CD3+CD8+) (Fig. 3d). In contrast, the composition of CD4−CD26−CD127− lymphocytes was more complex and included Tc, B (CD19+) and CD56+ cells (NK, NKT, or γδ-T lymphocytes) (Fig. 3d).
Regarding the CD45RA and CCR7 markers, both CD26+ subpopulations (CD26intCD127+ and CD26highCD127+) displayed similar percentages of positive cells (Fig. 3e). Nevertheless, we noticed a decrease of CD45RA levels (mean fluorescence intensity/MFI) in CD26high cells (41.4 ± 4.2) compared to the CD26int subset (342.1 ± 198.2). This finding supports a differentiation of naïve cells (CD26int) towards a memory-like (CD26high) phenotype, compatible with the abundance of co-stimulatory molecules (CD27 and CD28) in both subsets (Fig. 3e). In contrast, only a small percentage of CD4−CD26−CD127− cells were CD27+, CD28+, or CCR7+. Strikingly, they showed a CD45RAhigh phenotype (Fig. 3e). Altogether, these characteristics define a TEMRA-like population within CD4−CD26−CD127− lymphocytes. Similar results were obtained when CD4− lymphocytes were segregated based on CD126 vs CD127 levels (Supplementary Fig. S3).
The CD26−/low subpopulation of γδ-T lymphocytes is augmented in non-allergic compared to allergic asthmatics
To ascertain the specific lymphocyte subset within the CD4− compartment showing altered proportions in NAA compared to AA and to limit possible cofounding effects (e.g. age, gender), we analysed blood samples from a second cohort study limited to AA and NAA donors (n = 12/each) with similar age and M/F proportions. The new analysis focused on CD8+ T (CD3+CD8+), NK (CD3−CD56+), NKT (CD3+CD56+), B (CD19+), and γδ-T cells (TCRγδ+CD3+) (Supplementary Fig. S4). There was no evidence for expansion of these circulating populations in NAA. However, this new study revealed an increased proportion of CD26−/low γδ-T lymphocytes (and an opposite pattern for CD26high γδ-T cells) in NAA compared to AA (Fig. 4). Once again, the percentage of γδ-T cells correlated inversely with the percentage of CD26+ cells within this subset (rs = −0.460, P = 0.024). Interestingly, there was also a negative correlation between the percentages of γδ-T cells and B lymphocytes (rs = −0.602, P = 0.002).
Non-allergic asthmatics exhibit augmented proportions of CD26−/low γδ-T lymphocytes compared with allergic asthmatics. (a) Gating strategy for CD3+γδ-TCR+ lymphocytes (γδ-T cells). After gating, expression of CD127 and CD26 was used to identify three subsets (CD26−/lowCD127−/+, CD26intCD127+, and CD26highCD127+) in γδ-T cells (right dot plot). (b–d) Percentage (median ± IQR1-3) of CD26−/lowCD127−/+ (b), CD26intCD127+ (c), and CD26highCD127+ cells (d) amongst γδ-T lymphocytes in allergic (AA; N = 12) and non-allergic (NAA; N = 12) asthmatics. Statistically significant differences between these two groups of donors are indicated (t-test: P < 0.05).
CD26 could act as a negative regulator of T-cell proliferation
CD26 down-modulation on T lymphocytes from both AA (CD4+ T cells) and NAA (CD4− γδ-T cells) patients could be: a) a consequence of different mechanisms that simply reduce the amount of this protein on the cell surface, like, for example, the dilution of CD26 molecules (half-life > 48 h) throughout the successive cytokinesis rounds; or b) a necessary condition to initiate the proliferation/differentiation programme of naïve or memory T cells. To distinguish between both possibilities, we tested the effect of RNA interference (RNAi) on the DPP4 gene during the proliferative response of T lymphocytes to mitogenic triggers. Peripheral blood mononuclear cells (PBMCs) were CFSE-labelled and cultured in vitro with either non-target or CD26/DPP4-specific Accell siRNAs. As CD26 up-regulation during T cell activation was mainly derived from the translocation of this protein from intracellular stores toward the cell surface, T-cell division was stimulated with phytohemagglutinin P (PHA) in the presence or absence of IL-12, a cytokine that promotes CD26 mRNA translation. Furthermore, it was required to extend the in vitro culture incubation for 6 days to observe the inhibitory effect of the CD26-specific siRNA on protein levels. As expected, CD26-specific siRNAs down-modulated the expression of CD26, but only in IL-12-stimulated PBMCs (Fig. 5a). After verification of compliance with CD26 down-modulation by RNAi, we estimated the percentage of cells that divided at least once. As Fig. 5b shows, those T cells where DPP4 gene silencing was more intense (i.e. IL-12-costimulated) were the ones showing an increase in the proliferation rate.
siRNA mediated depletion of CD26 mRNA leads to enhanced T-cell proliferation. PBMCs from healthy subjects were isolated and placed in culture for 6 days in 96 round-well plates. To promote T-cell division, Accell culture medium was supplemented with PHA ± IL-12. Besides, a CD26-specific or a non-targeting Accell siRNAs pool was also used. (a) Expression of CD26 (MFI; mean fluorescence intensity) on PBMCs was assessed by flow cytometry. Three representative assays are shown. 2-way ANOVA with Tukey’s multiple comparison test: *P < 0.05, ***P < 0.001, ****P < 0.0001; n.s., non-significant. (b) PBMCs from 2 representative donors were labelled with CFSE and T-cell proliferation induced by PHA ± IL-12 was assessed by CFSE-dilution assays. Responder frequency is the percentage of T lymphocytes that divided at least once. 2-way ANOVA with Tukey’s multiple comparison test: *P < 0.05, **P < 0.01, ****P < 0.0001; n.s., non-significant.
CD126/IL-6Rα down-modulation in neutrophils, monocytes, and lymphocytes is associated to disease severity and asthma control
Asthma severity could be responsible for changes in pulmonary and inflammatory parameters. To assess this, we segregated asthma patients into moderate-severe (n = 90) and intermittent-mild (n = 102) asthmatics; asthma control degree was also considered (Table 1). As expected, FEV1 and FEV1/FVC were decreased in moderate-severe and uncontrolled asthmatics (Supplementary Table S2), regardless of phenotype. Eosinophils count was also augmented in moderate-severe and badly-controlled asthmatics (Supplementary Table S2), although the statistical significance is only maintained in AA after segregation according to the phenotype. In contrast, badly-controlled NAA was only characterized by an increased neutrophils count (P = 0.032; data not shown). Interestingly, there was a decrease in the expression of CD126 in many leukocyte subsets (monocytes, neutrophils, CD4− and CD4+ lymphocytes) as the severity was higher (Fig. 6). This finding extends to Teff, Treg, and Tlow lymphocytes. Similar decreased levels were obtained for CD126 on leukocytes from badly-controlled asthmatics. Furthermore, we did not observe changes in CD26 levels on most leukocyte subpopulations with asthma severity, although the percentage of CD26+ Tregs was higher in moderate-severe NAA patients (Supplementary Fig. S5). Finally, the percentage of CD4+ lymphocytes was higher in badly-controlled AA (P = 0.006; data not shown).
Generalised down-modulation of CD126 in leukocytes from patients with moderate-severe asthma. Monocytes (a), neutrophils (b), and lymphocytes (c,d) were gated on a flow cytometry FSC/SSC plot. Afterwards, lymphocytes were further subdivided into CD4− (c) and CD4+ (d) cells. The number of CD126 antibodies bound per cell (ABC; median ± IQR1-3; see Material and Methods section) was measured on every population (a–d) in intermittent-mild (IMA; N = 102) and moderate-severe (MSA; N = 90) asthma patients. Statistically significant differences between these two groups of donors are indicated (t-test: P < 0.05).
Discussion
This study shows an intense correlation between the expressions of CD26/DPP4 and CD126/IL-6Rα, two molecules that identify naïve (CD26int and/or CD126+ cells), memory (CD26high and/or CD126+), and highly differentiated effector subsets (TEM and TEMRA cells; CD26−/low and/or CD126−). Precisely, the last subset is expanded in both groups of asthma patients: AA (CD4+ Tlow cells) and NAA (γδ-T cells). Probably as a result of this expansion, there is a reduction of sCD26 concentration in plasma that is shared by AA and NAA patients. Moreover, CD26 could be relevant to slow the rate of cell division of naïve or central-memory T lymphocytes and, conversely, its down-modulation necessary for rapid proliferation and differentiation into effector cells. Finally, down-regulation of CD126 on leukocytes may be related to asthma severity.
CD26 is an activation marker18 that identifies TH1 and especially TH17 cells11. Our results show an increase of “activated” CD4+ cells (CD26+) in asthma, in agreement with published work19. Despite the IL-13-dependent up-regulation of CD26/DPP4 on human bronchial epithelial cells43 or the potential role of TH17 cells in NAA8,44, both AA and NAA patients express similar levels of CD26 on CD4+ T cells. A possible explanation for this observation is that following antigen presentation to naïve CD4+ T cells in lymph nodes, these lymphocytes proliferate, differentiate, and move back to the peripheral circulation, where they still have an early differentiation state (CD26high; TH17 ≫ TH1 > TH2) and an extended half-life compared to innate leukocytes (CD26−/low; eosinophils, neutrophils) (Supplementary Table S3). Therefore, these circulating TH cells will still require the down-modulation of CD26 to become TEM/TEMRA cells and migrate into sites of inflammation; a similar event would occur with IL-6Rα/CD126 on T lymphocytes to favour the proinflammatory trans-signalling pathway.
Three major TH subsets coexist in circulation: Treg (CD25highCD26lowCD127low), “conventional” Teff (CD25lowCD26highCD127high), and Tlow cells (CD25−/lowCD26−/lowCD127−/low). The latter is a highly differentiated counterpart of “conventional” Teff cells. CD4+ Tlow cells are expanded in AA20 and their abundance is negatively correlated with CD26/CD126 levels, as it happens for other subpopulations (e.g. CD26−/low γδ-T lymphocytes). Despite the small expansion of the Tlow cell compartment, this might be relevant for AA pathogenesis considering two facts: (a) total lymphocytes, instead of antigen-specific cells, have been measured; and (b) patients were in stable phase (i.e. absence of exacerbations for 4 weeks before sample collection). On the other hand, even though there is a negative association between the percentage of CD26+/CD126+ cells and the percentage of Tlow cells, this does not necessarily imply CD26-dependent causation. Therefore, we performed T cell proliferation assays after siRNA-mediated depletion of CD26 mRNA. These results show a negative regulatory role for CD26 in T cell proliferation15. Thus, our data agree with the study of Yan et al. working with ovalbumin-induced CD26−/− C56BL/6 animals45, or Stephan et al. showing that oral administration of DPP4-inhibitors aggravates the airway inflammation in a rat model of asthma46.
A reduced expression of CD27, CD28, and CCR7 is the hallmark of highly-differentiated effector cells (TEM or TEMRA). Tlow cells fulfil these criteria; therefore, these lymphocytes might be part of a pool of “conventional” CD4+ Teff cells that remains upon antigen clearance and develop into senescent lymphocytes with short telomeres, low proliferative capacity, and presence of cytotoxic molecules47. Thus, in line with other works17,28, expression of CD26/DPP4 and CD126/IL-6Rα would identify cells with a naïve (CD45RA+, CD62L+, CCR7+) or a central-memory (CD45RO+, CD62L+, CCR7+) phenotype, while reduced levels would distinguish CD4+ T subsets expanded in AA with an TEM or TEMRA phenotype. Similarly, we found augmented proportions of peripheral blood CD4− γδ-T cells with a CD26−CD126−CD127− phenotype (likely V𝛿2/Vγ9+)48 in NAA compared to AA. Several studies have described the presence of different circulating V𝛿2/Vγ9+ T cells (naïve/CD45RA+CD27+, TCM/CD45RA−CD27+, TEM/CD45RA−CD27−, and TEMRA/CD45RA+CD27−)49,50, which is compatible with the existence of CD26− (TEM/TEMRA), CD26int (naïve), and CD26high (TCM) γδ-T subsets. Thus, an enlarged population of CD26− γδ-T lymphocytes (i.e. TEM/TEMRA) with a preferential production of TH17 or TH1 cytokines51 in NAA patients could explain the enhanced airway inflammation and the inverse relationship between γδ-T cell and B-cell proportions. However, it has been previously reported that most of circulating γδ-T cells are CD126−gp130− (i.e. they are not IL-6-responders)52, which raises the question about how they can become IL-17-producers.
Another caveat is if those phenotypic changes on T lymphocytes could be mirrored in serum samples. Different circulating molecules were measured (TGF, TNF, sCD25, sCD26) (Table 1; Fig. 1), but most of them remained unaltered. We did not quantify “soluble” IL-6R/CD126 (sIL-6R/sCD126), but several authors reported higher levels in stable asthma and especially upon flare-ups due to mechanisms involving sheddases (e.g. ADAM10/17)53,54,55, spliceosomes53,56, or vesicles54. In contrast, changes in sCD26 levels in asthma remain almost unexplored. Lun et al. reported an elevation of sCD26 in AA patients linked to the activated phenotype (CD26high) of CD4+ T lymphocytes19. However, reduced sCD26 levels have been recently described in severe asthmatics57 or a low eosinophilic TH2low severe asthma endotype58. Our results are in agreement with these last works and support a rather generalized (AA and NAA) sCD26 down-modulation. The underlying mechanism is likely the expansion of CD26− T subsets20 with a TEM or TEMRA phenotype: CD4+ T cells in AA and CD4− γδ-T cells in NAA. Reduced levels of CD26 on lymphocytes and the extracellular compartment could be concomitant with the loss of caveolin-1 (a CD26 ligand) in bronchial epithelial cells and monocytes from asthmatics15. Moreover, the decrease of CD26 levels may be important for the bioavailability of soluble factors (e.g. chemokines, adenosine) and to promote cell functions like proliferation, chemotaxis, and migration toward inflamed tissues15.
Treg cell function has been described as impaired in asthma59. Although we did not find deregulation of Treg numbers, they showed increased CD26 expression in asthmatic patients. This is relevant because CD39 is an ecto-enzyme expressed by CD26− Treg lymphocytes12,60 and involved in adenosine (Ado) production61. Ado is an immune-regulatory molecule whose synthesis is counteracted by adenosine deaminase (ADA), an ecto-enzyme anchored to CD2662,63. Therefore, a CD26high phenotype in Tregs could decrease local Ado concentration and exacerbate disease severity64. Indeed, the percentage of CD26+ Treg cells in NAA was higher in moderate-severe patients than intermittent-mild subjects. The percentage of CD127+ Treg cells, a phenotype correlated with a diminished suppressive capacity65, was also augmented in asthmatics compared to rhinitis and HC. However, future studies including the assessment of Treg function in NAA and AA will be necessary.
Asthma severity is also influencing CD126 levels on CD4+ T cells, neutrophils and monocytes. IL-6 acts via either IL-6R (classic-signalling) or sIL-6R/sCD126 (trans-signalling)22. Contrary to the anti-inflammatory role of the first pathway28, the trans-signalling route allows CD126−CD130+ cells to respond to IL-627 and is important in asthma through the maintenance of TH17 cells or the inhibition of T-cell apoptosis66. Naïve CD4+ T cells down-modulate IL-6R upon TCR-mediated activation, probably due to protein shedding28. This release mechanism has been observed in asthma, and sIL-6R levels have been directly associated with IgE levels, but negatively with lung function67. Therefore, reduction of CD126 expression in monocytes, neutrophils, and CD4+ cells from moderate-severe patients highlights the role of IL-6 trans-signalling in asthma severity.
In conclusion, our data provide evidence that both asthma phenotypes share common immune-pathologic mechanisms, with expansion of CD26−/low subsets in AA (CD4+ Tlow or “highly-differentiated” Teff cells) and NAA (CD4− T cells; γδ-T lymphocytes) and down-modulation of additional surface molecules (IL-6Rα/CD126, CD27, CD28, IL-7Rα/CD127, CCR7) to produce differentiated effector subsets and extracellular sCD26 reduction. This CD26/sCD26 down-modulation and the potential role in T-cell proliferation should be considered in the light of clinical usage of DPP4 inhibitors and anti-CD26 antibodies.
Material and Methods
Subjects
Adult patients with asthma or allergic rhinitis were recruited from hospital consultations for Pneumology in Galicia (Spain) between 2014 and 2016. The diagnosis of different asthma phenotypes and allergy was confirmed according to Global Strategy for Asthma Management and Prevention (GINA 2006, http://www.seicap.es/documentos/archivos/GINA2006general.pdf) criteria for at least one year prior to study initiation. A positive skin prick test and the presence of allergen-specific IgE were used to confirm sensitization in allergic patients. Lung function parameters (forced expiratory volume in the 1st second (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio) as well as eritro-sedimentation rate (ESR) were also analysed. All asthmatics were in a stable phase of the disease (i.e. absence of exacerbations for at least 4 weeks before sample collection). Healthy donors were subjects without allergy or systemic diseases, who were scheduled for minor surgeries (orthopedic surgery or inguinal hernia). A second cohort of patients was also included with 12 patients with AA (M/F proportion: 6/6; age: 52.75 ± 14.12) and 12 patients with NAA (M/F proportion: 6/6; age: 61.00 ± 10.71), recruited from hospital consultations for Pneumology in Galicia, Spain. The research project was approved by the Ethics Committee of Clinical Research of Galicia (2011/001), Spain, all subjects signed an informed consent, and all research was performed in accordance with the relevant guidelines and regulations.
Biochemical determinations
Biochemical determinations and nucleated cell counting were performed by using an ADVIA®1650 analyser (SIEMENS Healthcare Diagnostics S.L., Berlin, Germany) and an ADVIA®2120 haematology counter (SIEMENS Healthcare Diagnostics S.L., Berlin, Germany), repectively. Serum TGF-β1 levels were measured using ELISA plates (Human TGF-beta1 Platinum ELISA; ref. BMS249/4TEN; eBioscience) following commercial guidelines. Optical densities were assayed at 450 nm (Labsystem Multiscan MS), and protein concentration was calculated from standard curves.
Flow cytometry assays
Venous peripheral blood from each donor was collected in EDTA tubes (BD Vacutainer K2E). Then, FITC, PE, PE-Cy7, PerCP-Cy5.5 or AlexaFlour-647-labelled mouse IgG1 κ isotype antibodies (BD Bioscience) or specific antibodies were incubated with cells for 30 min in FACS buffer (PBS, pH 7.4, 2% FBS). We used BD FACSTM Lysing Solution (15 min; room temp.) to lyse red blood cells before sample collection. Finally, 200000 events were acquired on a BD FACSortTM flow cytometer and data were analysed using WinMDI 2.9 software (Joseph Trotter, La Jolla, CA. USA). A list of antibodies is shown in Supplementary Table S4. Isotype antibodies were used to determine the non-specific binding of the antibodies and therefore to set a threshold value to identify negative and positive populations. Single stained lymphocytes were used for fluorescence compensation.
Flow cytometry data are presented as either “percentage of positive cells” or “number of antibodies per cell” (ABC) instead of mean fluorencence intensity (MFI). We used the BD Quantibrite™ Beads PE kit (Fluorescence Quantitation Kit; BD Bioscience) to estimate ABC according to manufacturer instructions. In brief, we ran a BD Quantibrite PE tube with the same instrument settings as the assay. Therefore, MFI values in FL2 were converted into the number of PE molecules bound per cell. Finally, this number of PE molecules/cell was changed into ABC values by using known ratios of PE to antibodies. We took advantage of this transformation to minimise as much as possible the inter-day variation related to the working conditions of the flow cytometer.
In vitro proliferation assays and CD26 mRNA silencing
PBMCs were placed in RPMI 1640 at a cell density of 107 cell/mL and incubated with 5 μM CFSE for 8 min at RT in the dark. Then, FBS was added to stop the reaction and cells were thoroughly washed with RPMI 1640 before cell counting. Cell cultures were set up at 0.25 × 106 cells/mL in 96-well microplates (round-wells). Accell delivery media (ref. B-005000-500; Dharmacon) was used to culture these cells under non-serum conditions. The Accell delivery medium was supplemented or not with 1 μg/ml PHA (±2 ng/ml IL-12), in the presence of either DPP4-specific or non-targeting Accell siRNAs pools (Dharmacon). To achieve a partial gene silencing we used a commercial Accell SMART pool of 4 short interfering RNA (siRNA) designed to target the mRNA encoded by the human DPP4 gene (ref. E-004181-00-0005; Dharmacon); these siRNAs were designed to minimize the off-target effects. Besides, we also used two non-target siRNAs: a) an Accell green non-targeting siRNA (ref. D-001950-01-05; Dharmacon), which is a fluorescent unspecific siRNA used for assessment of Accell siRNA passive delivery effectiveness; b) a negative control Accell non-targeting siRNA pool of four siRNAs (ref. D-001910-10-05; Dharmacon) to control the background response to siRNA. All these siRNAs were initially resuspended at 100 μM by using a 1X siRNA buffer (ref. B-002000-UB-100; Dharmacon). Strikingly, the working concentration suggested by the manufacturer (1 μM) induced high cell mortality. Therefore, we titrated down the concentration of siRNA by using the Accell green non-targeting siRNA. A final concentration of 0.02 μM was selected to carry out the final experiments. This concentration was enough to label > 98% of cells and allow a high cell viability (>95%).
Upon 6 days of in vitro culture, CFSE fluorescence and the number of cell divisions were measured by flow cytometry (Supplementary Fig. S6), as well as the amount of CD26 protein with both specific anti-CD26 and isotype antibodies. Each condition was tested several times (n = 3–5 technical replicates). Unlabelled cells served as negative controls in cell proliferation assays. The calculated responder frequency (Rf) is the percentage of responder T cells that divided at least once (Supplementary Fig. S6).
Statistics
Descriptive data are presented as median (interquartile range; IQR1-3). To assess the changes between asthmatic groups, rhinitis, and healthy donors for non-normally distributed variables we used the Kruskal–Wallis test followed by Dunn’s multiple comparison test. Spearman’s test was used to measure association between these variables. For CFSE proliferation studies, a two-way ANOVA followed by a Tukey’s multiple comparison test were used. Finally, t-test was performed with data from Fig. 2c to assess changes in normally distributed variables between moderate-severe allergic asthmatics and healthy subjects. Mann-Whitney U test was used to assess changes in non-normally distributed variables. All analyses were conducted using GraphPad Prism 6.0 (GraphPad Software, Inc., San Jose, California, USA). The statistical significance was defined as P < 0.05.
Data Availability
The datasets analyzed are available from the corresponding author on justifiable request, and not publicly available due to protection of participant confidentiality.
References
Wenzel, S. E. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat. Med. 18, 716–725 (2012).
Chung, K. F. Defining phenotypes in asthma: a step towards personalized medicine. Drugs 74, 719–728 (2014).
Meyers, D. A., Bleecker, E. R., Holloway, J. W. & Holgate, S. T. Asthma genetics and personalised medicine. Lancet Respir. Med. 2, 405–415 (2014).
Canonica, G. W. et al. Asthma: personalized and precision medicine. Curr. Opin. Allergy Clin. Immunol. 18, 51–58 (2018).
Woodruff, P. G. et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 180, 388–395 (2009).
Stokes, J. R. & Casale, T. B. Characterization of asthma endotypes: implications for therapy. Ann. Allergy Asthma Immunol. 117, 121–125 (2016).
Newcomb, D. C. & Peebles, R. S. Jr. Th17-mediated inflammation in asthma. Curr. Opin. Immunol. 25, 755–760 (2013).
Cosmi, L., Liotta, F. & Annunziato, F. Th17 regulating lower airway disease. Curr. Opin. Allergy Clin. Immunol. 16, 1–6 (2016).
Chesné, J. et al. IL-17 in severe asthma. Where do we stand? Am. J. Respir. Crit. Care Med. 190, 1094–1101 (2014).
Lindén, A. & Dahlén, B. Interleukin-17 cytokine signalling in patients with asthma. Eur. Respir. J. 44, 1319–1331 (2014).
Bengsch, B. et al. Human Th17 cells express high levels of enzymatically active dipeptidyl peptidase IV (CD26). J. Immunol. 188, 5438–5447 (2012).
Salgado, F. J. et al. CD26: a negative selection marker for human Treg cells. Cytometry A 81, 843–855 (2012).
Ohnuma, K., Dang, N. H. & Morimoto, C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 29, 295–301 (2008).
Klemann, C., Wagner, L., Stephan, M. & von Hörsten, S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 185, 1–21 (2016).
Nieto-Fontarigo, J. J. et al. CD26 and Asthma: A Comprehensive Review. Clin. Rev. Allergy Immunol. 56, 139–160 (2019).
Ibegbu, C. C. et al. Differential expression of CD26 on virus-specific CD8(+) T cells during active, latent and resolved infection. Immunology 126, 346–353 (2009).
Bailey, S. R. et al. Human CD26high T cells elicit tumor immunity against multiple malignancies via enhanced migration and persistence. Nat. Commun. 8, 1961 (2017).
Ohnuma, K. et al. Role of CD26/dipeptidyl peptidase IV in human T cell activation and function. Front. Biosci. 13, 2299–2310 (2008).
Lun, S. W., Wong, C. K., Ko, F. W., Hui, D. S. & Lam, C. W. Increased expression of plasma and CD4+ T lymphocyte costimulatory molecule CD26 in adult patients with allergic asthma. J. Clin. Immunol. 27, 430–437 (2007).
Nieto-Fontarigo, J. J. et al. Expansion of a CD26low Effector TH Subset and Reduction in Circulating Levels of sCD26 in Stable Allergic Asthma in Adults. J. Investig. Allergol. Clin. Immunol. 28, 113–125 (2018).
Röhrborn, D., Eckel, J. & Sell, H. Shedding of dipeptidyl peptidase 4 is mediated by metalloproteases and up-regulated by hypoxia in human adipocytes and smooth muscle cells. FEBS Lett. 588, 3870–3877 (2014).
Taga, T. et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell 58, 573–581 (1989).
Zhou, L. et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 (2007).
Maddur, M. S., Miossec, P., Kaveri, S. V. & Bayry, J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am. J. Pathol. 181, 8–18 (2012).
Nish, S. A. et al. T cell-intrinsic role of IL-6 signaling in primary and memory responses. Elife 3, e01949 (2014).
Lust, J. A. et al. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine 4, 96–100 (1992).
Rose-John, S., Scheller, J., Elson, G. & Jones, S. A. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J. Leukoc. Biol. 80, 227–236 (2006).
Jones, G. W. et al. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J. Immunol. 184, 2130–2139 (2010).
Scheller, J., Ohnesorge, N. & Rose-John, S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand. J. Immunol. 63, 321–329 (2006).
Chalaris, A., Garbers, C., Rabe, B., Rose-John, S. & Scheller, J. The soluble Interleukin 6 receptor: generation and role in inflammation and cancer. Eur. J. Cell Biol. 90, 484–494 (2011).
Neurath, M. F. & Finotto, S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 22, 83–89 (2011).
Farahi, N. et al. Neutrophil-mediated IL-6 receptor trans-signaling and the risk of chronic obstructive pulmonary disease and asthma. Hum. Mol. Genet. 26, 1584–1596 (2017).
Ullah, M. A. et al. Allergen-induced IL-6 trans-signaling activates γδ T cells to promote type 2 and type 17 airway inflammation. J. Allergy Clin. Immunol. 136, 1065–1073 (2015).
Tamura-Yamashita, K. et al. Gamma-delta T cell is essential for allergen-induced late asthmatic response in a murine model of asthma. J. Med. Dent. Sci. 55, 113–120 (2008).
Chien, Y. H., Zeng, X. & Prinz, I. The natural and the inducible: interleukin (IL)-17-producing γδ T cells. Trends Immunol. 34, 151–154 (2013).
van Rensen, E. L. et al. Bronchial CD8 cell infiltrate and lung function decline in asthma. Am. J. Respir. Crit. Care Med. 172, 837–841 (2005).
den Otter, I. et al. Lung function decline in asthma patients with elevated bronchial CD8, CD4 and CD3 cells. Eur. Respir. J. 48, 393–402 (2016).
Dakhama, A. et al. IL-13-producing BLT1-positive CD8 cells are increased in asthma and are associated with airway obstruction. Allergy 68, 666–673 (2013).
Huber, M. & Lohoff, M. Change of paradigm: CD8+ T cells as important helper for CD4+ T cells during asthma and autoimmune encephalomyelitis. Allergo. J. Int. 24, 8–15 (2015).
Hatano, R., Ohnuma, K., Yamamoto, J., Dang, N. H. & Morimoto, C. CD26-mediated co-stimulation in human CD8(+) T cells provokes effector function via pro-inflammatory cytokine production. Immunology 138, 165–172 (2013).
Lee, N. et al. IL-6 receptor α defines effector memory CD8+ T cells producing Th2 cytokines and expanding in asthma. Am. J. Respir. Crit. Care Med. 190, 1383–1394 (2014).
Casrouge, A. et al. Lymphocytes are a major source of circulating soluble dipeptidyl peptidase 4. Clin. Exp. Immunol. 194, 166–179 (2018).
Zhen, G. et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am. J. Respir. Cell Mol. Biol. 36, 244–253 (2007).
Zhou, T. et al. Associations between Th17-related inflammatory cytokines and asthma in adults: A Case-Control Study. Sci. Rep. 7, 15502 (2017).
Yan, S., Gessner, R., Dietel, C., Schmiedek, U. & Fan, H. Enhanced ovalbumin-induced airway inflammation in CD26−/− mice. Eur. J. Immunol. 42, 533–540 (2012).
Stephan, M. et al. Effects of dipeptidyl peptidase-4 inhibition in an animal model of experimental asthma: a matter of dose, route, and time. Physiol. Rep. 1, e00095 (2013).
Di Mitri, D. et al. Reversible senescence in human CD4 + CD45RA + CD27- memory T cells. J. Immunol. 187, 2093–2100 (2011).
Bank, I. & Marcu-Malina, V. Quantitative peripheral blood perturbations of γδ T cells in human disease and their clinical implications. Clin. Rev. Allergy Immunol. 47, 311–333 (2014).
Pang, D. J., Neves, J. F., Sumaria, N. & Pennington, D. J. Understanding the complexity of γδ T-cell subsets in mouse and human. Immunology 136, 283–290 (2012).
Dieli, F. et al. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J. Exp. Med. 198, 391–397 (2003).
Caccamo, N. et al. Differentiation, phenotype, and function of interleukin-17-producing human Vγ9Vδ2 T cells. Blood 118, 129–138 (2011).
Oberg, H. H., Wesch, D., Grüssel, S., Rose-John, S. & Kabelitz, D. Differential expression of CD126 and CD130 mediates different STAT-3 phosphorylation in CD4 + CD25− and CD25high regulatory T cells. Int. Immunol. 18, 555–563 (2006).
Lamas, J. R. et al. Alternative splicing and proteolytic rupture contribute to the generation of soluble IL-6 receptors (sIL-6R) in rheumatoid arthritis. Cytokine 61, 720–723 (2013).
Schumacher, N. et al. Shedding of Endogenous Interleukin-6 Receptor (IL-6R) Is Governed by A Disintegrin and Metalloproteinase (ADAM) Proteases while a Full-length IL-6R Isoform Localizes to Circulating Microvesicles. J. Biol. Chem. 290, 26059–26071 (2015).
Yan, I. et al. ADAM17 controls IL-6 signaling by cleavage of the murine IL-6Ralpha from the cell surface of leukocytes during inflammatory responses. J. Leukoc. Biol. 99, 749–760 (2016).
Wolf, J. et al. A soluble form of the interleukin-6 family signal transducer gp130 is dimerized via a C-terminal disulfide bridge resulting from alternative mRNA splicing. Biochem. Biophys. Res. Commun. 470, 870–876 (2016).
Hemken, P. M. et al. Development and analytical performance of a new ARCHITECT automated dipeptidyl peptidase-4 immunoassay. Pract. Lab. Med. 9, 58–68 (2017).
Agache, I. et al. Monitoring inflammatory heterogeneity with multiple biomarkers for multidimensional endotyping of asthma. J. Allergy Clin. Immunol. 141, 442–445 (2018).
Hartl, D. et al. Quantitative and functional impairment of pulmonary CD4 + CD25hi regulatory T cells in pediatric asthma. J. Allergy Clin. Immunol. 119, 1258–1266 (2007).
Mandapathil, M. et al. CD26 expression and adenosine deaminase activity in regulatory T cells (Treg) and CD4(+) T effector cells in patients with head and neck squamous cell carcinoma. Oncoimmunology 1, 659–669 (2012).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
De Meester, I. et al. Binding of adenosine deaminase to the lymphocyte surface via CD26. Eur. J. Immunol. 24, 566–570 (1994).
Moreno, E. et al. Molecular Evidence of Adenosine Deaminase Linking Adenosine A2A Receptor and CD26 Proteins. Front. Pharmacol. 9, 106 (2018).
Li, P. et al. CD39+ regulatory T cells attenuate allergic airway inflammation. Clin. Exp. Allergy 45, 1126–1137 (2015).
Liu, W. et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203, 1701–1711 (2006).
Doganci, A. et al. The IL-6R alpha chain controls lung CD4 + CD25+ Treg development and function during allergic airway inflammation in vivo. J. Clin. Invest. 115, 313–325 (2005).
Wang, Y. et al. The IL6R gene polymorphisms are associated with sIL-6R, IgE and lung function in Chinese patients with asthma. Gene 585, 51–57 (2016).
Acknowledgements
This work was supported by grants from Sociedad Española de Neumología y Cirugía Torácica, (SEPAR) (121/2012) and Instituto de Salud Carlos III, Ministerio de Economía y Competitividad (Fondo de Investigación Sanitaria, FIS; co-financed by European Union ERDF funds) (PI13/02046). JJN-F is a recipient of a Xunta de Galicia Fellowship (co-financed by European Social Fund (ESF)).
Author information
Authors and Affiliations
Contributions
J.J.N.-F., F.J.S., M.E.S.-J. and M.J.C. performed flow cytometry experiments and serum soluble marker measurements. A.P.-D. and F.J.S. carried out the in vitro culture studies. J.J.N.-F., F.J.S., F.J.G.-B., M.N. and P.A. participated in the study design, statistical analysis, and article writing. L.V. and F.J.G.-B. carried out sample collection and the clinical part of the study. All authors critically reviewed the manuscript and approved the final draft to be published.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nieto-Fontarigo, J.J., Salgado, F.J., San-José, M.E. et al. Expansion of different subpopulations of CD26−/low T cells in allergic and non-allergic asthmatics. Sci Rep 9, 7556 (2019). https://doi.org/10.1038/s41598-019-43622-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43622-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.