Abstract
Muscle nonshivering thermogenesis (NST) was recently suggested to play an important role in thermoregulation of species lacking brown adipose tissue (BAT). The mechanism, which is independent of muscle contractions, produces heat based on the activity of an ATPase pump in the sarcoplasmic reticulum (SERCA1a) and is controlled by the protein sarcolipin. To evaluate whether muscle NST could indeed play an important role in thermoregulation in species lacking BAT, we investigated the thermogenic capacities of newborn wild boar piglets. During cold exposure over the first 5 days of life, total heat production was improved while shivering intensity decreased, indicating an increasing contribution of NST. Sampling skeletal muscle tissue for analyses of SERCA activity as well as gene expression of SERCA1a and sarcolipin, we found an age-related increase in all three variables as well as in body temperature. Hence, the improved thermogenesis during the development of wild boars is not due to shivering but explained by the observed increase in SERCA activity. Our results suggest that muscle NST may be the primary mechanism of heat production during cold stress in large mammals lacking BAT, strengthening the hypothesis that muscle NST has likely played an important role in the evolution of endothermy.
Similar content being viewed by others
Introduction
The regulation of a high and stable body temperature (Tb) independent of climatic conditions is one of the most important mechanisms that arose during the evolution of mammals and birds. After decades of intensive research, it is now well-understood how mammals possessing brown adipose tissue (BAT) - a specialised thermogenic organ - are able to maintain an optimal Tb even in cold environments by using nonshivering thermogenesis (NST)1. However, only ~20% of endothermic birds and mammals actually possess BAT2. NST in BAT requires thermogenic functional uncoupling protein 1 (UCP1) that alters proton conductance in the inner mitochondrial membrane, leading to heat generation instead of ATP production3,4. Functional UCP1 has not been found, however, in marsupials or monotremes5 and typically substantial amounts of BAT are present only in neonates of large-bodied mammals6,7, but see8. Furthermore, a recent study has shown that UCP1-inactivating mutations have occurred in various mostly large-bodied placental mammals9. Large mammals, such as pigs, are likely able to cope well with cold exposure even without possessing BAT. Their juveniles, on the other hand, have high surface area-to-volume ratios and need a high capacity for heat production to maintain a constantly high Tb10. Inside the mother´s womb juveniles are protected against thermal variations and they are exposed to a cold environment for the first time after birth. In fact, neonates of many large-bodied species in which BAT depots are negligible in adults, possess large amounts of BAT6,7.
It was long believed that species without thermogenic functional UCP1 rely solely on shivering, a process that on its own is in fact insufficient for the maintenance of a stable Tb during cold exposure in knockout mice11. However, a second mechanism of NST in muscle, which had been studied in vitro for a long time12,13,14,15, has recently been shown to play an important role in thermoregulation in mice lacking functional BAT11. The mechanism is so far only confirmed as an additional form of NST in rodents in which the wild type possesses BAT11,16,17,18,19,20, but is assumed to occur in all mammals2,21. Furthermore, muscle NST is discussed as a heat production mechanism that played an important role for the evolution of a high Tb, i.e. endothermy, in mammals2,21, as endothermy in this group evolved before UCP1- mediated NST22.
In short, muscle NST is based on the activity of the Ca2+-ATPase pump in the sarcoplasmic reticulum (SERCA). SERCA1a, the major isoform occurring in skeletal muscle23, is involved in muscle contraction via the transport of Ca2+-ions from the cell lumen into the sarcoplasmic reticulum24,25. But ATP hydrolysis by SERCA1a can be uncoupled from actual transmembrane transport of Ca2+ by the regulatory protein sarcolipin (SLN) causing the release of the Ca2+-ions bound to SERCA back to the cytoplasmic side of the membrane (so called “slippage”) rather than into the sarcoplasmic reticulum26. This results in increased ATP hydrolysis and heat production in muscle through SERCA1a activity without actual Ca2+-transport and without muscle contraction13,26,27,28,29. Studies on laboratory mice have shown that muscle NST can compensate for the loss of UCP1, but muscle NST in wild type mice is largely masked by heat production in BAT30,31. This raises the question whether the thermogenic capacity of muscle NST alone can enable mammals to maintain a stable Tb under cold conditions.
We investigated whether muscle NST is an important and effective mechanism to generate heat in feral, (i.e. wild type) mammals lacking BAT, using newborn wild boars (Sus scrofa), naturally lacking BAT32 and UCP1-dependent NST33,34 as model species. Importantly, juvenile wild boars are born in early spring, when ambient temperatures (Ta) can still be around or below 0 °C and thermoregulatory demands are high. We firstly hypothesized that, if NST plays a significant role for thermogenesis in piglets, heat production should increase during cold exposure, whereas shivering should remain constant or may even decrease. We secondly hypothesized that, if heat production is based on muscle NST, expression levels of SERCA1a and sarcolipin as well as SERCA activity should be elevated during cold exposure.
Material and Methods
Experimental setup
Piglets were born in March 2017 by five sows kept and bred in outdoor enclosures at the Research Institute of Wildlife Ecology (48.22°N, 16.28°E) of the University of Veterinary Medicine Vienna in Austria. Each sow was provided with a roofed shelter outfitted with straw in which they gave birth. Shelters were equipped with IP-cameras and activity of the sows was monitored to know the exact time of birthing. All animals were exposed to natural Ta but piglets typically huddled with each other or with adults. Mean daily Ta during the 5 days of our study ranged from 5.9 °C to 14.7 °C and nightly minimum Tas were between 1.5 °C and 9.4 °C.
We temporarily removed 19 of 29 newborn wild boars from their mothers within the first 24 hours after birth (age 8.5–24 h) and again four days later. Rectal Tb was taken within 5–10 minutes after removal from the mother by inserting a thermometer with rectal gel approximately 2 cm into the rectum. Piglets were weighed to an accuracy of 1 g (Sartorius, Göttingen, Germany) and equipped with a custom-made acceleration logger (see below) that was firmly attached to their abdomen with a cohesive bandage (Henry Schein, New York, USA) before being placed individually into metabolic chambers (40 × 25 × 22 cm, volume: 20 l). The metabolic chambers were located inside a walk-in climate chamber that was set to +10 °C (mean 10.3 ± 0.7 °C). Individuals were exposed to cold for approximately 60–90 min and heat production and shivering intensity during cold exposure were determined (see below). After measurements rectal Tb was determined again and piglets were transferred to a surgery room where muscle biopsies were taken (see below).
Metabolic measurements
All experiments were conducted between 0800 h and 1630 h. Metabolic measurements lasted for 60–90 min (depending on the activity level of the animal). Animals were allowed to acclimate to cold conditions for 10 min, before the measurement was started. Energy expenditure was determined by measuring the rate of O2 consumption (VO2) as a proxy of metabolic rate using an O2 and CO2 analyser (Servopro 4100, Servomex, Crowborough, UK). The metabolic chamber was connected to the analyser (pull mode; order: metabolic chamber, pump, needle valve, flow meter, O2 analyser, CO2 analyser) with airtight tubes. Water vapour was removed from the air prior to analysis using silica gel. A gas switch allowed measurement of air from six metabolic cages and one reference air channel for one minute each. The analyser was calibrated once a week using a high precision gas-proportioning pump (H. Wösthoff, Bochum, Germany, type 55A27/7a). Air was continuously drawn through the cages with pumps at a flow rate of 250 on day 1 and −330 l h−1 on day 5. Flow rate through each metabolic chamber was measured using calibrated thermal mass-flow meters (FMA 3100, Omega Engineering, Stamford, CT, USA). VO2 was calculated by a self-written R program35 using equation 10.6 by Lighton36 and converted to heat production (watts) assuming 20.1 J ml−1 O2 consumption37. VO2 were computed from the mean of the three lowest consecutive values per measurement. To standardize measurements we only used the first 30 min of the measurement for calculations.
Shivering
We successfully measured shivering on day 1 and day 5 of 12 cold-exposed piglets by attaching a custom-made acceleration logger (three axis acceleration sensor ADXL345; 3 × 1 × 45 mm; Li-polymer rechargeable Battery LP-402025-1S-3; weight with battery of 6.4 g). The sensor of our acceleration logger measured acceleration in three orthogonal axes (x, y, and z). Sampling rate of the acceleration sensor was set to 1600 Hz, acceleration range was ±16 g and had a resolution of 4 mg per axis. The battery had a capacity of 155 mAh limiting the runtime to approx. 6 hours, which was sufficient for our needs. We analyzed the acceleration sensor data entirely in the frequency domain. For this purpose, we calculated the resulting acceleration from the x, y and z-components:
Then we subtracted the mean acceleration stemming from gravitation and applied a Fast Fourier Transformation (FFT) in R (V3.4.2)35 (fft in package ‘signal’)38 to consecutive segments of 6 second intervals in the time domain (see Supplementary Fig. S1). From every FFT output we extracted the following parameters:
-
Maximum Amplitude (m/s²)
-
Frequency at Maximum Amplitude (in Hz)
Exposure to 10 °C without the opportunity to huddle with conspecifics caused clearly visible shivering with short intermitted phases of suspension of shivering. To calculate shivering intensity (the maximum amplitude) we used the same time frame as for metabolic rate calculations, i.e. a total of 30 min, starting after 10 min of acclimatization. The frequency of muscle activation during shivering thermogenesis is correlated with body mass in mammals39 and is reported to be:
where f is the mean shivering frequency in Hz and m is the mean body mass in g39. Based on this equation the shivering frequency of day 1 to day 5 old piglets with a mean body mass of 1700 g ± 306 g is predicted to be 18.55 Hz. We therefore restricted our analyses to frequencies between 10 Hz and 30 Hz and excluded frequencies above or below this range from the analysis. Measured mean shivering frequency at maximum amplitude for piglets was with 17.97 ± 1.66 Hz (N = 24) in the predicted range and did not differ between days (χ2 = 0.540, df = 1, p = 0.462).
Biopsies
80–100 mg muscle tissue was taken with a biopsy needle from the thigh region of the piglets (Musculus semimembranosus) under standard surgical conditions. The procedure was conducted under general anaesthesia in combination with local anaesthesia. The anaesthesia was induced by placing a mask over the piglets mouth and nose, using an Isofluran gas (Isofluran, Isoba, MSD animal health, Vienna, Austria) inhalation machine and medical oxygen. Pain relief was achieved by injecting local anaesthetics (Lidocain, Xylocain 2% with Epinephrin 1:100,000. Mibe GmbH, Brehna, Germany) 10 min prior biopsy in the proximal region of the M. semimembranosus and the skin. Anti-inflammatory and pain relief treatment was furthermore gained by injecting Meloxicam 10 min prior biopsy intramuscularly (Meloxicam 0.4 mg/kg, Metacam 2% inj., Boehringer Ingelheim Vetmedica, Ingelheim, Germany). During the entire procedure, vital parameters (respiration rate, peripheral haemoglobin oxygen saturation as measured by pulse oximetry (SpO2), heart rate, Tb) and anaesthetic depth were monitored. The skin and muscle fascia incisions were closed separately in two layers with absorbable sutures (Surgicryl USP 2/0 PGA, SMI AG, Hünningen, Belgium). Piglets were marked with an ear tag for further individual recognition. After the biopsy the juveniles were monitored and kept in a warm environment for recovery from anaesthesia until they were moved to their enclosure. Piglets were fed with 1 mL of glucose solution orally (50% glucose infusion, B. Braun AG, Melsungen, Germany) before being returned to their mothers. Piglets were never kept away from their mother for more than 3–4 hours. The muscle tissue sample was split for biochemical as well as genetic analyses. We were able to obtain samples on both days of 17 piglets. The two animals for which we only obtained one sample were excluded from further analyses regarding SERCA1a and SLN. For two further animals tissue material was too small to be used for biochemical analyses, which reduced the sample size for SERCA activity to N = 15.
Biochemical analysis
Approximately 50 mg of muscle tissue of 15 piglets were snap frozen within 10 min and later stored at −80 °C until used to prepare muscle homogenates according to the procedures described in Giroud et al.40. Homogenates were used to measure SERCA activities by a standard coupled enzyme assay, in which the rate of SERCA ATP hydrolysis was calculated from spectrophotometric recording (method previously described by Simonides et al.41). While this assay is measuring overall ATPase activity of all isoforms of SERCA present in the sample, SERCA activity is assumed to primarily reflect SERCA1a activity, because SERCA1a is the major isoform found in fast twitching muscles, such as M. semimembranosus23. SERCA activity was divided by total protein concentration (determined with Bradford method)42 in order to normalize the ATPase activity values for variations in total protein concentrations among samples. More details on isolation of muscle homogenates and SERCA activity measurements can be found in the Supplementary Methods.
Gene expression analyses
Further, 30 mg muscle tissue of 17 piglets were directly stored in RNAlater®, kept in the fridge for 24 hours and stored at −80 °C until RNA was extracted using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Hilden, Germany). Gene expression levels for SLN and SERCA1a were analysed from cDNA via Droplet Digital PCR (ddPCR™). RNA was reverse-transcribed with MultiScribe™ Reverse Transcriptase (High Capacity cDNA Reverse Transcription Kits, ThermoFisher Scientific) using random hexamer primers. Primer sequences for the target gene, SLN, were available from Vangheluwe et al.43. No primer sequence was available for SERCA1a so we designed suitable primers from the reference sequence NM_001204393.1 with the assistance of the NCBI primer design tool44. Primers for candidate reference genes Hypoxanthine Phosphoribosyltransferase 1 (HPRT1) and Glucuronidase Beta (GUSB) were available from45. All primer sequences as well as additional methodological details can be found in the Supplementary Methods and Table S1. Data acquisition was accomplished by the QX200™ Droplet Reader (Bio-Rad), and analysed using the Bio-Rad Droplet Digital™ PCR QuantaSoft software. Expression levels are given as the relative ratio of the concentration (copies/µl) of the assay target gene over the concentration of the reference gene. We did not determine protein levels, but SLN protein expression has been shown to correlate well with SLN mRNA expression31.
Data analyses
Data are presented as mean ± 1 S.E.; N denotes the number of individuals. Data were analysed in R (V3.4.2)35. We first tested whether SERCA1a gene expression, SLN gene expression, SERCA activity and Tb increased with age (using group “day 1” and “day 5”) by employing a linear-mixed effect model followed by type II sum-of-squares ANOVA (lme in library ‘nlme’46; Anova in library ‘car’47). All models were corrected for non-independence by including the individual’s mother as well as the individual´s ID as nested random effects. Models on SERCA1a gene expression and SERCA activity were additionally corrected for heteroscedasticity. To investigate whether SERCA activity was explained by SLN and SERCA1a gene expression, we additionally computed linear-mixed effect models including random effects as described above (pseudo r2 was calculated using sem.model.fits in library ‘piecewiseSEM’)48. For the cold exposure experiments we tested whether there was an age depended change in heat production, shivering intensity and shivering frequency as described above. In tests for changes of total heat production, we included body mass as a covariate. Mass-specific rates of heat production were computed for graphical presentation, but not used for statistical tests. To test Tb regulation over the time of cold exposure we computed a linear mixed effect model, again with ID and mother as random effects, Tb at the end of exposure as the response variable, and day (1 or 5), initial Tb, and duration of cold exposure (60–90 min) as fixed effects. We used Shapiro-Wilk tests to access the normality of model residuals. If needed, data were Box-Cox transformed.
Results
Cold exposure experiment
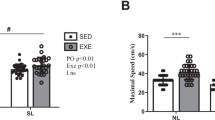
Heat production during cold exposure did not significantly differ between day 1 and day 5 when adjusted for the increase in body mass (χ2 = 1.92, df = 1, p = 0.165; N = 19; Fig. 1A), although shivering intensity decreased between both measurements by 50% (χ2 = 9.00, df = 1, p = 0.003; N = 12; Figs 1B and 2A,B). Tb after cold exposure was dependent on Tb at start of the experiment (Table 1; χ2 = 8.01, df = 1, p = 0.0046) but did not significantly differ between the days (χ2 = 3.33, df = 1, p = 0.0679) and was not dependent on the duration of cold exposure (χ2 = 1.86, df = 1, p = 0.173).
Change in (A) heat production and (B) shivering between day 1 and day 2. Different letters indicate significant differences. Heat production, adjusted for the increase in body mass, did not significantly differ between days (N = 19; χ2 = 1.92, df = 1, p = 0.165), whereas shivering significantly decreased (N = 12; χ2 = 9.00, df = 1, p = 0.003).
SERCA1a, SLN and SERCA activity
SERCA activity, as well as SERCA1a and SLN mRNA expressions increased significantly from day 1 to day 5 of the juveniles´ life (Fig. 3, Table 1; SERCA1: χ2 = 15.93, df = 1, p < 0.0001; SLN: χ2 = 9.64, df = 1, p = 0.002; SERCA activity: χ2 = 16.81, df = 1, p < 0.0001) and SERCA1a and SLN expression levels were positively correlated (χ2 = 32.2, df = 1, p < 0.0001, marginal pseudo r2: 0.51; Fig. 4). There was generally more variability in the gene expression data at day 5 than at day 1 (Fig. 3A,B). Importantly, the age-related increase of SERCA activity was linked to the increase in SLN gene expression, when tested in a single-predictor model (χ2 = 13.68, df = 1, p < 0.001) as well as in multiple regression together with SERCA1 (χ2 = 6.57, df = 1, p = 0.01; Fig. 5A); SERCA1a gene expression was only significantly influencing SERCA activity when used in a single-predictor model (χ2 = 7.23, df = 1, p = 0.007), but not when tested together with SLN (χ2 = 0.002, df = 1, p = 0.967; Fig. 5B). Tb of piglets increased significantly from day 1 to day 5 (χ2 = 41.01, df = 1, p < 0.001; Table 1) and this increase was linked to SERCA activity (χ2 = 5.22, df = 1, p = 0.02).
Change in (A) SERCA1a and (B) SLN gene expression (copies per µl target gene/copies per µl reference gene (HPRT1)) between day 1 and day 5 (N = 17 per day). Depicted is the range of individual data (dots) as well as the mean values (line). SERCA1a and SLN gene expression were significantly increased on day 5. Statistical test results are reported in the text.
Partial regression plots of the effect of (A) SERCA1a and (B) SLN gene expression on SERCA activity. The increase of SERCA activity was linked to SLN gene expression (χ2 = 6.57, df = 1, p = 0.01), while SERCA1a gene expression had no significant effect on SERCA activity when tested together with SLN (χ2 = 0.002, df = 1, p = 0.967). Gene expression is reported in copies per µl target gene/copies per µl reference gene (HPRT1), SERCA activity as ATP hydrolyses per minute and mg total protein.
Discussion
Our study revealed that shivering intensity decreases from day 1 to day 5 in juveniles exposed to 10 °C, while heat production during cold is increasing proportional to body mass and the level at which Tb is maintained increases. This result is clear evidence for an increasing contribution of NST to thermogenesis during cold exposure in piglets over the first days of life. The finding that SERCA activity and the expression of SERCA1a and SLN were recruited simultaneously points to muscle NST and increased SERCA activity as the principal source of heat production, as we can rule out UCP1- mediated NST in this species. We observed a high variability in the gene expression data at day 5, suggesting that there is some heterogeneity to the individual response rate to cold exposure. Alternatively, this could reflect different micro-environmental conditions (e.g., benefits through huddling depending on litter size) that the piglets experienced within the enclosures during early development. Further strong evidence would be provided from measurements of increased SLN protein content. Unfortunately, in this study, the limitation of sample material available for analyses and the lack of sufficiently high quality antibodies for wild boar precluded us from adding this extra confirmation of the mechanism.
Our statistical analysis suggests that the increase in SERCA activity between day 1 and day 5 was mainly due to an SLN controlled upregulation of ATP hydrolysis by SERCA1a instead of an increase in SERCA1a molecules. This is in accordance with previous data on mice in which upregulated SLN expression led to an increasing contribution of SERCA-based Ca2+-slippage to heat production11,17. However, we cannot exclude that there are also other processes or tissues involved. For example, a study on cold-acclimatization of UCP1-knockout mice has found changes in gene expression levels in inguinal white adipose tissue between cold-adapted wild type and knockout mice, while no change was detected in skeletal muscle49.
In mice and rats, in which neonates are born blind and naked (‘altricial’ neonates), the transcription and translation of SLN is highest after birth and gradually decreases with development when kept at normal housing conditions (~23 °C)16,31, while the amount of BAT is successively recruited1. However, when kept under cold conditions juvenile mice keep SLN upregulated for improved thermoregulatory capacity31, suggesting that both mechanisms of NST are necessary for an effective maintenance of a high Tb during cold exposure in newborn rodents. In adult mice, however, both mechanisms of NST, UCP1-mediated as well as muscle NST, can compensate for the loss of one system50. In contrast, our data indicate that muscle NST, in combination with some shivering, is already sufficient to maintain a stable Tb for short-term cold exposure in juvenile wild boar, which are markedly larger than juvenile mice and are already born with fur and much better thermogenic abilities (‘precocial’ juveniles). Interestingly, in other precocial species, such as sheep and goats that possess functional UCP1, BAT is recruited already before birth1. Furthermore, reconstituted function of UCP1 can further improve thermoregulatory function of cold exposed 6-months old Bama pigs, a cold-sensitive pig breed.
Our finding that muscle NST is involved in thermoregulation of juvenile wild boars and allows a near stable Tb even during short-term cold exposure supports the hypothesis that muscle NST may be the primary mechanism of heat production during cold-exposure in large mammals lacking BAT2,21. While the evolution of BAT has often been related to the ability of small placental mammals to colonize colder habitats4,51,52, a recent study has shown that UCP1-inactivating mutations have occurred in at least eight of the 18 placental mammalian orders, mainly in larger-bodied species9, such as pigs. It therefore appears that the combination of shivering and muscle NST is sufficient for heat production in large mammals. Pigs, for example, likely lost UCP1 function and the ability to use BAT for thermoregulation because of absent or only weak selection for this mechanism in a warm climate33; all species except the wild boar live only in tropical or subtropical habitats. In addition to heat production via muscle NST, wild boar apparently evolved compensatory mechanisms to cope with adverse thermal conditions in northern habitats, such as larger adult body size53, building insulating nests for offspring, and synchronizing reproduction within social groups or enabling piglets to huddle in large groups of combined litters33,54. Behavioural thermoregulation is less energetically costly than NST55 and a study on winter mortality of juvenile wild boar has shown that the negative effects of cold winters can be compensated by high availability of food resources53.
In addition to our finding that SLN-mediated NST in skeletal muscle is involved in piglet thermoregulation, recent studies on domestic pig breeds suggest that SERCA2b (another isoform of SERCA) and UCP3 might also influence pig thermoregulation56,57. However, so far the importance of both mechanisms is unclear2 and the evolution of a compensatory mechanism after the pigs colonized cold habitats is likely56, while muscle NST is discussed as a potentially evolutionary old heat production mechanism2,21. Whether and to what extend domestic pig breeds also possess muscle NST remains speculative. While piglets of wild boar are accustomed to deal with temperatures around or below zero degrees, domestic pigs are kept under warm conditions (20–35 °C). Therefore, it cannot be ruled out that the extreme susceptibility of pigs to cold is partly due to inadvertent selection against high thermogenic capacity during domestication. An earlier study on thermoregulation of young domestic pigs (~13 days old) showed that piglets dealt with a five day cold exposure with a decrease in core Tb and an increase in insulation by postural changes58. Previous studies on thermoregulation of juvenile domestic pigs have also found that shivering intensity decreased during the first days after birth while heat production and blood flow to muscles simultaneously increased59,60,61. While this was originally attributed to an increase in shivering efficiency59,60, it seems questionable whether an increased thermogenesis by increased efficiency of shivering is physically possible. Our data now suggest that the improved thermogenesis found in domestic pigs, similarly to wild boar, was not due to an increase in shivering efficiency, but explained by an increase in muscle NST.
Taken together, our data show for the first time that muscle-based NST via SERCA1a plays a role in the thermoregulation of wild type mammals lacking BAT and that muscle NST can replace UCP1-mediated NST. The function of UCP1 as a thermogenic protein has occurred after the divergence between placental and marsupial mammals22, suggesting that the evolution of endothermy in ancestral mammals was independent of heat production in BAT. Although the earth was likely warmer, ancestral mammals still would have experienced daily and yearly fluctuations in Ta, likely similar to temperatures found in tropical areas today, which can get rather cold during the night. Therefore, while heat produced as a by-product of metabolic processes as well as basking62 would have allowed the establishment of a stable Tb during a big part of the day, muscle NST was likely important during the colder night hours.
Ethics statement
The study was approved by the institutional ethics and animal welfare committee and the national authority according to §§ 26ff. of Animal Experiments Act, Tierversuchsgesetz 2012 – TVG 2012 (BMWFW-68.205/0171-WF/V/3b/2016).
Data Availibility
The data that support the findings are available in figshare at https://doi.org/10.6084/m9.figshare.6839591.
References
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol Rev 84, 277–359, https://doi.org/10.1152/physrev.00015.2003 (2004).
Nowack, J., Giroud, S., Arnold, W. & Ruf, T. Muscle non-shivering thermogenesis and its role in the evolution of endothermy. Frontiers in Physiology 8, https://doi.org/10.3389/fphys.2017.00889 (2017).
Hansen, E. & Knudsen, J. Parallel measurements of heat production and thermogenin content in brown fat cells during cold acclimation of rats. Biosci Rep 6, 31–38, https://doi.org/10.1007/bf01145176 (1986).
Heaton, G., Wagenvoord, R., Kemp, A. J. & Nicholls, D. Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur J Biochem 82, 515–521 (1978).
Hayward, J. S. & Lisson, P. A. Evolution of brown fat: its absence in marsupials and monotremes. Can J Zool 70, 171–179, https://doi.org/10.1139/z92-025 (1992).
Vatnick, I., Tyzbir, R. S., Welch, J. G. & Hooper, A. P. Regression of brown adipose tissue mitochondrial function and structure in neonatal goats. American Journal of Physiology - Endocrinology and Metabolism 252, E391–E395, https://doi.org/10.1152/ajpendo.1987.252.3.E391 (1987).
Alexander, G., Bennett, J. & Gemmell, R. Brown adipose tissue in the new‐born calf (Bos taurus). The Journal of Physiology 244, 223–234, https://doi.org/10.1113/jphysiol.1975.sp010793 (1975).
Gilsanz, V., Hu, H. H. & Kajimura, S. Relevance of brown adipose tissue in infancy and adolescence. Pediatric research 73, 3–9, https://doi.org/10.1038/pr.2012.141 (2013).
Gaudry, M. J. et al. Inactivation of thermogenic UCP1 as a historical contingency in multiple placental mammal clades. Science Advances 3, e1602878, https://doi.org/10.1126/sciadv.1602878 (2017).
Barnett, S. A. & Neil, A. C. The growth of infant mice at two temperatures. J Reprod Fertil 29, 191–201, https://doi.org/10.1530/jrf.0.0290191 (1972).
Bal, N. C. et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nature Medicine 18, 1575–1579 (2012).
de Meis, L. Ca2+-ATPases (SERCA): energy transduction and heat production in transport ATPases. J Membrane Biol 188, 1–9, https://doi.org/10.1007/s00232-001-0171-5 (2002).
de Meis, L. Uncoupled ATPase activity and heat production by the sarcoplasmic reticulum Ca2+-ATPase. Regulation by ADP. J Biol Chem 276, 25078–25087, https://doi.org/10.1074/jbc.M103318200 (2001).
de Meis, L. Role of the sarcoplasmic reticulum Ca2+-ATPase on heat production and thermogenesis. Biosci Rep 21, 113–137, https://doi.org/10.1023/A:1013640006611 (2001).
de Meis, L., Arruda, A. & Carvalho, D. Role of sarco/endoplasmic reticulum Ca2+-ATPase in thermogenesis. Biosci Rep 25, 181–190, https://doi.org/10.1007/s10540-005-2884-7 (2005).
Babu, G. J., Bhupathy, P., Carnes, C. A., Billman, G. E. & Periasamy, M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. Journal of Molecular and Cellular Cardiology 43, 215–222, https://doi.org/10.1016/j.yjmcc.2007.05.009 (2007).
Bal, N. C., Maurya, S. K., Singh, S., Wehrens, X. H. T. & Periasamy, M. Increased reliance on muscle-based thermogenesis upon acute minimization of brown adipose tissue function. J Biol Chem 291, 17247–17257, https://doi.org/10.1074/jbc.M116.728188 (2016).
Pant, M., Bal, N. C. & Periasamy, M. Sarcolipin: a key thermogenic and metabolic regulator in skeletal muscle. Trends in Endocrinology & Metabolism 27, 881–892, https://doi.org/10.1016/j.tem.2016.08.006 (2016).
Anderson, K. J. Multi-omic analysis of hibernator skeletal muscle and regulation of calcium handling Master of Science thesis, University of Minnesota (2016).
Anderson, K. J. et al. Proteogenomic analysis of a hibernating mammal indicates contribution of skeletal muscle physiology to the hibernation phenotype. Journal of Proteome Research 15, 1253–1261, https://doi.org/10.1021/acs.jproteome.5b01138 (2016).
Rowland, L. A., Bal, N. C. & Periasamy, M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol Rev 10.1111/brv.12157, https://doi.org/10.1111/brv.12157 (2014).
Saito, S., Saito, C. T. & Shingai, R. Adaptive evolution of the uncoupling protein 1 gene contributed to the acquisition of novel nonshivering thermogenesis in ancestral eutherian mammals. Gene 408, 37–44, https://doi.org/10.1016/j.gene.2007.10.018 (2008).
Periasamy, M. & Kalyanasundaram, A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve 35, 430–442, https://doi.org/10.1002/mus.20745 (2007).
Hasselbach, W. & Makinose, M. Die Calciumpumpe der Erschlaffungsgrana des Muskels und ihre Abhängigkeit von der ATPSpaltung. Biochemische Zeitschrift 333, 518–528 (1961).
Periasamy, M. & Huke, S. SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. Journal of Molecular and Cellular Cardiology 33, 1053–1063, https://doi.org/10.1006/jmcc.2001.1366 (2001).
Mall, S. et al. The presence of sarcolipin results in increased heat production by Ca2+-ATPase. J Biol Chem 281, 36597–36602, https://doi.org/10.1074/jbc.M606869200 (2006).
Asahi, M. et al. Sarcolipin regulates sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) by binding to transmembrane helices alone or in association with phospholamban. PNAS 100, 5040–5045, https://doi.org/10.1073/pnas.0330962100 (2003).
Maurya, S. K. et al. Sarcolipin is a key determinant of the basal metabolic rate, and its overexpression enhances energy expenditure and resistance against diet-induced obesity. J Biol Chem 290, 10840–10849, https://doi.org/10.1074/jbc.M115.636878 (2015).
MacLennan, D. H., Asahi, M. & Tupling, A. R. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Annals of the New York Academy of Sciences 986, 472–480, https://doi.org/10.1111/j.1749-6632.2003.tb07231.x (2003).
Bal, N. C. et al. Mild cold induced thermogenesis: are BAT and skeletal muscle synergistic partners? Biosci Rep 37, BSR20171087, https://doi.org/10.1042/BSR20171087 (2017).
Pant, M., Bal, N. C. & Periasamy, M. Cold adaptation overrides developmental regulation of sarcolipin expression in mice skeletal muscle: SOS for muscle-based thermogenesis? J Exp Biol 218, 2321–2325, https://doi.org/10.1242/jeb.119164 (2015).
Jastroch, M. & Andersson, L. When pigs fly, UCP1 makes heat. Molecular Metabolism 4, 359–362, https://doi.org/10.1016/j.molmet.2015.02.005 (2015).
Berg, F., Gustafson, U. & Andersson, L. The uncoupling protein 1 gene (UCP1) is disrupted in the pig lineage: a genetic explanation for poor thermoregulation in piglets. PLoS Genet 2, e129, https://doi.org/10.1371/journal.pgen.0020129 (2006).
Hou, L. et al. Pig has no uncoupling protein 1. Biochemical and Biophysical Research Communications 487, 795–800, https://doi.org/10.1016/j.bbrc.2017.04.118 (2017).
R Development Core Team. R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2014).
Lighton, J. R. B. Measuring metabolic rates. A manual for scientists. (Oxford University Press, 2008).
Schmidt-Nielsen, K. Animal physiology: adaptation and environment. (Cambridge University Press, 1983).
Kienzle, P. et al. Signal: signal processing. Available from, http://r-forge.r-project.org/projects/signal/ (2014).
Spaan, G. & Klussmann, F. W. Die Frequenz des Kältezitterns bei Tierarten verschiedener Größe. Pflügers Archiv 320, 318–333, https://doi.org/10.1007/bf00588211 (1970).
Giroud, S. et al. Membrane phospholipid fatty acid composition regulates cardiac SERCA activity in a hibernator, the Syrian hamster (Mesocricetus auratus). PloS One 8, e63111, https://doi.org/10.1371/journal.pone.0063111 (2013).
Simonides, W. S. & van Hardeveld, C. An assay for sarcoplasmic reticulum Ca2+-ATPase activity in muscle homogenates. Analytical Biochemistry 191, 321–331, https://doi.org/10.1016/0003-2697(90)90226-Y (1990).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254, https://doi.org/10.1016/0003-2697(76)90527-3 (1976).
Vangheluwe, P. et al. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J 389, 151–159, https://doi.org/10.1042/bj20050068 (2005).
Ye, J. et al. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13, 134–134, https://doi.org/10.1186/1471-2105-13-134 (2012).
Charron, S. et al. Identification of region-specific myocardial gene expression patterns in a chronic swine model of repaired tetralogy of fallot. PloS One 10, e0134146, https://doi.org/10.1371/journal.pone.0134146 (2015).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Core Team. Linear and nonlinear mixed effects models. R package version 3.1-137, https://CRAN.R-project.org/package=nlme (2014).
Fox, J. & Weisberg, S. An companion to applied regression. 2 edn, (Sage, 2011).
Lefcheck, J. S. PiecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol Evol 7, 573–579, https://doi.org/10.1111/2041-210X.12512 (2016).
Ukropec, J., Anunciado, R. P., Ravussin, Y., Hulver, M. W. & Kozak, L. P. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. J Biol Chem 281, https://doi.org/10.1074/jbc.M606114200 (2006).
Rowland, L. A., Bal, N. C., Kozak, L. P. & Periasamy, M. Uncoupling protein 1 and sarcolipin are required to maintain optimal thermogenesis, and loss of both systems compromises survival of mice under cold stress. J Biol Chem 290, 12282–12289, https://doi.org/10.1074/jbc.M115.637603 (2015).
Chaffee, R. R. J. et al. Studies on thermogenesis in brown adipose tissue in temperature-acclimated Macaca mulatta. Comp Biochem Physiol A 50, 303–306, https://doi.org/10.1016/0300-9629(75)90017-1 (1975).
Foster, D. & Frydman, M. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can J Physiol Pharmacol 56, 110–122 (1978).
Vetter, S. G., Ruf, T., Bieber, C. & Arnold, W. What is a mild winter? Regional differences in within-species responses to climate change. PloS One 10, e0132178, https://doi.org/10.1371/journal.pone.0132178 (2015).
Graves, H. B. Behavior and ecology of wild and feral swine (Sus scrofa). Journal of Animal Science 58, 482–492, https://doi.org/10.2134/jas1984.582482x (1984).
Terrien, J., Perret, M. & Aujard, F. Behavioral thermoregulation in mammals: a review. Frontiers in Bioscience 16, 1428–1444 (2011).
Lin, J. et al. Cold adaptation in pigs depends on UCP3 in beige adipocytes. Journal of Molecular Cell Biology, https://doi.org/10.1093/jmcb/mjx018 (2017).
Ikeda, K. et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nature Medicine 23, 1454, https://doi.org/10.1038/nm.4429 (2017).
Heldmaier, G. Cold adaptation by short daily cold exposure in the young pig. J Appl Physiol 36, 163–168 (1974).
Berthon, D., Herpin, P., Bertin, R., De Marco, F. & le Dividich, J. Metabolic changes associated with sustained 48-hr shivering thermogenesis in the newborn pig. Comp Biochem Physiol B 114, 327–335, https://doi.org/10.1016/0305-0491(96)00044-2 (1996).
Berthon, D., Herpin, P. & Le Dividich, J. Shivering thermogenesis in the neonatal pig. J Therm Biol 19, 413–418, https://doi.org/10.1016/0306-4565(94)90040-X (1994).
Herpin, P., Damon, M. & Le Dividich, J. Development of thermoregulation and neonatal survival in pigs. Livest Prod Sci 78, 25–45, https://doi.org/10.1016/S0301-6226(02)00183-5 (2002).
Geiser, F., Stawski, C., Wacker, C. B. & Nowack, J. Phoenix from the ashes: fire, torpor, and the evolution of mammalian endothermy. Frontiers in Physiology, https://doi.org/10.3389/fphys.2017.00842 (2017).
Acknowledgements
We thank Peter Steiger and Michaela Salaba for their help with animal maintenance, Joy Einwaller, Jessica S. Cornils and Arne Müller for help during the experiments, Omid Hekmat and Michael Hämmerle for biochemical analyses and Martin Olesch, Thomas Paumann and Radovan Kovacki for infrastructural support. The study was supported by funding from the Humboldt foundation to JN and by the University of Veterinary Medicine, the City of Vienna, and the Government of Lower Austria.
Author information
Authors and Affiliations
Contributions
J.N. and T.R. designed the experiments, J.N. conducted the experiments, analysed the data and wrote the manuscript, S.V. was involved in the performance of the experiments and helped with statistical analyses, G.S. and J.P. performed the biopsies, M.L. and O.H. conducted the biochemical analyses, M.K. and S.S. conducted the genetic analyses, J.P. designed the accelerometers and computed the shivering intensities, C.B. designed the enclosures and organised logistics, C.B. and W.A. were involved in the discussion of the experimental plan. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nowack, J., Vetter, S.G., Stalder, G. et al. Muscle nonshivering thermogenesis in a feral mammal. Sci Rep 9, 6378 (2019). https://doi.org/10.1038/s41598-019-42756-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42756-z
This article is cited by
-
Cold exposure alters lipid metabolism of skeletal muscle through HIF-1α-induced mitophagy
BMC Biology (2023)
-
Trans-anethole Induces Thermogenesis via Activating SERCA/SLN Axis in C2C12 Muscle Cells
Biotechnology and Bioprocess Engineering (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.