Abstract
Chlamydia pecorum is an established and prevalent infection that produces severe clinical disease in many koala populations, contributing to dramatic population declines. In wild South Australian koala populations, C. pecorum occurrence and distribution is unknown. Here, C. pecorum-specific real-time quantitative PCR (qPCR) was applied to ocular and urogenital swabs from targeted surveys of wild koalas from the mainland Mount Lofty Ranges (MLR) (n = 75) and Kangaroo Island (KI) (n = 170) populations. Historical data from 13,081 KI koalas (1997–2018) provided additional evidence for assessing the absence of C. pecorum infection. In the MLR population, 46.7% (CI: 35.1–58.6%) of koalas were C. pecorum positive by qPCR but only 4% had grade 3 clinical disease. MLR koala fertility was significantly reduced by C. pecorum infection; all reproductively active females (n = 16) were C. pecorum negative, whereas 85.2% of inactive females (n = 23) were positive (P < 0.001). KI koalas were C. pecorum negative and the population was demonstrated to be free of C. pecorum infection with 95% confidence. C. pecorum is a real threat for the sustainability of the koala and KI is possibly the last isolated, large C. pecorum-free population remaining in Australia. These koalas could provide a safeguard against this serious disease threat to an iconic Australian species.
Similar content being viewed by others
Introduction
Chlamydia pecorum is recognised as the most significant pathogen causing mortality in koalas and a key contributor to the dramatic population declines in northern Australia (Queensland and New South Wales)1,2. Chlamydia are obligate intracellular bacteria, of which two species, C. pecorum and C. pneumoniae, can infect and cause disease in wild and captive koalas3. C. pecorum is the most pathogenic species, causing conjunctivitis and blindness4,5, pneumonia6, urinary tract infections (cystitis and nephritis) associated with urinary incontinence7,8; and reproductive tract infections resulting in infertility in both females and males due to, paraovarian cysts, endometritis and vaginitis9, and orchitis, epididymitis and prostatitis, respectively10. C. pneumoniae infection can cause pneumonia and respiratory tract infections, however pneumonia is more commonly reported in captive koala populations, and the prevalence appears lower in wild koalas7,11. In northern koalas, C. pecorum is a prevalent pathogen (up to 90%) and severe overt chlamydial disease is commonly observed1,3.
In South Australia, the two largest koala populations12,13 are found on the mainland in the Mount Lofty Ranges (MLR) and on Kangaroo Island (KI) (Fig. 1). Although these populations are presumed to be healthy based on the overabundance of koalas within these populations12,13, there have been limited reports of the occurrence of infectious diseases.
In MLR, two small scale surveys to investigate Chlamydia prevalence were performed two decades ago using conventional PCR; the first reported all six tested koalas to be positive for Chlamydia, of unknown species14, and in the second 88% (15/17) of animals were C. pecorum positive with a low incidence of clinical disease reported3. The first clinical cases of C. pecorum disease were only recently reported in three MLR free-ranging koalas that all presented with severe conjunctivitis15. Subsequently, a post mortem survey of rescued koalas found a high prevalence of C. pecorum detection (88%, 57/65) in the MLR. However 28% (n = 16) of positive koalas had no disease associated with infection, 21% (n = 12) showed mild clinical signs of conjunctivitis and/or urinary tract infections and the remaining 51% (n = 29) had inapparent, predominantly microscopic, C. pecorum infection only16.
On KI, previous investigations of Chlamydia used outdated techniques with low diagnostic sensitivity and specificity in comparison to PCR, which is regarded as the gold standard3. Radiography showed potential paraovarian cysts in eleven female koalas in 198417. Serological studies reported conflicting results, with one study finding no anti-Chlamydia antibodies in 63 koalas in 198918 and the second a seroprevalence of 18% in 1997 (n = 201)19. The first survey to use direct detection of C. pecorum DNA was conducted in 1999 and surveyed ten koalas that were all found to be C. pecorum PCR negative3. Given that koalas were introduced into KI from French Island, Victoria in the 1920’s20, which was considered Chlamydia-free21, KI has also been regarded as Chlamydia-free. However, C. pecorum has recently been detected in two French Island koalas22 and other reports of introduction of koalas from Queensland to KI in the 1940s23 raise doubts on the true status of C. pecorum in the KI koala population.
At a time when other mainland koala populations are seriously jeopardised by C. pecorum infection, disease and infertility, MLR koalas appear to have lower levels of overt disease, whilst KI may be the last large C. pecorum-free population in Australia. Hence this study aimed to determine the prevalence of C. pecorum in wild ranging koalas from the MLR and KI populations in South Australia and to describe any clinical disease associated with infection.
Results
Mount Lofty Ranges targeted survey
In the MLR, 30 male and 45 female koalas were captured and sampled. C. pecorum was detected by qPCR in 46.7% (35/75, Binomial Exact 95% CI: 35.1–58.6%) of koalas. C. pecorum was more likely to be detected with higher loads at the urogenital site (median, (range)) (34/35; 170 (10–30,600) copies/µL) compared to the ocular site (3/35; 30 (17–2,020) copies/µL). There was no significant difference in prevalence between sexes (females: 55.5%, 25/45; males: 36.7% 11/30; P = 0.156) or between sex and the site of infection (P = 0.339). Despite this, the three koalas that were qPCR positive at the ocular site were all female (3/45). At the urogenital site, females had a higher chlamydial load with median 409 copies/µL (range: 28–30,600 copies/µL) compared to males, 77 copies/µL (range: 10–645 copies/µL). Only 4% (3/75) of koalas presented with overt C. pecorum clinical disease and all were classified as severe (grade 3). These koalas were all female; one case of mucopurulent pyometra (TWC II), one case of cystitis with urine soiling and scalding of the rump of the koala (TWC VI) and one case of unilateral severe, conjunctivitis (TWC V) (Fig. 2a). Koalas which did not fit the definition of a clinical case included; grade 1 urogenital signs observed as fur discolouration with no scalding of the perineum in koalas qPCR positive (n = 10) and negative (n = 5) for C. pecorum infection, and grade 1 ocular signs observed in 22 C. pecorum PCR negative koalas (Fig. 2b).
Kangaroo Island targeted survey
From the targeted survey, 170 female koalas were sampled over a 3-year period (2014–17) and all found to be C. pecorum negative by qPCR. The DEW koala program recorded observations for 13,373 individual koalas, surgically sterilised over a 22-year period (1997–2018). None of the clinical records fitted the definition of a clinical chlamydial disease case. Records for two koalas from the targeted survey and 292 koalas from the DEW koala program (all negatives) were not included in the demonstration of freedom analysis as information for age or sex was missing. Data for 10,160 females and 2,921 males were incorporated from the DEW koala program. Details about demographic strata (sex, age class), number of koalas sterilised each year and each surveillance component were reported into the model accessible elsewhere (https://figshare.com/s/590fe1b98c52a4778f83).
Fecundity of female koalas
Reproductive activity was recorded in female koalas on KI by pregnancy (observed during laparoscopic sterilisation) and the presence of pouch and back young and in MLR by the presence of pouch young, as back young had matured by this time and were not observed. On KI 79.2% (118/149) of sexually mature females were reproductively active, with 41 back young, 66 pouch young and 28 pregnant. Six koalas had both a back and pouch young, and eleven had a back young and were pregnant. The youngest pregnant female was aged 2–3 years (TWC II) and weighed 3.78 kg.
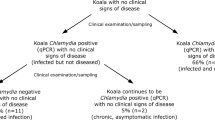
In the MLR, reproduction was significantly reduced due to C. pecorum infection, where only 37.2% (16/43) of female koalas had pouch young. No reproductively active females were infected with C. pecorum; while females without pouch young were significantly more likely to be infected with C. pecorum (P < 0.001). In addition, reproductively active females were five times more likely to be infected (RR = 5.0). Of the inactive females, 85.2% (23/27) were infected. Reproductively active females from both populations were more likely to be in excellent body condition (P = 0.040). There was no association between reproductive activity and age in either population (Fig. 3).
Grade 1 clinical disease observed in KI
In both the targeted survey and the DEW koala program, no KI koalas fitted the definition of a clinical case (>grade 2), however, some koalas were graded 1 for the ocular and urogenital sites. In the targeted survey, 12 (7%) koalas were observed with very mild ocular changes (qPCR negative for C. pecorum) which were graded with an ocular clinical score of 1. Within the DEW koala program clinical records, 1.08% of koalas (n = 141) were found to have clinical records graded at 1. Ocular changes were recorded in 0.5% of koalas (n = 67), with records such as conjunctivitis (n = 11), corneal scar, ulcer or opacity (n = 35) and periocular inflammation or oedema (n = 21). Urinary tract changes were recorded in 0.03% of female koalas (n = 4), with cystitis (n = 1), haematuria (n = 2) and kidney disease (n = 1) reported. Testicular aplasia was recorded in 0.2% of males (n = 27) and one male with testicular infection. Reproductive changes were recorded in 0.33% of female koalas (n = 43), where records were brief and lacked significant detail. ‘Cystic ovary’ was the most common record (n = 30), and of these koalas 13 were reproductively active; ‘enlarged uterus’ was reported in one female with pregnancy status not recorded; and ‘reproductive adhesions or inflammation’ was also recorded in females with unrecorded pregnancy status (n = 12).
Demonstration of freedom simulation model
The evidence of absence of C. pecorum on KI was collected through the 22 year DEW program and targeted surveys including qPCR over a 3-year period. This data supported that the KI koala population is free from C. pecorum infection in 2018, with an estimated probability of freedom with at least 95% confidence if the prevalence of C. pecorum on KI was at least 2% and if the yearly probability of introduction of the bacterium in the population is at most 7%. It was not possible to reach a minimum of 95% probability of freedom if the design prevalence was 1% or less. If the design prevalence is >2%, the probability of freedom was at least 95% regardless of the probability of introduction (Table 1).
The evolution over time of the sensitivity of the surveillance (SSei) and the probability of freedom (95% CI), with a 2% design prevalence and a risk of introduction at 7% is represented in Fig. 4. The SSe displayed is the average of 10,000 iterations in the model. The SSe was highly depended on the surveillance effort (i.e. the number of koalas screen and accuracy of detection). Over the first two years (1997–1998), the SSe was above 80%, but dropped to below 70% from 1999 to 2004 due to a smaller number of records for each year (maximum 564) and no koalas captured in 2001, reflecting the SSe to 0%. From 2005–2018, the SSe remained above 50%. The highest SSe was above 90% in 2006 and 2007 with the largest number of koalas observed in the DEW program, with 1,522 and 1,705 observed, respectively. The targeted survey from 2015 to 2017 increased the SSe to above 80% from below 70% in 2014 with a similar number of koalas captured.
Demonstration of freedom from Chlamydia pecorum infection in the KI koala population. Mean estimates for surveillance sensitivity (SSe) and probability of freedom, P(free)i from C. pecorum between 1997 and 2018. The mean P(free) (solid, dark green line) with 95% CI as lower (shorted, dashed, dark green line) and upper (long, dashed, dark green line) confidence limits and SSe (long, dashed, light blue line) with a probability of introduction, P(intro) of 7% (solid double red line) and a design prevalence (P*) of 2%, concluded in 2018 above the 95% limit (solid double yellow line).
The lower limit of the P(free)i was below 90% up until 2007. In 2007, with the large number of koalas captured, minimum P(free)i increased to 96.0% and approximated 95% with the last P(free)2018 of 95.1% (Fig. 4).
Discussion
Chlamydia pecorum is a pathogen of critical, national importance to the conservation of the koala species. Koala populations in northern Australia (Queensland and New South Wales) are experiencing significant declines that have been attributed to a number of factors, including deforestation, urbanisation, trauma (vehicular and dog attacks)1,24 and C. pecorum25. In the present study, we have shown that C. pecorum is well established in the wild Mount Lofty Ranges (MLR) population, with 46.7% of the surveyed koalas positive by PCR, but clinical disease was only observed in 4% of koalas. In KI koalas, the population was demonstrated to be free of C. pecorum infection with 95% confidence.
In wild MLR koalas, the prevalence of C. pecorum infection was found to be high, but was half of the prevalence reported in the previous study of necropsied koalas from the same area16. Overt C. pecorum disease was only observed in three koalas, which is substantially less than that seen in northern populations, where 20.8% (5/24) of free-ranging koalas from Queensland populations presented with overt C. pecorum disease26. Urogenital infections (2/3) were more common than ocular infections (1/3), which is also the trend reported in Victorian koalas27. Although C. pecorum infection was reported in the MLR population two decades ago3,14, clinical disease associated with infection is only recently being reported more frequently with increasing disease severity15,16. Prior to 2012, overt clinical disease was not observed by local veterinarians treating rescued koalas from the MLR population (N. Speight pers. comm.). The increasing prevalence may be due to increased awareness of chlamydial disease but may also suggest a possible change in pathogenicity of C. pecorum or increased host susceptibility. Possible causes include the introduction of a new pathogenic variant of C. pecorum28; an upregulation in the transmission of a virulence plasmid, pCpec29; or the recent introduction of the Koala retrovirus30, which may predispose koalas to develop clinical disease through immunosuppression31,32. Evidence has shown immunologically naïve koalas exposed to C. pecorum are at risk of high morbidity and mortality33,34,35. The implications for C. pecorum infection in the MLR population is unknown, but chlamydial infection was found in this study to significantly reduce female koala fecundity. Hence monitoring of the MLR population should continue to detect any future changes in chlamydial prevalence and pathogenicity that could cause the population to decline similarly to that observed in northern koala populations.
In KI, the model has demonstrated that the DEW koala program and targeted survey had sufficient surveillance sensitivity to detect an established C. pecorum infection in the KI population if the prevalence was greater than 2%. Hence it remains possible that C. pecorum may be present in the population at a very low prevalence, for instance if it had been recently introduced. The risk of introduction of C. pecorum into KI is unknown. Possible routes of introduction include from infected koalas, domestic livestock or other native species. C. pecorum is thought to transmit between koalas by direct contact3,35, which is likely to include sexual transmission26 and mother to offspring27,36. As the introduction of infected koalas to KI is unlikely to occur due to strict DEW regulations on koala care and movement, koala faeces infected with C. pecorum37 may pose a threat, based on what is known in other species, as the faecal-oral route is thought to be the most likely route of transmission between domestic cattle (Bovis taurus) infected with C. pecorum38.
This highlights the potential introduction of C. pecorum into KI through livestock. While the koala has a distinct clade of C. pecorum isolates which infect and cause disease in the koala, there are some genetically similar strains of C. pecorum shared between domestic cattle, sheep (Ovis aries) and the koala39,40. The recently discovered C. pecorum isolates in French Island koalas were found to be genetically related to livestock C. pecorum genotypes22, suggesting potential transmission between species. Sheep farming is a common activity on KI, with up to 680,000 sheep41, and while the presence of C. pecorum in KI sheep remains unknown, C. pecorum has been found in sheep in south-eastern South Australia42. Investigation into the presence of C. pecorum in livestock on KI is underway.
Transmission may occur either directly by exposure of koalas to infected livestock faeces when they travel on the ground between trees, or via an intermediate species such as has been hypothesised with possums43. It has been found that the mountain brushtail possum (Trichosurus caninus) in Victoria can be infected with C. pecorum44 and due to its arboreal nature, it may introduce contaminated livestock faeces into the eucalypt trees on which koalas feed. While this species of possum is not present on KI, the common brushtail possum (Trichosurus vulpecula) is highly abundant and has also been shown to share some intestinal parasites with sheep on KI45. To ensure trans-species transmission does not occur with the koala on KI, C. pecorum in domestic livestock and native species on KI should be investigated through targeted surveillance, in conjunction with increased biosecurity measures implemented to ensure that C. pecorum is not brought onto the island.
The DEW sterilisation program on KI provides a sensitive surveillance tool for both ocular and urogenital disease. Review of the clinical data from the KI DEW koala program found 141 koalas (1.08%) with possible signs of chlamydial disease that were assigned a grade 1 chlamydial score. This was due to minimal details of clinical signs recorded, that no diagnostic PCR performed to confirm C. pecorum infection and that although these changes were consistent with chlamydial infection, they were not pathognomonic for C. pecorum, and could be explained by other causes. Ocular and urinary tract disease has been reported in southern Australian koalas without C. pecorum infection16,21,27, and with recent analysis of the ocular and urogenital microbiomes in the koala, there are possibly other pathogens which cause similar clinical signs46,47. Kidney disease may be due to oxalate nephrosis, which is highly prevalent in the MLR population48,49, while testicular aplasia has been reported in association with reduced genetic diversity in KI koalas50. A common report was “ovary cyst” with no additional description of the cysts, such as size or quantity. As some koalas were also reproductively active, it cannot be determined if these described cysts were pathological9, or a result of normal reproduction, such as large follicles51.
The impacts of C. pecorum on the eastern Australian koala populations are devastating with considerable morbidity and mortality as a result of infection and declines in population numbers due to infertility. We have estimated freedom from infection on KI over a 22-year period with >95% confidence. Hence this large, isolated C. pecorum-free population of koalas holds significant importance as insurance for the future of the species. Every effort should be made to ensure the population remains C. pecorum-free so that these koalas could be used, in conjunction with the newly developed C. pecorum vaccine52, to re-populate declining populations, and may ultimately ensure the survival of the koala for generations.
Materials and Methods
Chlamydia pecorum targeted surveys
In the MLR, 75 wild koalas were captured in April 2016 using ropes and poles as described previously19, and relocated to a nearby sampling site. On KI, 170 wild koalas were sampled between November 2014 and February 2017 in conjunction with the South Australian Department for Environment and Water (DEW) Koala Sterilisation Program. The Koala Sterilisation Program was implemented in 1996 to monitor the overabundant KI population and uses surgical sterilisation as a means for population management53. These surveys were approved by The University of Adelaide Animal Ethics committee (S-2013-198, S-2015-138) with State Government DEW Scientific Research permits (Y26054-6, U26431-1) and completed in accordance with the University of Adelaide and State Government guidelines and regulations.
For clinical examination, MLR koalas were anaesthetised with either alfaxalone (Alfaxan, Jurox, United Kingdom) (3.5 mg/kg) IM or alfaxalone (2 mg/kg) with medetomidine (Domintor, Vetquinol, United Kingdom) (40 µg/kg) IM, and on KI, isoflurane (2–5%) and oxygen (2%/min) was used. Each koala was aged by the degree of wear of the upper premolar (Tooth wear class (TWC) I, 1–2 years; II, 2–3 years; III, 4 years; IV, 5–6 years; V, 10–12 years; VI, 12+ years54). For KI females, laparoscopic sterilisation was performed allowing visualisation of the reproductive tract for pregnancy or any pathological changes. Reproductive activity was recorded in MLR females by the presence of pouch young and on KI by pregnancy, pouch and/or back young. Koalas were classified as sexually immature if their bodyweight <3.90 kg19. Each koala was graded for ocular and urogenital clinical signs consistent with C. pecorum using a 4 scale system (grade 0, no disease; 1, mild disease; 2, moderate disease; 3, severe disease)55. Two dry aluminium shaft swabs (Copan Italia, Brescia, Italy) of the conjunctiva and cloaca were collected7 and stored at −80 °C until C. pecorum detection.
Historical clinical examination on KI
The DEW koala program collected clinical examination data during routine surgical sterilisations of koalas conducted every year from approximately November to March for 22 years (1997–2018). The clinical data included sex (male or female), age class (as described above) and signs of disease. Individual clinical records were reviewed retrospectively and ocular and urogenital findings consistent with C. pecorum infection were graded as described above55. A koala was classified as a positive clinical case if the clinical record described grade 2 disease or above for either the ocular or urogenital sites. This case definition favours the specificity of the classification to minimise possible false positive cases from mild, non-pathognomonic clinical signs46,48,50,51.
Chlamydia pecorum molecular detection
Ocular and urogenital swabs from individual koalas were screened for C. pecorum detection. DNA was extracted using Qiagen DNA Mini kit (Qiagen, Hilden, Germany) and extracted DNA was pooled and amplified using a C. pecorum-specific qPCR targeting a 209 bp fragment of C. pec HP gene56. Positive pooled swabs were re-tested separately. Briefly, the qPCRs were performed in a final volume of 20 µl, including 10 µl iTaq master mix (Bio-Rad, California, USA), 1 µl of 10 µM each of forward and reverse primer (Sigma-Aldrich, Australia), 3 µl dH2O and 5 µl template DNA. Cycling conditions consisted of 15 min at 95 °C, followed by 35 cycles of 15 sec at 94 °C, 15 sec at 57 °C and 30 sec extension at 72 °C. Samples were tested in duplicates, and negative control (dH2O) and positive control (C. pecorum Marsbar DNA) were included in each assay. In each assay, infectious load was quantified by plotting the crossing points against a standard curve produced using a serial dilution from 106 to 100 copies/µl of the known standard, C. pecorum target amplicon. C. pec HP gene amplicon was characterised with a high-resolution melt (HRM) of 77.5 ± 0.5 °C. DNA quality was assessed by detecting a 122 bp fragment of the koala β–actin gene. The reaction was performed in 25 μl containing 5 μl of 5X Taq polymerase buffer (Bioline, Australia), 2 mM of magnesium chloride, 0.1 mM of dNTP mix, 1 mM of each of the published β-actin primers, 5′-AGATCATTGCCCCACCT-3′ (sense) and 5′-TGGAAGGCCCAGATTC-3′ (anti-sense)57, 0.25 µL of MyTaq DNA polymerase (Bioline, Australia), and 10 µL of DNA template. The PCR conditions were performed as recommended by the Bioline PCR kit, with a 58 °C primer annealing temperature and final extension at 72 °C for 10 minutes.
Univariate analysis
Statistical analysis was performed using SPSS v.24 to determine significance based on sex, age and C. pecorum status (α = 0.05). For continuous Chlamydia load variables, a Shapiro-Wilk test was performed to determine Gaussian distributions. For variables with normal distribution, an F-test was performed to determine equal variance prior to a two-way independent t-test. For non-parametric variables a Kruskal-Wallis H analysis was performed with post-hoc Mann-Whitney U test. Chi-squared analysis was performed to determine relationships and odds ratio between C. pecorum infection, sex, age and reproduction status.
Demonstration of freedom from Chlamydia pecorum on KI
The probability that the KI koala population is C. pecorum-free was estimated by collating qPCR results from the targeted survey and DEW koala program historical clinical data in a scenario tree modelling approach58. The probability of freedom of a given year of surveillance i (P(free)i) was calculated using the Bayesian approach from the probability of freedom from the prior year i − 1 (P(free)i−1) and the surveillance system sensitivity (SSei) and specificity (SSpi), reflecting the strength of the evidence collected during the year i58:
P(free)i was then adjusted for the probability of introduction (P(intro)i) during the same year i58:
Surveillance System Specificity (SSp)
The diagnostic specificity of both diagnostic methods, qPCR and clinical examination, were perfect (100%) at the individual animal level. Therefore, the SSp relying on these two methods at the population level was deduced as also 100%. This deduction was further supported by the fact that none of the surveyed KI koalas were classified as positive (i.e. no potential false-positive).
Surveillance System Sensitivity (SSe)
The SSe was calculated based on 12 month periods to match the yearly cycle of the surveillance. In a given year i, the SSei was calculated from the sensitivity of the C. pecorum targeted survey (CSeSurveyi) and DEW koala program (CSeDEWi) surveillance components as follow58:
System Component Sensitivity (CSe)
In a given year, CSei was calculated from the component unit sensitivity (CSeUi, probability of detecting a single infected koala if sampled at a given period) of each surveillance component as follow58:
where n is the total number of koalas sampled during a given activity and given year.
Component Unit Sensitivity (CSeU)
For each year i, the CSeUi was calculated from scenario trees representing all possible scenarios, including risk categories (sex and age class) and detection outcomes (positive or negative)59. Two separate trees were built for each surveillance component, the targeted survey and DEW koala program (Fig. 5). The probability of each scenario tree branch was calculated by multiplying the representation of its risk category class in the total sample, the adjusted risk (AR) of the risk category class, the design prevalence (assumed minimum prevalence of C. pecorum when the infection is established in a koala population) (P*) and the probability of detection according to the diagnostic method accuracy (i.e. diagnostic sensitivity and specificity, DSe and DSp respectively).
Adjusted Risk (AR)
For each risk category classes (sex and age classes), an ARageXsex was calculated by multiplying the AR for sex and the AR for age class. The AR for sex were calculated using the relative risk of C. pecorum infection (RR) of males compared to females and the assumed population representation (PrP) of each sex as follow58:
The AR for the six age classes were calculated similarly using the relative risk (RR) of each age class compare to the first age class (TWC I) and the assumed population representation (PrP) of each class for a given sex58:
where J is the age class ranging from TWC II to VI.
Diagnostic sensitivity and specificity (DSe and DSp)
DSe and DSp of the qPCR (DSePCR and DSpPCR) were estimated in-house by comparing the proportions of positive and negative results when screening known positive and negative standards respectively. The DSe and DSp of the clinical examination (DSeClin and DSpClin) were deduced by crossing the results from the clinical examination with the qPCR from koalas surveyed in the MLR (population infected with C. pecorum). DSeClin and DSpClin were calculated using the following formulae60:
where n is the total number of koalas tested in the MLR; n1 is the count of individuals with clinical signs present (positive clinical cases); n0 is the count with no clinical signs (negative clinical cases); b is the count of PCR positive koalas without clinical signs; c is the count of PCR negative koalas with clinical signs; m1 is the total count of PCR positive and m0 is the total count of PCR negative koalas.
Model stochasticity
To account for the uncertainty about model parameters, stochasticity was built into the model by allocating a distribution to the parameters using the PopTools Excel add-in v3.2 (PopTools 2011). Distribution used in the model and the source of the information used to parametrise the distribution are reported in Table 2. Probability parameters were allocated Beta distribution parametrised with BetaBuster v1.0 software (Su 2012 https://www2.vetmed.ucdavis.edu/cadms/local_resources/docs/betabuster012006.zip) according to their exact Binomial 95%CI. Other model parameters were allocated a Pert distribution according to their reported 95% CI. The simulation was run for 10,000 iterations and the model outputs’ distributions were reported with the mean, 2.5th and 97.5th percentiles. A population of koalas was deemed free from the infection if the 2.5th percentile (lower limit) the estimated probability of freedom was >95%.
Sensitivity Analysis
No robust estimates of the design prevalence (P*) and the probability of introduction (P(intro)) could be sourced. A sensitivity analysis was conducted by varying both parameters and assessing the impact of the final probability of freedom estimate and 95% CI. As the risk of introduction may vary with time, we assumed in the model that the probability of introduction was at its maximum possible value for the entire surveillance period.
The model to assess freedom from C. pecorum was implemented in MS Excel (2013) and a copy is accessible online (https://figshare.com/s/590fe1b98c52a4778f83).
Data Availability
The dataset generated and analysed during this study is available in the Figshare repository [https://figshare.com/s/590fe1b98c52a4778f83]. Further data generated during this study is available from the corresponding author on reasonable request.
References
Griffith, J. E., Dhand, N. K., Krockenberger, M. B. & Higgins, D. P. A retrospective study of admission trends of koalas to a rehabilitation facility over 30 years. J. Wildl Dis 49, 18–28, https://doi.org/10.7589/2012-05-135 (2013).
Wilson, D. P., Craig, A. P., Hanger, J. & Timms, P. The paradox of euthanizing koalas (Phascolarctos cinereus) to save populations from elimination. J. Wildl Dis. 833–842, https://doi.org/10.7589/2014-12-278 (2015).
Polkinghorne, A., Hanger, J. & Timms, P. Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Vet Microbiol 165, 214–223, https://doi.org/10.1016/j.vetmic.2013.02.026 (2013).
Cockram, F. A. & Jackson, A. R. Isolation of a Chlamydia from cases of keratoconjunctivitis in koalas. Aust Vet J 50, 82–83 (1974).
Hemsley, S. & Canfield, P. J. Histopathological and immunohistochemical investigation of naturally occurring chlamydial conjunctivitis and urogenital inflammation in koalas (Phascolarctos cinereus). J. Comp Pathol 116, 273–290 (1997).
Mackie, J. T., Gillett, A. K., Palmieri, C., Feng, T. & Higgins, D. P. Pneumonia due to Chlamydia pecorum in a koala (Phascolarctos cinereus). J. Comp Pathol. https://doi.org/10.1016/j.jcpa.2016.07.011 (2016).
Blanshard, W. & Bodley, K. Koalas in Medicine of Australian Mammals (eds L Vogelnest & R Woods) 227–328 (CSIRO Publishing, 2008).
Canfield, P. J. A survey of urinary tract disease in New South Wales koalas. Aust Vet J 66, 103–106 (1989).
Obendorf, D. L. & Handasyde, K. A. Pathology of chlamydial infection in the reproductive tract of the female koala (Phascolarctos cinereus) in Biology of the Koala (eds A. Lee, K. A. Handasyde, & G. D. Sanson) 255–259 (Surrey Beatty & Sons, 1990).
Johnston, S. D. et al. Orchitis and epididymitis in koalas (Phascolarctos cinereus) infected with Chlamydia pecorum. Vet Pathol. https://doi.org/10.1177/0300985815570069 (2015).
Wardrop, S., Fowler, A., O’Callaghan, P., Giffard, P. & Timms, P. Characterization of the koala biovar of Chlamydia pneumoniae at four gene loci - ompAVD4, ompB, 16S rRNA, groESL spacer region. System. Appl Microbiol 22, 22–27, https://doi.org/10.1016/s0723-2020(99)80024-1 (1999).
Masters, P., Duka, T., Berris, S. & Moss, G. Koalas on Kangaroo Island: from introduction to pest status in less than a century. Wildl Res 31, 267–272, https://doi.org/10.1071/WR03007 (2004).
Sequeira, A. M., Roetman, P. E., Daniels, C. B., Baker, A. K. & Bradshaw, C. J. Distribution models for koalas in South Australia using citizen science-collected data. Ecol Evol 4, 2103–2114, https://doi.org/10.1002/ece3.1094 (2014).
Houlden, B. A. & St John, B. J. Genetic diversity and disease status in koalas of South Australia in Wildlife Conservation Fund, Final report, Project 2516, University of New South Wales, Sydney (2000).
Funnell, O. et al. Conjunctivitis associated with Chlamydia pecorum in three koalas (Phascolarctos cinereus) in the Mount Lofty Ranges, South Australia. J. Wildl Dis 49, 1066–1069, https://doi.org/10.7589/2013-03-066 (2013).
Speight, K. N. et al. Prevalence and pathologic features of Chlamydia pecorum infections in South Australian koalas (Phascolarctos cinereus). J. Wildl Dis 52, 301–306, https://doi.org/10.7589/2015-05-120 (2016).
Brown, A. S., Carrick, F. N., Gordon, G. & Reynolds, K. The diagnosis and epidemiology of an infertility disease in the female koala Phascolarctos cinereus (Marsupialia). Vet Radio 25, 242–248 (1984).
Robinson, A. C., Spark, R. & Halstead, C. The distribution and management of the koala (Phascolarctos cinereus) in South Australia. SA Nautral 64, 4–24 (1989).
Whisson, D. & Carlyon, K. Temporal variation in reproductive characteristics of an introduced and abundant island population of koalas. J. Mammal 91, 1160–1167, https://doi.org/10.1644/09-MAMM-A-384.1 (2010).
Phillips, B. Koalas: The Little Australians We’d All Hate To Lose (AGPS Press, 1990).
Patterson, J. L. et al. The prevalence and clinical significance of Chlamydia infection in island and mainland populations of Victorian koalas (Phascolarctos cinereus). J. Wildl Dis, https://doi.org/10.7589/2014-07-176 (2015).
Legione, A. R. et al. Chlamydia pecorum infection in free-ranging koalas (Phascolarctos cinereus) on French Island, Victoria, Australia. J. Wildl Dis 52, 426–429, https://doi.org/10.7589/2015-10-276 (2016).
Lindsay, H. A. Re-establishing the koala in South Australia. Wild. Life 12, 257–262 (1950).
Gonzalez-Astudillo, V., Allavena, R., McKinnon, A., Larkin, R. & Henning, J. Decline causes of koalas in south east Queensland, Australia: a 17-year retrospective study of mortality and morbidity. Sci Rep 7, 42587, https://doi.org/10.1038/srep42587 (2017).
Rhodes, J. R. et al. Using integrated population modelling to quantify the implications of multiple threatening processes for a rapidly declining population. Biol Conserv 144, 1081–1088, https://doi.org/10.1016/j.biocon.2010.12.027 (2011).
Jackson, M., White, N., Giffard, P. & Timms, P. Epizootiology of Chlamydia infections in two free-range koala populations. Vet Microbiol 65, 255–264 (1999).
Legione, A. R. et al. Identification of unusual Chlamydia pecorum genotypes in Victorian koalas (Phascolarctos cinereus) and clinical variables associated with infection. J. Med Microbiol 65, 420–428, https://doi.org/10.1099/jmm.0.000241 (2016).
Kollipara, A. et al. Genetic diversity of Chlamydia pecorum strains in wild koala locations across Australia and the implications for a recombinant C. pecorum major outer membrane protein based vaccine. Vet Microbiol 167, 513–522, https://doi.org/10.1016/j.vetmic.2013.08.009 (2013).
Jelocnik, M. et al. Genetic diversity in the plasticity zone and the presence of the chlamydial plasmid differentiates Chlamydia pecorum strains from pigs, sheep, cattle, and koalas. BMC genomics 16, 893, https://doi.org/10.1186/s12864-015-2053-8 (2015).
Fabijan, J. et al. Lymphoma, koala retrovirus infection and reproductive chlamydiosis in a koala (Phascolarctos cinereus). J. Comp Pathol 157, 188–192, https://doi.org/10.1016/j.jcpa.2017.07.011 (2017).
Tarlinton, R., Meers, J., Hanger, J. & Young, P. Real-time reverse transcriptase PCR for the endogenous Koala retrovirus reveals an association between plasma viral load and neoplastic disease in koalas. J. Gen Virol 86, 783–787, https://doi.org/10.1099/vir.0.80547-0 (2005).
Waugh, C. et al. Infection with koala retrovirus subgroup B (KoRV-B), but not KoRV-A, is associated with chlamydial disease in free-ranging koalas (Phascolarctos cinereus). Sci Rep 7, 134–137, https://doi.org/10.1038/s41598-017-00137-4 (2017).
Lee, A. K. & Martin, R. W. The koala: a natural history (University of New South Wales Press, 1996).
McColl, K. A., Martin, R. W., Gleeson, L. J., Handasyde, K. A. & Lee, A. K. Chlamydia infection and infertility in the female koala (Phascolarctos cinereus). Vet Rec 115, 655 (1984).
Brown, A. & Grice, R. Experimental transmission of Chlamydia psittaci in the koala in Chlamydial infections (eds D. Oriel et al.) 349–352 (Cambridge University Press, 1986).
Russell, I. et al. Prevalence of Chlamydia pecorum in juvenile koalas (Phascolarctos cinereus) and evidence for protection from infection via maternal immunization. J. Wildl Dis, https://doi.org/10.7589/2017-07-183 (2018).
Wedrowicz, F., Karsa, M., Mosse, J. & Hogan, F. E. Reliable genotyping of the koala (Phascolarctos cinereus) using DNA isolated from a single faecal pellet. Mol Ecol Resour 13, 634–641, https://doi.org/10.1111/1755-0998.12101 (2013).
Reinhold, P., Sachse, K. & Kaltenboeck, B. Chlamydiaceae in cattle: commensals, trigger organisms, or pathogens? Vet J 189, 257–267, https://doi.org/10.1016/j.tvjl.2010.09.003 (2011).
Bachmann, N. L. et al. Comparative genomics of koala, cattle and sheep strains of Chlamydia pecorum. BMC genomics 15, 667, https://doi.org/10.1186/1471-2164-15-667 (2014).
Jelocnik, M., Frentiu, F. D., Timms, P. & Polkinghorne, A. Multilocus sequence analysis provides insights into molecular epidemiology of Chlamydia pecorum infections in Australian sheep, cattle, and koalas. J. Clin Microbiol 51, 2625–2632, https://doi.org/10.1128/jcm.00992-13 (2013).
Australian Bureau of Statistics (ABS). National Regional Profile: Kangaroo Island (Statistical Subdivision), http://www.abs.gov.au/AUSSTATS/abs@.nsf/Previousproducts/41010Industry12004-2008 (2018).
Yang, R. et al. Longitudinal prevalence and faecal shedding of Chlamydia pecorum in sheep. Vet J 201, 322–326, https://doi.org/10.1016/j.tvjl.2014.05.037 (2014).
Burnard, D. & Polkinghorne, A. Chlamydial infections in wildlife-conservation threats and/or reservoirs of ‘spill-over’ infections? Vet Microbiol 196, 78–84, https://doi.org/10.1016/j.vetmic.2016.10.018 (2016).
Bodetti, T. J. et al. Wide range of Chlamydiales types detected in native Australian mammals. Vet Microbiol 96, 177–187, https://doi.org/10.1016/S0378-1135(03)00211-6 (2003).
O’Callaghan, M. & Moore, E. Parasites and serological survey of the common brushtail possum (Trichosurus vulpecula) from Kangaroo Island, South Australia. J. Wildl Dis 22, 589–591 (1986).
Legione, A. R. et al. Variation in the microbiome of the urogenital tract of Chlamydia-free female koalas (Phascolarctos cinereus) with and without ‘wet bottom’. Plos One 13, e0194881, https://doi.org/10.1371/journal.pone.0194881 (2018).
Vidgen, M. E., Hanger, J. & Timms, P. Microbiota composition of the koala (Phascolarctos cinereus) ocular and urogenital sites, and their association with Chlamydia infection and disease. Sci Rep 7, 5239, https://doi.org/10.1038/s41598-017-05454-2 (2017).
Speight, K. N. et al. Pathological features of oxalate nephrosis in a population of koalas (Phascolarctos cinereus) in South Australia. Vet Pathol 50, 299–307, https://doi.org/10.1177/0300985812456215 (2012).
Speight, K. N. et al. Necropsy findings of koalas from the Mount Lofty Ranges population in South Australia. Aust Vet J 96, 188–192, https://doi.org/10.1111/avj.12690 (2018).
Cristescu, R. et al. Inbreeding and testicular abnormalities in a bottlenecked population of koalas (Phascolarctos cinereus). Wildl Res 36, 299–308, https://doi.org/10.1071/WR08010_CO (2009).
Obendorf, D. L. Pathology of the female reproductive tract in the koala, Phascolarctos cinereus (Goldfuss), from Victoria, Australia. J. Wildl Dis 17, 587–592 (1981).
Khan, S. A. et al. Antibody and cytokine responses of koalas (Phascolarctos cinereus) vaccinated with recombinant chlamydial major outer membrane protein (MOMP) with two different adjuvants. Plos One 11, e0156094, https://doi.org/10.1371/journal.pone.0156094 (2016).
Molsher, R. Kangaroo Island koala population survey 2015 (Department of Enivronment, Water and Natural Resources, 2017).
Martin, R. W. Age-specific fertility in three populations of the koala, Phascolarctos cinereus Goldfuss, in Victoria. Wildl Res 8, 275–283 (1981).
Wan, C. et al. Using quantitative polymerase chain reaction to correlate Chlamydia pecorum infectious load with ocular, urinary and reproductive tract disease in the koala (Phascolarctos cinereus). Aust Vet J 89, 409–412, https://doi.org/10.1111/j.1751-0813.2011.00827.x (2011).
Jelocnik, M. et al. Development and evaluation of rapid novel isothermal amplification assays for important veterinary pathogens: Chlamydia psittaci and Chlamydia pecorum. PeerJ 5, e3799, https://doi.org/10.7717/peerj.3799 (2017).
Tarlinton, R. E., Meers, J. & Young, P. R. Retroviral invasion of the koala genome. Nature 442, 79–81, https://doi.org/10.1038/nature04841 (2006).
Cameron, A., Njeumi, F., Chibeu, D. & Martin, T. Risk-based disease surveillance (Food and Agriculture Organization of the United Nations, 2014).
Martin, P. A., Cameron, A. R. & Greiner, M. Demonstrating freedom from disease using multiple complex data sources 1: a new methodology based on scenario trees. Prev Vet Med 79, 71–97, https://doi.org/10.1016/j.prevetmed.2006.09.008 (2007).
Dohoo, I., Martin, S. & Stryhn, H. Veterinary Epidemiologic Research (VER Inc, 2009).
Acknowledgements
This project was funded by the Morris Animal Foundation (Grant ID D16ZO-829). The authors wish to thank Dr Debra Lehmann and staff from Kangaroo Island Veterinary Clinic; and Andrew Schofield, Jason van Weenen and Brodie Philp, Department for Environment and Water; Merridy Montarello and volunteers of Fauna Rescue of South Australia Inc.; Dr Jennifer McLelland, ZoosSA and Dr Katherine Adriansse for their field work assistance. We also wish to thank Dr Kandarp Patel and Patrick Taggart for figure assistance and Dr Ian Beckman, Rebecca Summerton and Adrian Hines, Veterinary Diagnostics Laboratory, Roseworthy campus for their technical assistance.
Author information
Authors and Affiliations
Contributions
J.F. contributed to the study design, development and implemented the field work, processed samples for PCR, interpreted the targeted survey and KI historical data, assisted with the development of the demonstration of freedom model and wrote the manuscript. C.C. developed the demonstration of freedom model. M.J. and A.P. performed qPCR. W.S.J.B. contributed to study design and performed clinical examinations on MLR koalas. E.N. and G.J. performed current and historical clinical examinations on KI koalas. R.M. assisted in koala capture and provided the KI historical data. L.W. assisted in classifying clinical chlamydial disease. P.T., G.S., F.H. and D.J.T. contributed to the study design. N.S. was awarded the project funds, contributed to the study design and development of sampling, performed clinical examinations on MLR and KI koalas, assisted in data interpretation and analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fabijan, J., Caraguel, C., Jelocnik, M. et al. Chlamydia pecorum prevalence in South Australian koala (Phascolarctos cinereus) populations: Identification and modelling of a population free from infection. Sci Rep 9, 6261 (2019). https://doi.org/10.1038/s41598-019-42702-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42702-z
This article is cited by
-
Dynamics of Antimicrobial Resistance Carriage in Koalas (Phascolarctos Cinereus) and Pteropid Bats (Pteropus Poliocephalus) Before, During and After Wildfires
Microbial Ecology (2024)
-
A retrospective study on antibacterial treatments for koalas infected with Chlamydia pecorum
Scientific Reports (2023)
-
Efficacy of a synthetic peptide Chlamydia pecorum major outer membrane protein vaccine in a wild koala (Phascolarctos cinereus) population
Scientific Reports (2023)
-
Transcriptomic and genomic variants between koala populations reveals underlying genetic components to disorders in a bottlenecked population
Conservation Genetics (2021)
-
Koala immunogenetics and chlamydial strain type are more directly involved in chlamydial disease progression in koalas from two south east Queensland koala populations than koala retrovirus subtypes
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.