Abstract
The maintenance of homeostasis in the gut is a major challenge for the immune system. Here we demonstrate that the transcription factor MAF plays a central role in T cells for the prevention of gastro-intestinal inflammation. Conditional knock out mice lacking Maf in all T cells developed spontaneous late-onset colitis, correlating with a decrease of FOXP3+RORγt+ T cells proportion, dampened IL-10 production in the colon and an increase of inflammatory TH17 cells. Strikingly, FOXP3+ specific conditional knock out mice for MAF did not develop colitis and demonstrated normal levels of IL-10 in their colon, despite the incapacity of regulatory T cells lacking MAF to suppress colon inflammation in Rag1−/− mice transferred with naïve CD4+ T cells. We showed that one of the cellular sources of IL-10 in the colon of these mice are TH17 cells. Thus, MAF is critically involved in the maintenance of the gut homeostasis by regulating the balance between Treg and TH17 cells either at the level of their differentiation or through the modulation of their functions.
Similar content being viewed by others
Introduction
Maf encodes for a transcription factor belonging to the AP-1 family (MAF or c-MAF). It has been studied for many years in T cells, and more specifically in CD4+ T helper (TH) cell differentiation. Its role in Il4 transcriptional regulation and TH2 regulation was first established using a transgenic mouse system for Maf1,2. Maf was also shown to play a prominent role in TH17 cells in both mice and human, through the regulation of Il10 and Il23r expression3,4 and in TFH cells together with the transcription factor Bcl65. MAF inactivation in T cells affects susceptibility to disease in a context-specific manner, depending on the T cell polarization induced by this disease with a general tendency to increase inflammatory responses over tolerance6. In invariant natural killer T (iNKT) cells, MAF regulates the expression of IL-17A and RORγt7. Interestingly, Maf was found upregulated in CD8+ T cells infiltrated in human and murine melanoma. This expression led to intratumoral T cell dysfunction through the regulation of genes involved in T cell exhaustion8.
The potential role of Maf in regulatory T cells (Treg) is less clear. MAF cooperates with AhR in FOXP3− T regulatory 1 (TR1) cells to control Il10 expression9. Recently, a subset of Treg expressing both FOXP3 and RORγt, the master transcription factor of TH17 cells, has been characterized in more detail10. Mostly present in the gut, RORγt+ Treg have enhanced suppressive activity compared to RORγt- Treg and maintain gut homeostasis to microbiota11,12,13. The development of this population, described in both mice and human11,12, is tightly linked to the presence of the microbiota but not to dietary antigens14. Transcriptomic analysis showed an enriched expression of Maf in RORγt+ Treg13 and it was shown that the inactivation of Maf in Treg strongly affects its function and differentiation into RORγt+ Treg15,16. Recent studies have linked MAF to the modulation of a large immunoregulatory and tissue-residency program in human TH17 cells producing Il-10. These results are reminiscent of the role of MAF in the regulation of tolerance in the gut that is dependent on induced Treg cells17. MAF can bind in the vicinity of many genes expressed in recently activated TH17 cell subsets. This study suggests that binding with other transcriptional partners to regulatory regions of the genome would explain the different effect of MAF on the expression of genes encoding either tolerogenic or inflammatory molecules.
In an attempt to clarify the role of Maf in T cells in vivo, we studied mice inactivated for Maf in all T cells (MafΔTcells). Interestingly, these mice developed late onset colitis correlating with a decrease of RORγt+ Treg and an increase of TH17 cells in the colon and the mesenteric LNs. The disease was associated with increased production of TNFα, IFNγ, IL-1β and a lack of IL-10 in the colon of MafΔTcells mice. Using an adoptive cell transfer model in Rag1−/− mice, we showed that Maf-deficient Treg are inefficient in preventing colitis, demonstrating the role of Maf in regulating Treg function. Moreover, we observed that MafΔTcells mice develop exacerbated TH17 response against Helicobacter pylori, a human pathobiont colonizing the stomach mucosa, leading to the inability of the bacteria to establish a chronic state of infection in MafΔTcells mice. Strikingly, mice deficient for Maf in Treg alone (MafΔTreg mice) did not develop colitis and demonstrated normal levels of IL-10 in their colon. Compared to MafΔTcells, we observed that TH17 cells of MafΔTreg mice produce IL-10. Taken together, our data demonstrated that Maf is playing a major role in the maintenance of gastro-intestinal homeostasis through the regulation of functions of both Treg and TH17 cells. Maf regulates the differentiation of RORγt+ Treg, the suppressive activities of Treg as well as the activity of TH17 cells from the gut.
Results
T cell specific Maf-deficient mice develop spontaneous colitis
To study the role of Maf in T cells, we generated CD4cre Maffl/fl mice (MafΔTcells), in which all T cells (CD4+ and CD8+) are inactivated for the expression of Maf. Interestingly, these mice spontaneously developed strong colitis, associated with a defect in weight gain starting at 16 weeks of age (Fig. 1A), concomitant with the appearance of diarrhea. These symptoms increased as the mice got older – with up to a 15% difference of body weight at 26 weeks of age between MafΔTcells mice and Cre-negative littermates (Maff/f) (Fig. 1A). Macroscopic and microscopic examination of the gastrointestinal system revealed severe inflammation of all the parts of the colon with a clear enlargement in most of the mice (Fig. 1B,C) but no clear difference in the length of the colon (Supplementary Fig. 1A). We did not observe similar features in other organs such as liver or kidney (Supplementary Fig. 1B-D). Hematoxylin and eosin staining on histological sections of colon samples showed massive infiltration of cells in the lamina propria and submucosal spread (Fig. 1C). The scoring of histological sections revealed that the majority of mice had severe colitis (4/7 mice with the score of 5) (Fig. 1D). We found a significant increase in the number of total immune cells both in the mesenteric LN (mLN) and in the colon of MafΔTcells mice compared to the one from Maff/f littermates (Fig. 1E). Both CD4+ and CD8+ T cells were more abundant in the colon of MafΔTcells mice compared to Maff/f littermates (Fig. 1F). MafΔTcells mice treated with antibiotics developed no or only mild colitis (Supplementary Fig. 1E,F) demonstrating the requirement of microbiota in the development of this phenotype. Thus, the absence of Maf in T cells leads to spontaneous colitis in mice.
MafΔTcells mice develop late-onset spontaneous colitis. (A) Body weight curves of Maff/f (n = 8) and MafΔTcells (n = 6) male littermates. Statistical significances were calculated by a Bonferroni test after a significant two-way ANOVA. (B) Representative pictures of colons from Maff/f and MafΔTcells mice. (C) Haematoxylin and eosin-stained sections of colons (upper picture: scale bar 200 µm; bottom pictures: scale bar 100 µm) and (D) histological scores (from 0 to 6) of microscopic changes in the colon from Maff/f and MafΔTcells mice (n = 7 per group). (E) Total immune cell numbers in mLN (left) and colon (right) of Maff/f and MafΔTcells mice (n > 10 per group). (F) Absolute cell numbers of CD4+ and CD8+ T cells in colons of Maff/f and MafΔTcells mice (n > 10 per group). (D–F) Each symbol represents an individual mouse. (E,F) Data are representative of at least 2 independent experiments with at least 4 mice per group. All mice were over 20 weeks old. All graphs indicate means. Error bars display Standard Error Mean (SEM).
In vitro differentiation of Maf-deficient CD4+ T cells in RORγt+ Treg is defective
As previously described, MAF has been shown to regulate cytokine production in various CD4+ TH subsets. To determine whether MAF deficiency in CD4+ T cells can also alter the differentiation of any of these subsets, we differentiated naïve CD4+ T cells with polarizing medium to obtain TH1, TH2, Treg and TH17 (FOXP3− or FOXP3+) cells in vitro (Supplementary Fig. 2A–C). Comparison of the expression of the master transcription factors TBET (for TH1), GATA3 (for TH2), FOXP3 (for Treg) and RORγt (for TH17) between CD4+ T cells from MafΔTcells and Maff/f mice did not show significant differences (Fig. 2A,B). However, in the TH17 polarizing condition, we observed the development of two distinct populations when analyzing FOXP3 expression. For the FOXP3+ population, also named RORγt+ Treg, there was a significant decrease in RORγt level in CD4+ T cells from MafΔTcells mice compared to the one from Maff/f mice, from 35% to 10% of CD4+ T cells respectively (Fig. 2A,B). In these polarizing conditions, MAF levels were the highest in RORγt+ Treg and TH17, with approximately 80% of MAF- expressing cells in both subsets. MAF is also expressed in RORγt- Treg, though at lower level - around 8% of the cells - whereas it is almost absent in TH1 and TH2 (Fig. 2C,D). These in vitro experiments of differentiation suggest that MAF plays a role for the differentiation of RORγt+ Treg and might impact the physiological functions of TH17 cells.
In vitro differentiation of Maf-deficient CD4+ T cells in RORγt+ Treg is defective. CD4+ T cells isolated from splenocytes of Maff/f and MafΔTcells mice were cultured for 5 days in TH1 (IL-12 + anti-IL-4), TH2 (IL-4 + anti-IFN-γ), Treg (TGF-β +IL-2) or TH17 (TGF-β +IL-6) polarizing conditions. (A) Representative histograms for the expression of master transcription factors associated with each helper subset: TBET (TH1), GATA3 (TH2), FOXP3 (Treg) and RORγt (TH17 FOXP3+ and FOXP3-) from Maff/f and MafΔTcells mice. (B) Percentage of each master transcription factor according to the condition of polarization among CD4+ T cells (C) Representative histograms for the expression of MAF in in vitro differentiated CD4+ T cell subsets according to the expression of their master transcription factor from Maff/f and MafΔTcells mice. MFI is indicated for T cells from Maff/f (grey) or MafΔTcells (black) mice. (D) Percentage of MAF+ cells among each TH subset according to the expression of their master transcription factor from WT mice. Each symbol represents an individual mouse. Data are representative of 2 independent experiments with at least 2 mice per group. All graphs indicate means. Error bars display SEM.
T cell specific Maf-deficient mice are deprived of RORγt+ Treg
To confirm the results obtained in vitro, we first looked at the abundance of transcripts encoding for cytokines in the total colon of aged Maff/f or MafΔTcells mice (20 weeks or more). We found higher expression of Tnfa, Ifng and Il1b in MafΔTcells mice compared to Maff/f littermates (Fig. 3A), in accordance with a higher infiltration of immune cells and a stronger inflammation in the colon. The transcripts encoding for Il4 were slightly less abundant in MafΔTcells mice and, strikingly, Il10 expression level was much lower in colons from MafΔTcells mice (Fig. 3A). We then determined the in vivo expression of MAF in the various subsets. MAF was expressed in RORγt+ FOXP3+ Treg and RORγt+ FOXP3− TH17 cells- around 40% and 20%, respectively - from the colon (Fig. 3B). In the colon, around 15% of RORγt− Treg expressed MAF (Fig. 3B). The proportion of RORγt+ Treg was dramatically decreased in spleens, mLN and colons of MafΔTcells mice, while we found a significant increase in percentages as well as total numbers of RORγt− Treg from these organs compared to Maff/f mice (Fig. 3C,D and Supplementary Fig. 3A). We also observed elevated percentages and cell numbers of TH17 cells in spleens, mLN and colons of MafΔTcells mice compared to Maff/f mice (Fig. 3C,D and Supplementary Fig. 3A). In Maff/f mice, RORγt+ Treg proportions increased with age to reach 10% of the total CD4+ T cell population in the colon at 25-weeks age mice (Fig. 3E). The defect in RORγt+ Treg in MafΔTcells mice is already detected at 2 weeks of age (i.e. the weaning period) compared to Maff/f mice (Fig. 3E). Interestingly, the level of MAF in these three populations followed the same upward trend with time (Fig. 3F). RORγt− Treg increase was already present in MafΔTcells mice at the age of 2 weeks and tended to expand over time (Fig. 3E). However, the increase of TH17 cells was only observed at an advanced age in MafΔTcells mice, correlating with the development of colitis. These data indicate that the deletion of MAF in T cells alters differentiation of highly suppressive RORγt+ Treg whereas it favors RORγt− Treg accumulation and later TH17 cells expansion. Loss of the equilibrium between these subsets in the colon is associated with colitis onset.
MafΔTcells mice are deprived of RORγt+ Treg. (A) Quantitative RT–PCR of colonic tissues from Maff/f (n = 6) and MafΔTcells (n = 8) mice for the indicated transcripts. Gene expression levels were normalized to Beta2microglobulin. (B) Representative histogram of the expression of MAF in mLN (left) and percentage of MAF+ cells (right) in mLN and colon in RORγt− Treg, RORγt+ Treg and TH17 cells from Maff/f mice (n > 10). (C) Representative contour plot of the expression of FOXP3 and RORγt in CD4+ T cells from spleens, mLN and colons of Maff/f and MafΔTcells mice. (D) Percentage of RORγt− Treg, RORγt+ Treg and TH17 cells from spleens, mLN and colons of Maff/f and MafΔTcells mice (n > 10 per group). (E) Percentage of RORγt− Treg, RORγt+ Treg and TH17 cells in CD4+ T cells from colons of Maff/f and MafΔTcells mice at indicated ages (n > 4 per group). (F) Percentage of MAF+ cells among RORγt− Treg, RORγt+ Treg and TH17 cells from mLN and colons of Maff/f and MafΔTcells mice at indicated ages (n > 4 per group). (A–C) Each symbol represents an individual mouse. Data are representative of at least 2 independent experiments with at least 3 mice per group. (E,F) Data are representative of at least 2 independent experiments with at least 2 mice per group. All graphs indicate means. Error bars display SEM.
Maf-deficient Treg fail to prevent colitis development in vivo
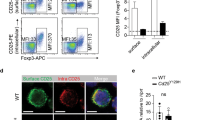
The dramatic decrease in RORγt+ Treg was associated with an increase in classical RORγt− Treg in MafΔTcells mice (Fig. 3D). However, the development of spontaneous colitis in MafΔTcells mice implies that Maf-deficient Treg are unable to offset the decrease in highly suppressive RORγt+ Treg. This would suggest that the remaining Maf-deficient Treg are dysfunctional and thus inefficient at suppressing TH17 cells. To test this hypothesis, we examined the capacity of wild type (WT) or Maf-deficient Treg to suppress gut inflammation driven by the injection of naïve CD4+ T cells into Rag1−/− mice18. We transferred either WT or Maf-deficient naïve CD4+ T cells alone or in the presence of either WT or Maf-deficient Treg into Rag1−/− recipient mice. Both WT and Maf-deficient naïve CD4+ T cells induced weight loss and cell infiltration in the colon when transferred alone (Fig. 4A,B) without any significant difference in the histologic score between the two groups (Fig. 4C). When we co-transferred WT Treg, the weight loss and the infiltration of cells in the colon were prevented with WT and Maf-deficient naïve CD4+ T cells (Fig. 4A,B). Strikingly, Maf-deficient Treg failed to prevent colitis in all transferred groups compared to WT Treg, with a significant difference for the weight loss and the histological score (Fig. 4A–C). Histological sections of colons from recipient mice transferred with Maf-deficient Treg displayed massive infiltration of cells in the associated lamina propria, with a disorganized architecture that was not observed in mice transferred with WT Treg (Fig. 4B,C). We confirmed the presence of Treg in all co-transferred groups (Fig. 4D) and the expression of MAF in WT transferred Treg (Fig. 4E). Taken together, these results demonstrated that Maf-deficient Treg are at least partially dysfunctional since they cannot prevent inflammation and colitis development.
Maf-deficient Treg fail to prevent colitis in vivo. CD4+CD45RBhiCD25lo cells (0.5 × 106) were intravenously injected alone or in combination with CD4+CD45RBloCD25hi cells (0.4 × 106) into males and females Rag1−/− mice from 10 to 15 weeks age. (A) Body weight curve of Rag1−/− mice injected with either WT (upper) or MafΔTcells naïve CD4+ T cells (bottom) alone or in combination with WT or MafΔTcells Treg. Statistical significances were calculated by a Bonferroni test after a significant two-way ANOVA. (B) Haematoxylin and eosin-stained sections of colons (Upper picture: scale bar, 200 µm. Bottom pictures: scale bar, 100 µm) and (C) Histological scores (from 0 to 6) for microscopic changes in the colons of indicated groups of mice. (D) Absolute cell numbers of total FOXP3+ T cells in the colons of the indicated groups of mice (n > 5 per group). (E) Percentage of MAF+ cells among FOXP3+ T cells in the colons of the indicated groups of mice (n > 5 per group). (A,C,D,E) All results are representative of 2 independent experiments with at least 2 mice per group. (C–E), Each symbol represents an individual mouse. All graphs indicate means. Error bars display SEM.
Treg specific Maf-deficient mice do not develop spontaneous colitis
We showed that both Treg differentiation and function were largely affected in MafΔTcells mice (Figs 3 and 4). To test whether the defect of MAF in Treg was sufficient to recapitulate the phenotype of colitis, we generated Foxp3YFPcre Maffl/fl mice (MafΔTreg), in which only Treg are inactivated for the expression of Maf. We observed a strong decrease in percentages as well as total numbers of RORγt+ Treg in the colon and in the mLN, mirrored by a slight increase in percentages of RORγt− Treg and TH17 cells (Fig. 5A,C and Supplementary Fig. 3). We confirmed the complete deletion of Maf only in Treg from MafΔTreg mice (Fig. 5B). However, we did not observe any signs of colitis, confirmed by histological colon section and body weight measurement of these mice compared to Maff/f mice (Fig. 5D,E) even in aged (6–12 months) MafΔTreg mice (Fig. 5F). Similarly, the number of immune cells infiltrated in the colon of Maff/f and MafΔTreg was not different (Fig. 5G). We noticed a slight increase in cells in mLN (Fig. 5G) and no difference in the number of CD4+ or CD8+ T cells in the colon of MafΔTreg mice compared to WT (Fig. 5H). This showed that Maf inactivation in Treg is not sufficient to induce spontaneous colitis.
MafΔTreg mice do not develop colitis. (A) Representative contour plot of the expression of FOXP3 and RORγt in CD4+ T cells from spleens, mLN and colons of Maff/f and MafΔTreg mice. (B) Percentages of RORγt- Treg, RORγt+ Treg and TH17 cells from spleens, mLN and colons of Maff/f and MafΔTreg mice (n > 8 per group). (C) Percentage of MAF+ cells among RORγt- Treg, RORγt+ Treg and TH17 cells from mLN of Maff/f and MafΔTcells (n > 10 per group). (D) Haematoxylin and eosin-stained sections of colons from Maff/f and MafΔTreg mice (Upper picture: scale bar, 200 µm; bottom pictures: scale bar, 100 µm). (E) Histological scores (from 0 to 6) of microscopic changes in the colon from Maff/f and MafΔTcells mice at the age of 6 to 10 months (n = 5 per group). (F) Body weight of Maff/f and MafΔTreg male mice of 10 and 22 weeks old (n > 6 per group). (G) Total cell numbers in mLN (left) and colons (right) of Maff/f and MafΔTreg mice (n > 10 per group). (H) Absolute cell numbers of CD4+ and CD8+ T cells in colons of Maff/f and MafΔTreg mice (n > 10 per group). (B,C,E,H) Each symbol represents an individual mouse. (B,C,G,H) Data are representative of at least 2 independent experiments with at least 4 mice per group. All mice were over 20 weeks old. All graphs indicate means.
Maf deletion impairs IL-10 production by Treg and TH17 cells
To uncover why MafΔTreg mice do not develop colitis, we measured transcript levels of cytokines encoding genes by quantitative RT-PCR in total colon of Maff/f, MafΔTreg or MafΔTcells mice aged of more than 20 weeks. As expected, we found higher expression of transcripts encoding for Maf in colon from MafΔTreg mice compared to colon from MafΔTcells mice, confirming that conventional T cells infiltrated in the colon still expressed Maf in MafΔTreg mice (Fig. 6A). When looking at transcripts encoding for Il10, we found a similar pattern of expression than the one for Maf, with a higher level of transcript in Maff/f and MafΔTreg mice compared to MafΔTcells mice (Fig. 6A). To confirm that Treg from MafΔTreg are really deficient for Maf and Il10 production, Treg from the spleen and LN of Maff/f, MafΔTreg or MafΔTcells mice were sorted using flow cytometry. We measured high levels of transcripts for Maf and Il10 in Treg cells from Maff/f mice, while the levels of transcripts for these genes were low in Treg cells from both MafΔTreg and MafΔTcells mice (Fig. 6B). Similarly, we confirmed that upon restimulation, Treg from mLN and colon of MafΔTreg or MafΔTcells mice expressed decreased levels of IL-10 compared to Maff/f counterparts (Fig. 6C,D). This reduction is largely observed in RORγt+ Treg but also to a lesser extent in RORγt− Treg from mLN of MafΔTreg mice, suggesting a dependence for MAF for IL-10 production in Treg. These data suggest that Maf expression in FOXP3− T cells is able to compensate for sufficient amounts of Il10 production to prevent colitis development in MafΔTreg mice. To determine which other T cell type could produce IL-10, we stimulated ex-vivo CD4+ T cells from mLN and colon of mice. We observed that IL-10 production by TH17 cells from mLN of MafΔTcells mice was decreased compared to production from Maff/f and MafΔTreg mice (Fig. 6E). This suggests that IL-10 production by TH17 cells in the gut is also regulated by Maf. The reduction of IL-10 expression not only by TH17 cells, but also Treg, could explain colitis onset observed only in mice where all the T cells are inactivated for Maf. Furthermore, we observed an increased percentage in IFNγ+TH17 cells while the percentage of IL-17A+ TH17 cells remained similar in MafΔTcells mice compared to Maff/f or MafΔTreg mice (Fig. 6F). Together, these data demonstrate that Maf is an essential driver of Treg and TH17 immunoregulatory function, especially through IL-10 regulation.
IL-10 expression is regulated by MAF in Treg and TH17 cells. (A) Quantitative RT–PCR of colonic tissues from Maff/f (n = 3), MafΔTcells (n = 4) and MafΔTreg (n = 5) mice for the indicated transcripts. Gene expression levels were normalized to Beta2microglobulin. (B) Quantitative RT–PCR of isolated Treg (CD4+CD25+CD45RBlo) of spleens and LN of Maff/f (n = 5), MafΔTcells (n = 6) and MafΔTreg (n = 6) mice for the indicated transcripts. Gene expression levels were normalized to Beta2microglobulin. (C) Representative contour plot of the expression of IL-10 and RORγt in Treg from mLN of Maff/f, MafΔTcells and MafΔTreg mice. (D) Percentage of IL-10+ cells among RORγt- (left) and RORγt+ Treg (right) in mLN and colon of Maff/f, MafΔTcells and MafΔTreg mice after ex vivo restimulation (n > 8 per group). (E) Percentage of IL-10+ cells among TH17 cells from mLN of Maff/f, MafΔTcells and MafΔTreg mice after ex vivo restimulation (n > 5 per group). (F) Percentage of IFN-γ+ and IL-17A+ cells among TH17 cells from colons of Cre-negative littermates, MafΔTcells and MafΔTreg mice after ex vivo restimulation (n > 6 per group). (A,B,D–F) Each symbol represents an individual mouse. Data are representative of 2 independent experiments. All mice were over 20 weeks old. All graphs indicate means. Statistical significances were calculated by a Tukey test after a significant one-way ANOVA.
Maf ΔTcells mice eliminate Helicobacter pylori through exacerbated TH17 response
Recently, Gabrysova L et al. demonstrated that TH17 response was inhibited in MafΔTcells mice in an experimental autoimmune encephalomyelitis (EAE) model6. This conclusion is not in line with our observation that MafΔTcells mice develop a colitis (Fig. 1C) characterized by the accumulation of TH17 cells in the colon lamina propria (Fig. 3D). In order to re-evaluate our results in a different experimental setting, we infected MafΔTcells mice with the human pathobiont Helicobacter pylori. H. pylori infects the stomach mucosa and relies on Treg and IL-10 to chronically infect its host19,20. Moreover, it has been established that H. pylori can be cleared from the stomach mucosa by a TH17 response triggered by vaccination21. Two months after infection, quantification of the bacterial burden by numeration of colony-forming unit (CFU) in the mouse stomach showed that MafΔTcells mice cannot be infected by H. pylori (Fig. 7A). Remarkably, as compared to infected Maff/f mice, we observed increased mRNA levels encoding for Cd4, Tnfα, Inos, Il17a, Il22 and the antimicrobial peptides RegIIIβ and RegIIIγ in the stomach mucosa of MafΔTcells mice (Fig. 7B–E). This pattern of increased expression, which is characteristic of a TH17 response, is very similar to the vaccine-induced TH17 response that clear H. pylori infection in WT mice22. In addition, we found higher Il2 production as well as higher Foxp3 level in MafΔTcells mice (Supplementary Fig. 4). Collectively, these data suggest that MafΔTcells mice can eliminate H. pylori infection by generating an exacerbated TH17 inflammatory program.
Elimination of Helicobacter pylori by MafΔTcells mice through exacerbated TH17 response. (A) Numeration of CFU from stomachs of Helicobacter pylori infected Maff/f (n = 6) and MafΔTcells (n = 5) mice. (B–E) Quantitative RT–PCR of stomachs from non-infected Maff/f (n = 6) and MafΔTcells (n = 6) mice and Helicobacter pylori infected Maff/f (n = 6) and MafΔTcells (n = 5) mice for the indicated transcripts. Gene expression levels were normalized to Gapdh. Each symbol represents an individual mouse. Data are representative of at least 2 independent experiments with at least 2 mice per group. Statistical significances were calculated by a Tukey test after a significant one-way ANOVA.
Discussion
Our study shows that the deletion of Maf in all T cells (MafΔTcells mice) drives spontaneous late-onset colitis (Fig. 1). To date, no study has shown that the deletion of Maf in T cells was sufficient and necessary to drive colitis. The development of the colitis is associated with the dysregulation of the Treg-TH17 equilibrium (Fig. 3D,E) and a large decrease in the production of IL-10 (Fig. 3A). Inactivation of Maf in T cells impaired the differentiation of RORγt+ Treg (Fig. 3C) and favoured the accumulation of colitogenic TH17 cells in the colon of the mice (Fig. 3D). The onset of the colitis is dependent on the presence of microbiota since MafΔTcells mice treated with antibiotics develop only mild colitis (Supplementary Fig. 1E,F). The first visible signs of colitis developed around the age of 15 weeks (Fig. 1A). It correlates with the natural differentiation and/or accumulation of RORγt+ Treg and MAF expression in these cells in the colon of WT animals (Fig. 3E,F). This RORγt Treg population is preferentially found in the colon (Fig. 3C) and represents the main producer of IL-10 (Fig. 6D)10,16. The role of MAF in the regulation of the RORγt Treg population was demonstrated very recently using mice in which Maf is deleted in Treg (MafΔTreg)15,16. In these two studies, the authors showed that the differentiation of this particular regulatory population is altered in the absence of Maf.
The low proportion of RORγt+ Treg in the colon of MafΔTcells mice was paradoxically associated with an increase in proportion and numbers of RORγt− Treg. We provide evidence that Treg in MafΔTcells mice are not fully functional. Indeed, compared to WT Treg, Maf-deficient Treg do not control the development of colitis induced by the transfer of naïve CD4+ T cells into Rag1−/− mice (Fig. 4). Moreover, we observed that Maf-deficient RORγt− Treg produce lower level of IL-10 as compared to WT RORγt− Treg (Fig. 6C,D). These findings are in appearance not entirely in line with the study of Xu et al. showing that Maf -deficient nTreg are still able to suppress colon inflammation in Rag1−/− mice transferred with naïve CD4+ T cells15. However, given the nature of their experimental setting in which Maf -deficient nTreg were isolated from H. hepaticus colonized mice and transferred in H. hepaticus infected mice, we assume that the antigen specificity of some Maf-deficient nTreg could compensate for their partial loss of function. One other possibility would be the effect of potentially contaminating Maf-deficient T cells transferred with sorted Maf-deficient Treg. We assume that this potentially pathogenic T cells are also present in the “naïve” Maf-deficient T cells transferred in the mice. However, control Treg transferred together with “naïve” Maf-deficient T cells are still able to control colitis, which make this possibility unlikely. Altogether, it can be suggested that despite their normal differentiation, Maf-deficient RORγt− Treg are unable of preventing colitis due to their loss of function (decreased IL-10 production), combined to their low specificity to the gut microbiota in our experimental setting.
An impaired differentiation of colon RORγt+ Treg was also observed in MafΔTreg mice (Fig. 5A). Despite this absence, the mice did not develop colitis even at old ages (Fig. 5D–F). The absence of colitis onset is associated with a very limited accumulation of TH17 cells (Fig. 5C) and normal expression level of IL-10 in the colon (Fig. 6A). This result is similar to what is described previously in these mice, with only a mild inflammation detected in H. hepaticus free animals aged of 6 months15.
One of the differences between MafΔTreg and MafΔTcells mice is that RORγt+ TH17 cells do not express Maf in MafΔTcells mice (Fig. 2C). It has been shown that MAF plays a prominent role in TH17 cells through the regulation of IL-10 production4,17. We indeed observed in vivo that RORγt+ TH17 cells from MafΔTcells mice do not produce IL-10 while Maff/f and MafΔTreg counterparts do (Fig. 6E). Furthermore, we showed an increase of IFN-γ+ TH17 cells from MafΔTcells compared to Maff/f and MafΔTreg mice (Fig. 6F).
Maf can affect other T cell subtypes that might participate to colitis development. A recent study on the role of MAF in iNKT cells showed that MAF inactivation in these cells leads to a decrease in IL-17a production7. In our system this would mean that inactivation of MAF in these cells would limit the development of colitis, which is however not the case. MAF regulates IL-4 but not IL-10 production in TH2 cells23. The absence of MAF leads to a decreased expression of Il-423, which is not in accordance with an increased pathology. TH2 cells are a minority of the CD4 T cells that we find in the colon of the mice and do not increase among CD4 T cells in MafΔTcells (Supplementary Fig. 3C). Despite the involvement of TH2 cells in the development of ulcerative colitis in human24, we think for these reasons that it is unlikely that the development of colitis after MAF inactivation is related to TH2 cells. TR1 cells, may also be a source of IL-10. Indeed, it was described that MAF is present in this population and regulates the expression of Il10 together with AhR9. We did not find any IL-10 producing RORgt- Foxp3- CD4 T cells in the colon of our mice, which limits the potential role of Tr1 in our system (Supplementary Fig. 3C). Altogether, the absence of IL-10 production by both RORγt+/− Treg and TH17 cells associated with the increase production of IFN-γ by TH17 cells in MafΔTcells mice most probably lead to colitis onset.IL-10 production by Treg is essential to maintain homeostasis at environmental surfaces by directly suppressing pathogenic TH17 cells and TH17/TH1 cells25. Several years ago, work led by Rudensky et al. showed that selective disruption of Il10 in Treg leads to spontaneous colitis26. However, mice lacking IL10RA27 in Treg that show reduced expression of IL-10, develop a more severe colitis, suggesting that compensatory mechanisms orchestrated by non-Treg can participate to maintain homeostasis through regulation of Treg function. Although restricted to the colon, the phenotype observed in our model looks similar to the colitis observed in T cell–specific IL-10 mutant mice28 or Il10−/− mice29. This is in line with recent study showing that Maf is a common regulator of IL-10 in CD4+ T cells6. We were able to link this phenotype to decreased suppressive capacity of all Maf-deficient Treg, but particularly RORγt+ Treg, leading to uncontrolled TH17-driven inflammation.
Recent reports suggested that RORγt is induced after the differentiation of naïve cells into Foxp3+ Treg13,30. How MAF is regulating the development of these cells remains an open question. MAF is induced by TGF-β and IL-6 in CD4+ TH subsets and CD8+ T cells3,4,8,15. Wheaton et al. proposed that IL-6 can upregulate MAF in pre-differentiated Treg leading to RORγt acquisition. Indeed, RORγt has been identified as a direct target of MAF6. Observation that selective disruption of STAT3 in Treg leads to a decrease of RORγt+ Treg (11) in mice suggest that STAT3 may also be required in this process of differentiation. Thus, MAF appears to be at least in part regulated through STAT3 activating cytokines such as IL-6. However, what is exactly regulating MAF expression in the gut remains to be precisely determined.
A recent study showed that additionally to its direct regulation of Il10 expression, Maf is also an inhibitor of Il26. In the Maf-deficient T cells, the increased production of IL-2 has been shown to inhibit the TH17 cell response in the EAE induced model6. In our study, we observed that after infection with H. pylori, the bacteria is cleared from the stomach mucosa of MafΔTcells mice but establish a chronic infection in Maff/f mice. We measured an increased level in transcripts coding for Il2 and Foxp3 in the stomach mucosa of H. pylori-infected MafΔTcells mice (Supplementary Fig. 4), leading to the possibility that increased number of classical Treg are recruited in the stomach mucosa of MafΔTcells mice through an Il2-dependent pathway. However, we also found high amount of Il17a and Il22, indicating the recruitment of TH17 cells (Fig. 7B,E) leading to the clearance of H. pylori from the stomach mucosa of MafΔTcells mice. These results clearly demonstrate that MAF plays a major role in maintaining the Treg -TH17 equilibrium not only in the colon but also in the gastric mucosa. In addition, it can be suggested that the development of a vaccine directed against a pathobiont, such as H. pylori, might be facilitated by a vaccine formulation that prevent MAF expression in primed T cells.
Altogether, we established that the role of Maf was not only restricted to Treg but also to conventional T cells, especially TH17 cells, establishing Maf as a major regulator of the Treg – TH17 balance in the gastro-intestinal tract. On a broader perspective, MAF expression in colonic lymphocytes appears to be strongly associated with an anti-inflammatory type of response. However, the implication of Maf in immune responses appears to be largely location- and context-dependent6.
Material and Methods
Mice
Rag1−/− and Foxp3YFPcre 26 mice were kindly provided by P.C Ho. Foxp3YFPcre and CD4cre 31 mice were crossed with Maffl/fl mice32 to generate Foxp3YFPcre Maffl/fl (MafΔTreg) and CD4cre Maffl/fl (MafΔTcells), respectively. Mice were bred and maintained in a specific-pathogen-free (SPF) environment, which excludes Helicobacter hepaticus, of the animal facility of the University of Lausanne. The animals used were cohoused and littermate controls are referred to as Maff/f mice. Experiments were performed in compliance with the University of Lausanne Institutional regulations and were approved by the veterinarian authorities of the Canton de Vaud (Switzerland).
Isolation of cells
Single-cell suspensions from spleens and mesenteric lymph nodes were obtained after mashing the organs through a 70 µM cell strainer. Colons were collected in calcium and magnesium free Hank’s Balanced Salt Solution (HBSS) (ThermoFisher Scientific) supplemented with 2% of Fetal Calf Serum (FCS) (HBSS 2%) on ice. Samples were further flushed with HBSS 2% and cut longitudinally into 3–4 pieces (2–3 cm). Minced tissues were treated with EDTA 1 mM (ThermoFisher Scientific) and DTT 1 µM (AppliChem) in HBSS 10% solution for 20 min under shaking at 37 °C. After intraepithelial lymphocytes (IEL) removal, cells from the lamina propria were washed twice in HBSS 10% and incubated with Collagenase D (1 mg/ml) (Roche) and complete RPMI (ThermoFisher Scientific) for 30 min under shaking at 37 °C. To isolate leucocytes, supernatants were centrifuged in density gradients 40%/70% Percoll (GE Healthcare Life Sciences) for 30 min at 2000 rpm. All isolated cells were washed in complete RPMI and filtrated before staining.
T cell isolation and transfer into Rag1 −/− mice
Donor cells were obtained from spleens and LN of both WT (obtained from Cre-negative littermates and C57Bl6 mice) and MafΔTcells female mice. CD4+ T cells were enriched using EasySep Mouse CD4+ T Cell Isolation Kit (StemCell Technologies) according to the manufacturer’s recommendations. CD4+CD45RBhiCD25lo and CD4+CD45RBloCD25hi were isolated by sorting on a FACS Aria cell sorter (BD Biosciences). CD4+CD45RBhiCD25lo cells (0.5 × 106) were intravenously injected alone or in combination with CD4+CD45RBloCD25hi cells (0.4 × 106) into males and females Rag1−/− mice from 10 to 15 weeks age. Mice were weighed and monitored weekly. 6 weeks after transfer, mice were sacrificed.
Histopathology and scoring
Colons were fixed with histological tissue fixative (Sigma), embedded in paraffin and stained with haematoxylin and eosin. Histopathological score (0–6) was calculated based on inflammatory cells infiltration (0–3) and tissues abnormalities (0–3) by a pathologist.
In vitro T cell differentiation
Naïve CD4+CD25loCD62hiCD44lo cells were isolated by using EasySep Mouse Naïve CD4+ T Cell Isolation Kit (Stemcell Technologies) from spleens and LN of Maff/f and MafΔTcells mice, and activated with plate-bound anti-CD3 (Biolegend, 5 μg/ml) and soluble anti-CD28 (Biolegend, 1 μg/ml) supplemented with mIL12 (10 ng/ml) and neutralizing antibody anti-IL-4 (clone 11B11, 10 μg/ml) (TH1), mIL-4 (10 ng/ml) and anti-IFN-γ (clone: XMG-121,10 μg/ml) (TH2), hIL-2 (50U/ml) and TGF-β (10 ng/ml) (Treg), TGF-β (5 ng/ml) and IL-6 (40 ng/ml) (FOXP3+ and FOXP3− TH17). Cells were incubated 5 days at 37 °C, 5% CO2.
Antibodies, intracellular staining and flow cytometry
All antibodies used are listed in Table 1. For intracellular cytokine staining, cells were restimulated with PMA (Sigma, 50 ng/ml) and Ionomycin (Sigma, 100 ng/ml) for 4 h at 37 °C in presence of Golgi Plug (BD Biosciences). After staining for viability using LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit (ThermoFisher Scientific) and extracellular markers, cells were fixed and permeabilized with the FOXP3 Transcription Factor Staining Buffer Set (eBiosciences) according to manufacturer’s recommendations. Following permeabilization, cells were intracellularly stained for specific cytokine and acquired on a LSRII flow cytometer (BD). Data were analysed with FlowJo software V10.
Real-time qPCR analysis
Colons were collected, dried and ground into a powder using liquid nitrogen. Total RNA was isolated using RNAeasy Mini Kit (Qiagen, #74194) according to the manufacturer’s recommendations. cDNA was retro-transcribed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, # 4368814) and used for quantitative PCR. The KAPA SYBR® FAST qPCR Master Mix (2×) Kit (Sigma, #KK4618) was used for SYBR analysis. The housekeeping gene beta2microglobulin was used to normalize gene expression. Sequences of primers used are listed in Table 2.
Bacteria and infection
H. pylori P49, kindly provided by Harry Kleanthous (Acambis, Cambridge, MA), is a human clinical isolate adapted to mice. H. pylori P49 expresses VacA but not CagA. H. pylori P49 was grown on Helicobacter pylori-selective agar plate (Oxoid, Basingstoke, UK) and brain heart infusion broth supplemented with 0.25% yeast extract and 10% fetal calf serum (PAA, Pasching, Austria) under microaerophilic conditions, as previously describe33. Mice were treated on day 4 and 5 after birth with 5 × 108 H. pylori P49, administered by orogastric gavage in 200 μL BHI.
Antibiotics treatment
Maff/f and MafΔTcells pregnant females were fed with antibiotics in their drinking water consisting of 0.5 mg/ml Amoxicillin (Mepha Pharma AG) and 5 mg/ml Enrofloxacine (Bayer). Antibiotic-containing drinking water was changed once a week until analysis. Offspring were then treated until their sacrifice at 17 weeks age.
Statistical analysis
Unless otherwise stated, unpaired non-parametric Mann-Whitney t tests were used to calculate statistical significance using GraphPad Prism software. P values: *P < 0.05, **0.01 < P < 0.05, ***P < 0.001.
References
Ho, I. C., Hodge, M. R., Rooney, J. W. & Glimcher, L. H. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell 85, 973–983, doi:S0092-8674(00)81299-4 [pii] (1996).
Ho, I. C., Lo, D. & Glimcher, L. H. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4-dependent and -independent mechanisms. J Exp Med 188, 1859–1866 (1998).
Rutz, S. et al. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nat Immunol 12, 1238–1245, https://doi.org/10.1038/ni.2134 (2011).
Xu, J. et al. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol 182, 6226–6236, https://doi.org/10.4049/jimmunol.0900123 (2009).
Kroenke, M. A. et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol 188, 3734–3744, https://doi.org/10.4049/jimmunol.1103246 (2012).
Gabrysova, L. et al. c-Maf controls immune responses by regulating disease-specific gene networks and repressing IL-2 in CD4(+) T cells. Nat Immunol 19, 497–507, https://doi.org/10.1038/s41590-018-0083-5 (2018).
Yu, J. S. et al. Differentiation of IL-17-Producing Invariant Natural Killer T Cells Requires Expression of the Transcription Factor c-Maf. Frontiers in immunology 8, 1399, https://doi.org/10.3389/fimmu.2017.01399 (2017).
Giordano, M. et al. Molecular profiling of CD8 T cells in autochthonous melanoma identifies Maf as driver of exhaustion. EMBO J 34, 2042–2058, https://doi.org/10.15252/embj.201490786 (2015).
Apetoh, L. et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol 11, 854–861, https://doi.org/10.1038/ni.1912 (2010).
Lochner, M. et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med 205, 1381–1393, https://doi.org/10.1084/jem.20080034 (2008).
Sefik, E. et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 349, 993–997, https://doi.org/10.1126/science.aaa9420 (2015).
Ohnmacht, C. et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 349, 989–993, https://doi.org/10.1126/science.aac4263 (2015).
Yang, B. H. et al. Foxp3(+) T cells expressing RORgammat represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol 9, 444–457, https://doi.org/10.1038/mi.2015.74 (2016).
Kim, K. S. et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351, 858–863, https://doi.org/10.1126/science.aac5560 (2016).
Xu, M. et al. c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373–377, https://doi.org/10.1038/nature25500 (2018).
Wheaton, J. D., Yeh, C. H. & Ciofani, M. Cutting Edge: c-Maf Is Required for Regulatory T Cells To Adopt RORgammat(+) and Follicular Phenotypes. J Immunol 199, 3931–3936, https://doi.org/10.4049/jimmunol.1701134 (2017).
Aschenbrenner, D. et al. An immunoregulatory and tissue-residency program modulated by c-MAF in human TH17 cells. Nat Immunol 19, 1126–1136, https://doi.org/10.1038/s41590-018-0200-5 (2018).
Mottet, C., Uhlig, H. H. & Powrie, F. Cutting edge: cure of colitis by CD4+CD25+regulatory T cells. J Immunol 170, 3939–3943 (2003).
Moyat, M. & Velin, D. Immune responses to Helicobacter pylori infection. World J Gastroenterol 20, 5583–5593, https://doi.org/10.3748/wjg.v20.i19.5583 (2014).
Velin, D., Bachmann, D., Bouzourene, H. & Michetti, P. Reduction of Helicobacter infection in IL-10-/- mice is dependent on CD4+T cells but not on mast cells. Helicobacter 13, 361–369, https://doi.org/10.1111/j.1523-5378.2008.00614.x (2008).
Velin, D. et al. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology 136, 2237–2246 e2231, https://doi.org/10.1053/j.gastro.2009.02.077 (2009).
Moyat, M. et al. IL-22-induced antimicrobial peptides are key determinants of mucosal vaccine-induced protection against H. pylori in mice. Mucosal Immunol 10, 271–281, https://doi.org/10.1038/mi.2016.38 (2017).
Kim, J. I., Ho, I. C., Grusby, M. J. & Glimcher, L. H. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity 10, 745–751, doi:S1074-7613(00)80073-4 [pii] (1999).
Fuss, I. J. et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 157, 1261–1270 (1996).
Huber, S. et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 34, 554–565, https://doi.org/10.1016/j.immuni.2011.01.020 (2011).
Rubtsov, Y. P. et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28, 546–558, https://doi.org/10.1016/j.immuni.2008.02.017 (2008).
Chaudhry, A. et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34, 566–578, https://doi.org/10.1016/j.immuni.2011.03.018 (2011).
Roers, A. et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med 200, 1289–1297, https://doi.org/10.1084/jem.20041789 (2004).
Kuhn, R., Lohler, J., Rennick, D., Rajewsky, K. & Muller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993).
Solomon, B. D. & Hsieh, C. S. Antigen-Specific Development of Mucosal Foxp3+RORgammat+T Cells from Regulatory T Cell Precursors. J Immunol 197, 3512–3519, https://doi.org/10.4049/jimmunol.1601217 (2016).
Sawada, S., Scarborough, J. D., Killeen, N. & Littman, D. R. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell 77, 917–929 (1994).
Wende, H. et al. The transcription factor c-Maf controls touch receptor development and function. Science 335, 1373–1376, https://doi.org/10.1126/science.1214314 (2012).
Velin, D., Bachmann, D., Bouzourene, H. & Michetti, P. Mast cells are critical mediators of vaccine-induced Helicobacter clearance in the mouse model. Gastroenterology 129, 142–155 (2005).
Acknowledgements
We thank the group of Dr. Caroline Pot (D. Duc; S. Vigne) for technical help and Dr. Connie Gilfillan for critical reading of the manuscript. This work was supported by grants from the Swiss Cancer League (G.V: 3679-08-2015), the Max Cloëtta Foundation (G.V), the Cancer Research Institute, USA (G.V, D.E.S), by the Swiss National Foundation grant (D.V: 310030_173197) and Ludwig Cancer Research, USA.
Author information
Authors and Affiliations
Contributions
C.I., M.M.L., D.V. and G.V. designed, performed and analysed the data. H.B. performed the analysis for the histology. G.V., C.I., D.V. and D.E.S. wrote, reviewed and revised the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Imbratta, C., Leblond, M.M., Bouzourène, H. et al. Maf deficiency in T cells dysregulates Treg - TH17 balance leading to spontaneous colitis. Sci Rep 9, 6135 (2019). https://doi.org/10.1038/s41598-019-42486-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42486-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.