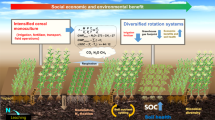
Abstract
Besides other deleterious effects, drought elevates ethylene level too in plants. Increased ethylene concentration reduces root elongation and development that consequently retard plant growth and yield. There are certain PGPR which produce ACC-deaminase. The ACC-deaminase converts ACC (an immediate precursor of ethylene biosynthesis in methionine pathway in higher plants) into ammonia and α-ketobutyrate instead of ethylene. Regularization of ethylene level in plants mitigate the effects of drought. On the other hand, biochar has been reported to be rich in nutrients and exhibiting higher water holding capacity. So, a pot study was conducted with the hypothesis that the combined application of ACC-deaminase producing PGPR and biochar would minimize the drought effects on wheat growth. The ACC-deaminase producing PGPR were applied on wheat seeds in combination with two biochar doses. Three moisture levels were maintained throughout the trial. The data obtained revealed that B. amyloliquefaciens + 2BC improved the chlorophyll a, chlorophyll b, photosynthetic rate, transpiration rate, 100-grain weight, and grain N, P and K up to 114%, 123%, 118%, 73%, 59%, 58%, 18% and 23%, respectively, under drought conditions. It is concluded that co-application of PGPR and biochar is an effective technique to mitigate the drought effects.
Similar content being viewed by others
Introduction
Various biotic (pests, pathogens) and abiotic (soil compaction, drought, salinity, waterlogging, heavy metals, poor nutrition etc.) stresses are a big cause of low crops productivity around the globe1. Drought stress is very common in worldwide arid and semi-arid areas. Moreover, climate change is going to create the worst situation in this regard2,3,4,5,6. The demand for irrigation water is expected to increase by 10% up to 20507. Under drought stress, growth and yield of crops are usually decreased due to less intake of nutrients, poor photosynthesis8 and limited supply of water9. In addition, drought accelerates the biosynthesis of ethylene10,11 which retards the roots elongation and development12,13,14.
Although, traditional breeding, water management and genetic engineering are thought to be useful tools to alleviate drought stress but high technicalities are involved to adopt and implement these approaches15. However, the use of plant growth promoting rhizobacteria (PGPR) is an alternative technique for mitigation of drought effects15. A large number of rhizospheric bacteria are well documented that show growth promotion in plants under stressful conditions14. As far as regularization of ethylene biosynthesis under drought stress is concerned, using 1-aminocyclopropane-1-carboxylate deaminase (ACC-deaminase) producing PGPR is found to be quite effective16,17,18,19,20. The ACC-deaminase cleaves the ACC (1-aminocyclopropane-1-carboxylic acid, an immediate precursor of ethylene biosynthesis through methionine pathway in higher plants) into ammonia and α-ketobutyrate instead of ethylene11,21,22. Besides ethylene regularization, the PGPR also help in better root development23, secretion of growth hormones (auxins or cytokinins)22 and solubilization of immobile nutrients (phosphorus, potassium etc.)24,25.
On the other hand, the imperative role of organic amendments in mitigation of drought stress by improving soil water holding capacity and availability of nutrients cannot be denied26,27. Biochar (BC), is a black carbon compound which is a good source of nutrients. It is produced through pyrolysis at high temperature under low or no supply of oxygen27,28,29. The physio-chemical properties of BC depend on the nature of waste material used and temperature of the pyrolysis30,31. High surface area and pore spaces of BC-structure improve soil water and nutrients holding capacity32,33,34,35.
Wheat (Triticum aestivum L.) is an important cereal crop and staple food in most parts of the world. It contains 55% carbohydrates and 8–12% proteins36. It is an important crop due to its worldwide trade too37. Cultivation of wheat under a limited supply of water significantly decreases the yield38 while its demand is increasing at the rate of 1.6%/annum39. The need of time is to enhance wheat yield even in the areas under drought stress.
In recent past, the researchers focused on the application of either ACC-deaminase containing PGPR or BC in separate to mitigate the drought stress. The novelty and aim of the present study are to examine the combined effect of ACC-deaminase producing PGPR and timber-waste BC for the alleviation of drought effects. Keeping in mind the importance of wheat, the current study was conducted with the hypothesis that the co-application of drought tolerant ACC deaminase producing PGPR and timber waste BC could be very effective to alleviate drought effects.
Results
Gas exchange attributes
Main effects of treatments (T) and various levels of drought (D) were significantly different, while their interaction (T × D) remained similar for the rate of photosynthesis and transpiration. For stomatal conductance, both main and interactive effects of T and D were significantly different. The photosynthetic rate was significantly improved as compared to the control where P. aeruginosa and B. amyloliquefaciens were applied (Table 1). The BC application without PGPR significantly enhanced photosynthetic rate as compared to the control but the application rate 2BC was more effective than 1BC for the improvement in photosynthetic rate. However, among all the treatments P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC remained the best to significantly increase the photosynthetic rate. For transpiration rate, a significant improvement was noted as compared to the control in all the treatments. Application of 1BC and 2BC without PGPR gave statistically similar results regarding transpiration rate. However, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC remained significantly better for transpiration rate as compared to L. adecarboxylata, A. fabrum, P. aeruginosa, B. amyloliquefaciens, 1BC and the control. Maximum increase in photosynthetic rate (118%) and transpiration (73%) rate was noted as compared to control where B. amyloliquefaciens + 2BC was applied. Application of 1BC and 2BC remained significantly better at severe drought (SD) level as compared to the control for stomatal conductance. However, at mild drought (MD) level, application of 2BC remained significantly better than 1BC and control regarding stomatal conductance. The B. amyloliquefaciens + 2BC at SD level while 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC at MD level performed significantly better among other treatments for stomatal conductance. However, stomatal conductance was maximum as compared to the control through 2BC (56%), B. amyloliquefaciens + 2BC (146%) and B. amyloliquefaciens + 2BC (5.62-fold) at normal moisture (NM), MD and SD levels, respectively.
Shoot length and electrolyte leakage
Both main and interactive effects of T and D were significantly different for shoot length and electrolyte leakage in wheat leaves. At SD level, the B. amyloliquefaciens + 2BC and P. aeruginosa + 2BC remained significantly better as compared to rest of the treatments for shoot length (Fig. 1). The 1BC and 2BC remained statistically alike but significantly different as compared to the control at SD for shoot length. However, B. amyloliquefaciens + 2BC also remained significantly better among other treatments for shoot length at MD level. Maximum increase, 153% and 73% in shoot length was noted at SD and MD levels respectively, as compared to the control where B. amyloliquefaciens + 2BC was applied (Fig. 1a). In case of electrolyte leakage at SD, A. fabrum + 2BC and B. amyloliquefaciens + 2BC were found to be significantly better as compared to the control. The 1BC and 2BC remained statistically alike but significantly different as compared to the control at SD for electrolyte leakage (Fig. 1b). The L. adecarboxylata, P. aeruginosa, B. amyloliquefaciens without BC also decreased the electrolyte leakage as compared to the control at SD. Maximum reduction (50%) in electrolyte leakage was noted as compared to the control where A. fabrum + 2BC and B. amyloliquefaciens + 2BC were applied at SD.
Effect of drought tolerant ACC deaminase containing PGPR and various levels of timber waste biochar on shoot length (a) and electrolyte leakage (b) in wheat leaves under various levels of drought (D). Means sharing the same letter are statistically similar. Error bars represent ± standard error. NM = Normal Moisture; MD = Mild Drought; SD = Severe Drought.
Carotenoids and proline
Both main and interactive effects of T and D were significantly different for carotenoids and proline contents. In case of carotenoids, the treatments, L. adecarboxylata + 2BC, A. fabrum + 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC were statistically alike but differed significantly as compared to the control for carotenoids at SD (Fig. 2). The 2BC proved significantly better as compared to 1BC and the control for carotenoids at SD. The 1BC, L. adecarboxylata + 1BC, A. fabrum + 1BC, P. aeruginosa + 1BC, B. amyloliquefaciens + 1BC, 2BC, L. adecarboxylata + 2BC, A. fabrum + 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC were statistically alike but significantly different as compared to the control for carotenoids at MD (Fig. 2a). Maximum increase i.e., 42%, 48% and 220% in carotenoids was noted at NM, MD and SD respectively as compared to the control where B. amyloliquefaciens + 2BC was applied. For proline reduction, A. fabrum, P. aeruginosa and B. amyloliquefaciens differed significantly as compared to the control at SD. The 1BC significantly decreased proline too as compared to control, but the 2BC was statistically far better for proline reduction as compared to 1BC at SD. The PGPR strains remained significantly better with 2BC for proline reduction as compared to with 1BC at SD (Fig. 2b). However, the B. amyloliquefaciens + 2BC showed maximum decrease in proline i.e. 34% at SD as compared to the control.
Effect of drought tolerant ACC deaminase containing PGPR and various levels of timber waste biochar on carotenoids (a) proline (b) in wheat leaves under various levels of drought (D). Means sharing the same letter are statistically similar. Error bars represent ± standard deviations. NM = Normal Moisture; MD = Mild Drought; SD = Severe Drought.
Photosynthetic pigments
Main effects of T and D were significantly different but their interactions remained similar for chlorophyll a, chlorophyll b and total chlorophyll contents in wheat leaves. The treatments L. adecarboxylata, A. fabrum, P. aeruginosa and B. amyloliquefaciens were statistically alike but differed significantly as compared to the control for chlorophyll a content. With and without PGPR, the application of 1BC and 2BC gave statistically similar results but remained significantly different as compared to the control for chlorophyll a (Table 2). For chlorophyll b contents, with and without 1BC, all the PGPR treatments (except B. amyloliquefaciens + 1BC) were statistically alike but differed significantly as compared to the control. Without PGPR, the 2BC was significantly better than 1BC as compared to the control for chlorophyll b contents. However, A. fabrum + 2BC and B. amyloliquefaciens + 2BC proved significantly better as compared to other treatments regarding chlorophyll b contents. In case of total chlorophyll contents, the treatments L. adecarboxylata, A. fabrum, P. aeruginosa and B. amyloliquefaciens differed significantly as compared to the control. Statistically, 2BC was significantly better than 1BC for total chlorophyll. However, the 1BC and 2BC significantly increased total chlorophyll as compared to the control. The 2BC, L. adecarboxylata + 2BC, A. fabrum + 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC were statistically alike and proved better among the other treatments for total chlorophyll contents. Maximum increase in chlorophyll a (114%), chlorophyll b (123%) and total chlorophyll (115%) was noted through B. amyloliquefaciens + 2BC as compared to the control.
N, P and K in shoot
Main effects of T and D were significantly different but their interaction remained similar for nitrogen (N) and phosphorus (P) concentration in shoot. For potassium (K) concentration in shoot, both main and interactive effects of T and D were significantly different. The treatments, L. adecarboxylata, A. fabrum, P. aeruginosa and B. amyloliquefaciens differed significantly as compared to the control for shoot N concentration. The 2BC remained significantly better as compared to 1BC for N concentration in shoot (Table 3). The PGPR (L. adecarboxylata, A. fabrum, P. aeruginosa and B. amyloliquefaciens) with 2BC performed significantly better as compared to 1BC (except B. amyloliquefaciens + 1BC) for N concentration in shoot. For phosphorus concentration in shoot, 1BC and 2BC significantly differed as compared to the control. In case of P concentration in shoot, L. adecarboxylata, A. fabrum and B. amyloliquefaciens were statistically alike but significantly different as compared to the control. The 2BC significantly increased (i.e., by 28%) P concentration in shoot as compared to 1BC. The treatments A. fabrum + 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC proved significantly better for P concentration in shoot. Maximum increase in N (73%) and P (150%) concentration in shoot was noted as compared to the control where B. amyloliquefaciens + 2BC was applied. In case of K concentration in shoot, all the treatments remained significantly better as compared to control at SD. The treatments 1BC and 2BC remained statistically alike without PGPR for K concentration in shoot at MD and SD. The B. amyloliquefaciens + 1BC, A. fabrum + 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC were significantly better for K concentration in shoot at MD. Maximum increase of 38%, 85% and 138% in K concentration in shoot was noted where B. amyloliquefaciens + 2BC was applied as compared to the control at NM, MD and SD, respectively.
Yield attributes
Both main and interactive effects of T and D were significantly different for grain yield pot−1 and straw yield pot−1. In case of 100-grain weight, main effects of T and D were significantly different but their interaction remained statistically similar. The PGPR with and without 1BC, as well as, 1BC and 2BC were statistically at par with the control at SD for grain yield pot−1. However, the L. adecarboxylata + 2BC, A. fabrum + 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC differed significantly as compared to the control for grain yield pot−1 at MD and SD (Table 4). The treatments B. amyloliquefaciens + 1BC also remained significantly better as compared to the control and other PGPR with 1BC at MD for grain yield pot−1. At NM, the B. amyloliquefaciens + 1BC, 2BC, L. adecarboxylata + 2BC, remained significantly different as compared to the control for grain yield pot−1. Maximum increase, 40%, 155% and 215% in grain yield pot−1 was noted at NM, MD and SD as compared to the control where L. adecarboxylata + 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC were applied, respectively. For 100-grain weight, all the treatments (except A. fabrum) remained significantly better as compared to the control. The 1BC and 2BC remained statistically alike for 100-grain weight. The 1BC, A. fabrum + 1BC, B. amyloliquefaciens + 1BC, 2BC, L. adecarboxylata + 2BC, A. fabrum + 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC remained significantly better for 100-grain weight. Maximum increase (59%) in 100-grain weight was noted as compared to the control where B. amyloliquefaciens + 2BC was applied. In case of straw yield pot−1, the PGPR with and without BC remained significantly better as compared to the control at NM, MD and SD. However, 1BC and 2BC remained statistically alike for straw yield pot−1. The L. adecarboxylata + 1BC, A. fabrum + 1BC, P. aeruginosa + 1BC, B. amyloliquefaciens + 1BC, 2BC, L. adecarboxylata + 2BC, A. fabrum + 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC remained significantly better for straw yield pot−1. Maximum increase i.e., 181% and 178% in straw yield pot−1 was noted as compared to the control where B. amyloliquefaciens + 2BC and A. fabrum + 2BC were applied at SD and MD respectively.
N, P and K in grains
Both main and interactive effects of T and D were significantly different for N, P and K concentration in grain. At SD, the L. adecarboxylata, A. fabrum, P. aeruginosa and B. amyloliquefaciens with and without 1BC and 2BC differed significantly as compared to the control for grain nitrogen (Table 5). The B. amyloliquefaciens, 1BC, L. adecarboxylata + 1BC, A. fabrum + 1BC, P. aeruginosa + 1BC and B. amyloliquefaciens + 1BC, 2BC, L. adecarboxylata + 2BC, A. fabrum + 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC remained significantly better as compared to the control for grain nitrogen at MD. Application of A. fabrum + 2BC proved significantly better as compared to the control for grain nitrogen at NM. For grain phosphorus, all the treatments (except P. aeruginosa) were significantly different as compared to the control at SD. However, at MD and NM all the treatments were statistically similar to the control for grain P. In case of grain K, all the treatments were significantly different as compared to the control at SD. The A. fabrum and P. aeruginosa at 2BC remained significantly better than at 1BC for grain K at SD. At MD, inoculation of PGPR with 1BC and 2BC proved significantly better than the control for grain K. The performance of A. fabrum was significantly better at 2BC than at1BC for grain K at MD. Among all the treatments A. fabrum + 1BC, B. amyloliquefaciens + 1BC, 2BC, L. adecarboxylata + 2BC, A. fabrum + 2BC, P. aeruginosa + 2BC, B. amyloliquefaciens + 2BC remained significantly better at NM. The 2BC proved significantly better than 1BC for grain K at NM, MD and SD. Maximum increase in grain N (58%), P (18%) and K (23%) was noted through B. amyloliquefaciens + 2BC as compared to the control at SD.
Discussion
In the current study, reduction in shoot length of wheat at MD and SD in the control might be due to competition for water and nutrients between root and shoot. Gargallo-Garriga et al.40 stated that drought stress deactivated shoot metabolic activity to conserve water and food which would have facilitated roots elongation. The reduction in water and nutrients movement in shoot, enhanced the up-regulation of ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC)41 while significant amount of ACC generated ethylene restricted the root elongation12. Glick et al.11 suggested the mechanism for reduction in stress ethylene through the activity of ACC-deaminase11. According to Glick et al.11, the synthesis of indole acetic acid (IAA) by PGPR stimulates the elongation of plants cells and activates ACC synthase which converts S-adenosyl methionine to ACC. A significant amount of ACC is exuded by plants roots and seeds in rhizosphere which is hydrolyzed by ACC deaminase into NH3 and α-ketobutyrate, resulting in better roots elongation. Secretion of roots exudates (phytosiderophores, sugars, organic acids, amino acids, vitamins, nucleosides and mucilage) also attracts PGPR that colonize roots and facilitates better uptake of water and nutrients42,43. Besides imperative role of PGPR, high surface area, ion exchange capacity, nutrients and water holding capacity of BC make it an effective amendment for better intake of nutrients and water in plants44,45,46. A significant improvement in photosynthetic rate, transpiration rate and stomatal conductance under MD and SD, signified the effectiveness of co-application of L. adecarboxylata, A. fabrum, P. aeruginosa, B. amyloliquefaciens with 2BC, comparative to their sole application and the control. The increase in gas exchange attributes might be due to the better uptake of water and nutrients, improvement in soil water holding capacity (WHC), PGPR colonization and reduction in ethylene through the co-application of 2BC47,48 and ACC deaminase containing PGPR49,50. According to Zheng et al.51 and Borch et al.52 the deficiency of nitrogen and phosphorus significantly decrease the growth of crops. Siddique et al.53 suggested the less stomatal conductance as main cause of reduction in rate of transpiration under drought. Tholen et al.54 argued that drought stress decreased the intake of CO2 due to less stomatal conductance which restricted the carboxylation. Hence reduced the rate of photosynthesis55. However, A significant improvement in chlorophyll a, chlorophyll b, total chlorophyll, shoot and grain nutrients concentration validated the efficacious functioning of co-application of ACC deaminase producing PGPR and 2BC that also significantly increased the yield attributes (100-grains weight, straw yield and grains yield pot−1) of wheat plants at MD and SD. Richardson et al.56 and Zahir et al.57, suggested better roots elongation and secretion of organic acids by PGPR for P and K solubilization as key factors, responsible for better nutrients uptake, improvement in dry weight and yield of crops. Moreover, the biochar ability to sorb nutrients also reduced the losses of N and improved its uptake in plants58,59. Chan et al.60 suggested that the high surface area of biochar is a basic reason for improved cation exchange sites in soil which resulted in better bioavailability of nutrients. Results of the current study also showed that electrolyte leakage in the wheat plants leaves was decreased where L. adecarboxylata, A. fabrum, P. aeruginosa, B. amyloliquefaciens with 2BC were applied comparative to the control under MD and SD. The reduction in electrolyte leakage might be due to the activity of ACC deaminase, better availability of water and nutrients by co-application of PGPR and 2BC. Senaratna and McKersie61 observed a significant increase in electrolyte leakage because of cell membrane damage by drought stress which made it more permeable. According to Matile et al.62, cell usually lost its membrane integrity as a result of lipid degradation by ethylene. When the lipids in cell membrane become degraded, the ethylene comes in contact with the chloroplast and activates the chlorophyllase (chlase) gene which causes severe damage to the chlorophyll62. However, the addition of 2BC and PGPR in combination significantly decreased the electrolyte leakage which might be due to activity of ACC deaminase, better availability of water and nutrients uptake.
Conclusion
It is concluded that the co-application of drought tolerant ACC deaminase producing PGPR and 2BC is comparatively better approach than their sole application to mitigate drought stress in wheat. Though Leclercia adecarboxylata and Pseudomonas aeruginosa were also effective enough but Agrobacterium fabrum and Bacillus amyloliquefaciens with 2BC gave maximum increase in the gas exchange attributes, nutrients concentration in shoot and grain, photosynthetic pigments and yield of wheat. More investigations are needed at field level to introduce A. fabrum and B. amyloliquefaciens with 2BC to improve growth and yield of wheat under drought stress.
Materials and Methods
Out of 23 initially screened rhizobacteria (Table 7) isolated from wheat rhizospheric soil, collected from Old Shujabad Road (30.11°N and 71.43°E) and Akramabad (30.16°N and 71.29°E), four most efficient drought-tolerant ACC-deaminase producing PGPR were screened out after a laboratory hydroponic trial in the Department of Soil Science, Bahauddin Zakariya University Multan, Pakistan. For screening of most effective drought tolerant PGPR strains, polyethylene glycol 6000 (PEG-6000) was used (0, 10 and 20%) to maintain osmotic potential (0.05, −0.23 and −0.78 MPa) to introduce drought stress63,64.
Molecular identification of the most efficient drought tolerant ACC deaminase producing PGPR was done by 16S rRNA gene sequencing using PCR primers 1492R 5′ (TAC GGY TAC CTT GTT ACG ACT T) 3′ and 27F 5′ (AGA GTT TGA TCM TGG CTC AG) 3′. The gene sequencing primers were 907R 5′ (CCG TCA ATT CMT TTR AGT TT) 3′ and 785F 5′ (GGA TTA GAT ACC CTG GTA) 3′. Finally, 16S rRNA gene sequences were aligned and relationships were deduced using BLAST analysis65. Most efficient drought-tolerant ACC-deaminase producing PGPR were identified as AbW1 = Leclercia adecarboxylata (NR_104933.1), CbW2 = Agrobacterium fabrum (NR_074266.1), CbW3 = Bacillus amyloliquefaciens (FN_597644.1) and AbW5 = Pseudomonas aeruginosa (CP012001.1). These PGPR strains were able to grow at the osmotic potential −0.78 MPa generated through 20% polyethylene glycol 6000 (PEG). The DF minimal salt medium (4.0 g KH2PO4, 6.0 g Na2HPO4, 0.2 g, MgSO4.7H2O, 2.0 g glucose, 2.0 g gluconic acid and 2.0 g citric acid with trace elements: 1 mg FeSO4.7H2O, 10 mg H3BO3, 11.19 mg MnSO4.H2O, 124.6 mg ZnSO4.7H2O, 78.22 mg CuSO4.5H2O, 10 mg MoO3, pH = 7.2 and 0.5 M ACC as a sole nitrogen source) was used to grow the strains66. For determination of indole acetic acid (IAA) with and without L-tryptophan, Glickmann and Dessaux67 method was adopted.
To confirm the presence of AcdS gene that plays key role in synthesis of ACC deaminase NCBI gene bank was consulted. From NCBI gene bank it was confirmed that B. amyloliquefaciens (https://www.ncbi.nlm.nih.gov/nuccore/KX709841.1/), A. fabrum (https://www.ncbi.nlm.nih.gov/protein/PZP48640.1/) and P. aeruginosa (https://www.ncbi.nlm.nih.gov/nuccore/CP014948.1/) have AcdS gene while work is yet continued on L. adecarboxylata. For assessing the ACC deaminase produced by PGPR methodology of El-Tarabily68 and Honma and Shimomura20 was used. Pikovskaya’s medium was used to examine the phosphorus solubilizing activity of PGPR as described by Vazquez et al.69. Potassium solubilizing activity of PGPR was assessed according to the methodology of Candra Setiawati and Mutmainnah70. Characterization of the PGPR isolates is provided in Table 7.
For the production of biochar, timber waste was collected from local timber market. The timber waste was initially sun-dried and then pyrolyzed at 389 °C for 80 min in an especially designed pyrolyzer as described by Qayyum et al.27. All the pyrolyzed material (biochar) was then crushed in a grinder and passed through 2 mm sieve. Finally, the fine powder of timber waste biochar (BC) was stored in air tight plastic jars27.
The pH and ECe of BC were determined by mixing the BC and water with the ration, 1:20 (w/v) as described by Qayyum et al.27. Di-acid (HNO3: HClO4) digestion71 of biochar was done for the analysis of total phosphorus following yellow color method on spectrophotometer72, and those of potassium and sodium on flame photometer73. For the determination of nitrogen, H2SO4 digestion72 was done followed by distillation on Kjeldahl’s distillation apparatus74. The volatile matter and ash content of biochar were analyzed according to Qayyum et al.75 by heating the biochar in muffle furnace at 450 °C and 550 °C respectively. The fixed carbon in biochar was assessed (Table 6) using the equation as follows76;
The plastic bag (30 cm deep × 20 cm in diameter) was used as a pot, having capacity to carry 8 kg soil. The soil was collected from the plough layer of bank of the Chenab River, Multan, Punjab, Pakistan. The soil of selected area was previously characterized as dark yellowish brown, moderately calcareous, weakly structured and well drained with Cambic subsurface horizon and an Ochric epipedon77. The soil texture was determined by hydrometer method78 which was sandy loam (USDA triangle) with mixed hyperthermic Haplocambids. The organic matter in soil was determined by Walkley79. The organic nitrogen in soil was determined using the equation:
For extractable soil P determination, Olsen and Sommers80 method was used. Similarly, the extractable K in soil was determined according to the method described by Nadeem et al.73 (Table 6).
In each plastic pot, 8 kg soil was filled. To fulfil macro nutrients requirement nitrogen (N), phosphorus (P) and potassium (K) fertilizers were added at the rate of 120: 90 and 60 kg ha−1 respectively, as recommended dose keeping in mind the nutrients concentration of biochar where it was applied81. The urea was added in three split doses. As far as diammonium phosphate (DAP) and muriate of potash (MOP) fertilizers are concerned, the recommended rates of fertilizers were applied in a single dose at the time of sowing. Timber waster biochar was added at three different rates including: control i.e., no biochar (0BC), 0.75% of soil (60 g biochar per 8 Kg soil) biochar (1BC) and 1.50% of soil (120 g biochar per 8 Kg soil) biochar (2BC).
The seeds of wheat (Galaxy-2013) were obtained from the certified seed dealer of the Government of Punjab, Pakistan. Healthy seeds were separated from broken and weak seeds. The seeds were surface-sterilized with sodium hypochlorite (5%) followed by 3 washes with ethanol (95%). Finally, all the seeds were washed three times with sterilized deionized water82. For PGPR inoculation, 10 ml of inoculum (0.5 optical density at 535 nm wavelength)83 was added along 10% sugar (glucose) in 100 g sterilized seeds. After proper mixing of seeds, inoculum and sugar solution, top dressing of seeds was done with a mixture of peat and clay (3:1 ratio) as described by Ahmad et al.84. Before inoculation of seeds, the peat and clay mixture was sterilized at 121 °C for 20 min in an autoclave83. All the control treatment seeds were also top dressed with peat and clay mixture along with 10% sugar solution without inoculum85.
In each pot, 10 seeds of wheat were initially sown. In control, the soil normal moisture (NM) was maintained at the level of 70% of field capacity (FC70) throughout the experiment on weight basis. However, to introduce mild drought (MD) and severe drought (SD) stress as per treatment plan, the soil moisture was maintained at the level of 50% and 30% of field capacity (FC50 and FC30), respectively, throughout the trial as suggested by Boutraa et al.86. After germination of seeds, five healthy seedlings were kept in each pot by thinning.
The pot experiment was conducted in the research area of the Department of Soil Science, Bahauddin Zakariya University Multan, Pakistan under drought stress on wheat. There were 15 treatments with 3 replications, following factorial completely randomized design (CRD). The treatments included: Control (No PGPR + No BC), L. adecarboxylata, A. fabrum, P. aeruginosa, B. amyloliquefaciens, 1BC, L. adecarboxylata + 1BC, A. fabrum + 1BC, P. aeruginosa + 1BC, B. amyloliquefaciens + 1BC, 2BC, L. adecarboxylata + 2BC, A. fabrum + 2BC, P. aeruginosa + 2BC and B. amyloliquefaciens + 2BC.
Leaf gas exchange parameters (net photosynthetic rate, net transpiration rate and stomatal conductance) were determined with the help of Infra-Red Gas Analyzer (CI-340 Photosynthesis system, CID, Inc. USA) by joining 4 leaves of wheat. On a sunny day, the readings were taken between 10:30 and 11:30 AM at saturating intensity of light87.
After 50 days of sowing, the seedlings were harvested from each pot for the measurement of shoot length and determination of electrolyte leakage, proline contents, photosynthetic pigments level and nutrients concentration in the shoot.
The electrolyte leakage (EL) was determined following the procedure given by Lutts et al.88. The leaves were washed with deionized water and then cut using a steel cylinder having diameter 1 cm. One gram of uniform sized leaf pieces were immersed in a test tube containing deionized water (20 ml) and incubated at 25 °C for 24 h. The electrical conductivity (EC1) was determined using pre-calibrated EC meter. The second EC (EC2) was noted heating the test tubes in a water bath at 120 °C for 20 min. The final value of EL was calculated using the equation as follows;
For proline assessment in wheat leaves, methodology stated by Bates et al.89 was followed. The proline was extracted from fresh (0.1 g) leaves in 2 ml of 40% methanol. After extraction, the 1 ml mixture of glacial acetic acid and 6 M orthophosphoric acid (3:2 v/v) was mixed in 1 ml extract along with 25 mg ninhydrin. Then the solution was incubated at 100 °C for 60 min. After cooling down, 5 ml Toluene was added. For the estimation of proline contents, absorbance was noted on spectrophotometer at 520 nm wavelength.
The chlorophyll a, chlorophyll b and total chlorophyll contents were determined in the fresh leaves of wheat according to the protocol given by Arnon90. The extract was taken from the leaves using acetone (80%) solution. For the estimation of chlorophyll a and chlorophyll b, the absorbance was taken at 663 and 645 nm wavelength, respectively on spectrophotometer. Final calculations were made using the following relations;
where, OD = Optical density (wavelength). V = Final volume made. W = Fresh leaf made (g).
The samples were digested with sulfuric acid72 followed by distillation on Kjeldahl’s distillation apparatus74. The yellow colour method was used for the determination of phosphorus concentration noting absorbance at 420 nm on spectrophotometer72. As far as the K concentration in wheat shoot and grain is concerned, the samples were digested and then run on flame photometer as described by Nadeem et al.73.
The wheat plants were harvested after 125 days of sowing for the determination of grains yield pot−1, straw yield pot−1 and 100-grain weight. Weight of 100 grains, straw and grains yield pot−1 were assessed on top weight balance. For straw yield, plants were harvested at 4 inches above the ground surface. Sun dried 100 grains of wheat were counted randomly and manually and then weighed on top weight balance. Total wheat grains collected from a single pot were weighed and considered as grain yield pot−1.
Statistical analyses of the data were carried out using standard statistical procedures91. All the treatments were compared using Tukey’s test at p ≤ 0.05.
Data Availability
No datasets were generated or analyzed during the current study. All the analyzed data can be accessed after publication by requesting the corresponding author.
References
Timmusk, S., Seisenbaeva, G. & Behers, L. Titania (TiO2) nanoparticles enhance the performance of growth-promoting rhizobacteria. Sci. Rep. 8, 617 (2018).
Anjum, S. A., Wang, L., Farooq, M., Xue, L. & Ali, S. Fulvic Acid Application Improves the Maize Performance under Well-watered and Drought Conditions. J. Agron. Crop Sci. 197, 409–417 (2011).
Zhang, S., Kang, H. & Yang, W. Climate Change-Induced Water Stress Suppresses the Regeneration of the Critically Endangered Forest Tree Nyssa yunnanensis. PLoS One 12, e0182012 (2017).
Griffin, M. T., Montz, B. E. & S Arrigo, J. Evaluating Climate Change Induced Water Stress: A Case Study of the Lower Cape Fear basin, NC. Appl. Geogr. 40, 115–128 (2013).
Mehran, A. et al. Compounding Impacts of Human-Induced Water Stress and Climate Change on Water Availability. Sci. Rep. 7, 6282 (2017).
Saikia, J. et al. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 8, 3560 (2018).
Wada, Y. et al. Multimodel Projections and Uncertainties of Irrigation Water Demand Under Climate Change. Geophys. Res. Lett. 40, 4626–4632 (2013).
Fahad, S. et al. Crop Production under Drought and Heat. Stress: Plant Responses and Management Options. Front. Pant Sci. 8, 1147 (2017).
Ludlow, M. M. & Muchow, R. C. A Critical Evaluation of Traits for Improving Crop Yields in Water-Limited Environments. Adv. Agron. 43, 107–153 (1990).
Johnson, P. R. & Ecker, J. R. The ethylene gas signal transduction pathway: a molecular perspective. Annu. Rev. Genet. 32, 227–254 (1998).
Glick, B., Penrose, D. & Li, J. A Model For the Lowering of Plant Ethylene Concentrations by Plant Growth-promoting Bacteria. J. Theor. Biol. 190, 63–68 (1998).
Mayak, S., Tirosh, T. & Glick, B. R. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 166, 525–530 (2004).
Morgan, P. W. & Drew, M. C. Ethylene and plant responses to stress. Physiol. Plant. 100, 620–630 (1997).
Danish, S., Zafar-ul-Hye, M., Hussain, M., Shaaban, M., Núñez-Delgado, A., Shahid Hussain, S. & Qayyum, M.F.Rhizobacteria with ACC-Deaminase Activity Improve Nutrient Uptake, Chlorophyll Contents and Early Seedling Growth of Wheat under PEG Induced Osmotic Stress. Intl. J. Agric. Biol. 2(1), 1212–1220 (2019).
Niu, X., Song, L., Xiao, Y. & Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid and their potential in alleviating drought stress. Front. Microbiol. 8 (2018).
Saraf, M. et al. The Role of ACC Deaminase Producing PGPR in Sustainable Agriculture. In Plant Growth and Health Promoting Bacteria (ed. Maheshwari, D.) 18, 365–385 (Springer, Berlin, Heidelberg, 2010).
Ngumbi, E. & Kloepper, J. Bacterial-mediated drought tolerance: Current and future prospects. Appl. Soil Ecol. 105, 109–125 (2016).
Saleem, M., Arshad, M., Hussain, S. & Bhatti, A. S. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J. Ind. Microbiol. Biotechnol. 34, 635–648 (2007).
Vurukonda, S. S. K. P., Vardharajula, S., Shrivastava, M. & SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 184, 13–24 (2016).
Honma, M. & Shimomura, T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 42, 1825–1831 (1978).
Glick, B. R. Bacterial ACC deaminase and the alleviation of plant stress. Adv. Appl. Microbiol. 56, 291–312 (2004).
Glick, B. R. et al. ACC deaminase is central to the functioning of plant growth promoting rhizobacteria. in Biology and Biotechnology of the Plant Hormone Ethylene II. (eds Kanellis, A. K. et al.) 293–298 (Springer, 1999).
Belimov, A. A. et al. Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 47, 642–652 (2001).
Alam, M. S., Talukder, N. M., Islam, M. T., Sarkar, A. & Hossain, M. M. Phosphate solubilizing rhizoplane bacteria on growth and yield of transplant aman rice. J. Agrofor. Environ. 2, 11–14 (2008).
Basak, B. B. & Biswas, D. R. Co-inoculation of potassium solubilizing and nitrogen fixing bacteria on solubilization of waste mica and their effect on growth promotion and nutrient acquisition by a forage crop. Biol. Fertil. Soils 46, 641–648 (2010).
Danish, S. et al. Influence of biochar on growth and photosynthetic attributes of Triticum aestivum L. under half and full irrigation. Int. J. Biosci. 5, 101–108 (2014).
Qayyum, M. F., Abid, M., Danish, S., Saeed, M. K. & Ali, M. A. Effects of various biochars on seed germination and carbon mineralization in an alkaline soil. Pakistan J. Agric. Sci. 51, 977–982 (2014).
Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 5, 381–387 (2007).
Singh, B., Singh, B. P. & Cowie, A. L. Characterisation and evaluation of biochars for their application as a soil amendment. Aust. J. Soil Res. 48, 516–525 (2010).
Navia, R. & Crowley, D. E. Closing the loop on organic waste management: biochar for agricultural land application and climate change mitigation. Waste Manag. Res. 28, 479–480 (2010).
Glaser, B., Lehmann, J. & Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal - a review. Biol. Fertil. Soils 35, 219–230 (2002).
Gundale, M. J. & DeLuca, T. H. Charcoal effects on soil solution chemistry and growth of Koeleria macrantha in the ponderosa pine/Douglas-fir ecosystem. Biol. Fertil. Soils 43, 303–311 (2006).
Warnock, D. D. Arbuscular mycorrhizal responses to biochars in soils - potential mechanisms of interaction and observed responses in controlled environments. (Division of Biological Sciences and the Microbial Ecology and Department of Crop and Soil Sciences. University of Montana and Cornell University., 2009).
Amonette, J. & Joseph, S. Characteristics of Biochar - Micro-chemical Properties. In Biochar for Environmental Management: Science and Technology (eds Amonette, J. & Joseph, S.) 33–52, https://doi.org/10.4324/9781849770552 (Earthscan, 2009).
Hartmann, M., Fliessbach, A., Oberholzer, H.-R. & Widmer, F. Ranking the magnitude of crop and farming system effects on soil microbial biomass and genetic structure of bacterial communities. FEMS Microbiol. Ecol. 57, 378–88 (2006).
Bos, C. et al. Postprandial metabolic utilization of wheat protein in humans. Am. J. Clin. Nutr. 81, 87–94 (2005).
FAO. Basic facts of the world cereal situation, Food Outlook No.4. Food and Agriculture Organization (FAO) of the United Nations. Food and Agriculture Organization (FAO) of the United Nations (2003).
Singh, G. P. & Chaudhary, H. B. Selection Parameters and Yield Enhancement of Wheat (Triticum aestivum L.) Under Different Moisture Stress Conditions. Asian J. Plant Sci. 5, 894–898 (2006).
Ortíz-Castro, R., Valencia-Cantero, E. & López-Bucio, J. Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal. Behav. 3, 263–265 (2008).
Gargallo-Garriga, A. et al. Opposite metabolic responses of shoots and roots to drought. Sci. Rep. 4, 6829 (2014).
Sobeih, W. Y., Dodd, I. C., Bacon, M. A., Grierson, D. & Davies, W. J. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J. Exp. Bot. 55, 2353–2363 (2004).
Shukla, K. P., Sharma, S., Singh, N. K., Singh, V. & Tiwari, K. Nature and role of root exudates: Efficacy in bioremediation. African J. Biotechnol. 10, 9717–9724 (2011).
Drogue, B., Combes-Meynet, E., Moënne-Loccoz, Y., Wisniewski-Dyé, F. & Prigent-Combaret, C. Control of the Cooperation Between Plant Growth-Promoting Rhizobacteria and Crops by Rhizosphere Signals. In Molecular Microbial Ecology of the Rhizosphere (ed. Bruijn, F. J. de) 1, 279–293 (Wiley-Blackwell, 2013).
Lehmann, J., Gaunt, J. & Rondon, M. Bio-char Sequestration in Terrestrial Ecosystems – A Review. Mitig. Adapt. Strateg. Glob. Chang. 11, 395–419 (2006).
Shenbagavalli, S. & Mahimairaja, S. Production and characterization of biochar from different biological wastes. Int. J. Plant, Anim. Environ. Sci. 2, 197–201 (2012).
Paetsch, L. et al. Effect of in-situ aged and fresh biochar on soil hydraulic conditions and microbial C use under drought conditions. Sci. Rep. 8 (2018).
Novak, J. M. et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 3, 195–206 (2009).
Lehmann, J. et al. Biochar effects on soil biota – A review. Soil Biol. Biochem. 43, 1812–1836 (2011).
Zafar-ul-Hye, M., Farooq, H. M., Zahir, Z. A., Hussain, M. & Hussain, A. Application of ACC-deaminase containing rhizobacteria with fertilizer improves maize production under drought and salinity stress. Int. J. Agric. Biol. 16, 591–596 (2014).
Akhtar, S. S., Andersen, M. N., Naveed, M., Zahir, Z. A. & Liu, F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 42, 770–781 (2015).
Zheng, Z., Parent, L. E. & MacLeod, J. A. Influence of soil texture on fertilizer and soil phosphorus transformations in Gleysolic soils. Can. J. Soil Sci. 83, 395–403 (2003).
Borch, K., Bouma, T. J., Lynch, J. P. & Brown, K. M. Ethylene: A regulator of root architectural responses to soil phosphorus availability. Plant, Cell Environ. 22, 425–431 (1999).
Siddique, M. R. B., Hamid, A. & Islam Drought stress effects on photosynthetic rate and leaf gas exchange of wheat. Bot. Bull. Acad. Sin. 40, 141–145 (1999).
Tholen, D., Pons, T. L., Voesenek, L. A. & Poorter, H. The role of ethylene perception in the control of photosynthesis. Plant Signal. Behav. 3, 108–109 (2008).
Chaves, M. M., Flexas, J. & Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 103, 551–560 (2009).
Richardson, A. E., Hocking, P. J., Simpson, R. J. & George, T. S. Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci. 60, 124–143 (2009).
Zahir, Z. A., Zafar-ul-Hye, M., Sajjad, S. & Naveed, M. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for coinoculation with Rhizobium leguminosarum to improve growth, nodulation, and yield of lentil. Biol. Fertil. Soils 47, 457–465 (2011).
Younis, U., Danish, S., Shah, M. H. R. & Malik, S. A. Nutrient shifts modeling in Spinacea oleracea L. and Trigonella corniculata L. in contaminated soil amended with biochar. Int. J. Biosci. 5, 89–98 (2014).
Decai, G. et al. Application of biochar in dryland soil decreasing loss of nitrogen and improving nitrogen using rate. Trans. Chinese Soc. Agric. Eng. 30, 54–61 (2014).
Chan, K. Y., Van Zwieten, L., Meszaros, I., Downie, A. & Joseph, S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 46, 437–444 (2008).
Senaratna, T. & McKersie, B. D. Characterization of Solute Efflux from Dehydration Injured Soybean (Glycine max L. Merr) Seeds. Plant Physiol. 72, 911–4 (1983).
Matile, P., Schellenberg, M. & Vicentini, F. Planta Localization of chlorophyllase in the chloroplast envelope. Planta 201, 96–99 (1997).
Piwowarczyk, B., Kamińska, I. & Rybiński, W. Influence of PEG generated osmotic stress on shoot regeneration and some biochemical parameters in Lathyrus culture. Czech J. Genet. Plant Breed. 50, 77–83 (2014).
Paul, S., Aggarwal, C., Manjunatha, B. & Rathi, M. S. Characterization of osmotolerant rhizobacteria for plant growth promoting activities in vitro and during plant-microbe association under osmotic stress. Indian J. Exp. Biol. 56, 582–589 (2018).
Siddikee, M. A., Chauhan, P. S., Anandham, R., Han, G. H. & Sa, T. Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J. Microbiol. Biotechnol. 20, 1577–1584 (2010).
Dworkin, M. & Foster, J. W. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 75, 592–603 (1958).
Glickmann, E. & Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 61, 793–796 (1995).
El-Tarabily, K. A. Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes. Plant Soil 308, 161–174 (2008).
Vazquez, P., Holguin, G., Puente, M. E., Lopez-Cortes, A. & Bashan, Y. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol. Fertil. Soils 30, 460–468 (2000).
Setiawati, T. C. & Mutmainnah, L. Solubilization of Potassium Containing Mineral by Microorganisms From Sugarcane Rhizosphere. Ital. Oral Surg. 9, 108–117 (2016).
Chapman, H. D. & Pratt, P. F. Methods of analysis for soils, plants and water (1961).
Jones, J. B., WolfH, B. & Mills, H. A. Plant Analysis Handbook: A Practical Sampling, Preparation, Analysis, and Interpretation Guide. (Micro-Macro Publishing Inc., 1991).
Nadeem, F. et al. Qualitative and Chemical Analysis of Rice Kernel To Time of Application of Phosphorus in Combination With Zinc Under Anaerobic Conditions. Asian J. Agric. Biol. 1, 67–75 (2013).
Van Schouwenberg, J. C. & Walinge, I. Methods of Analysis for Plant Material. (Agricultural University Wageningen, 1973).
Qayyum, M. F., Steffens, D., Reisenauer, H. P. & Schubert, S. Kinetics of Carbon Mineralization of Biochars Compared with Wheat Straw in Three Soils. J. Environ. Qual. 41, 1210 (2012).
Noor, N. M., Shariff, A. & Abdullah, N. Slow Pyrolysis of Cassava Wastes for Biochar Production and Characterization. Iran. J. Energy Environ. 3, 60–65 (2012).
Abid, M. et al. Biochar increased photosynthetic and accessory pigments in tomato (Solanum lycopersicum L.) plants by reducing cadmium concentration under various irrigation waters. Environ. Sci. Pollut. Res. 24, 22111–22118 (2017).
Gee, G. W. & Bauder, J. W. Particle-size analysis. In Methods of soil analysis. Part 1. Physical and mineralogical methods 383–411, https://doi.org/10.2136/sssabookser5.1.2ed.c15 (1986).
Walkley, A. An Examination of Methods for Determining Organic Carbon and Nitrogen in Soils. J. Agric. Sci. 25, 598 (1935).
Olsen, S. R. & Sommers, L. E. Phosphorus. In Method of soil analysis, Agron. No. 9, part 2: Chemical and microbiological properties (ed. Page, A. L.) 403–430 (American Society of Agronomy, 1982).
Adnan, M. et al. Integrated effects of rhizobial inoculum and inorganic fertilizers on wheat yield and yield components. Am. J. Plant Sci. 05, 2066–2073 (2014).
Ahmad, I. et al. Cadmium-tolerant bacteria induce metal stress tolerance in cereals. Environ. Sci. Pollut. Res. 21, 11054–11065 (2014).
Nadeem, S. M., Zahir, Z. A. & Naveed, M. Rhizobacteria containing ACC-deaminase confer salt tolerance in maize grown on salt-affected fields. Can. J. Microbiol. 55, 1302–1309 (2009).
Ahmad, M. T., Asghar, N., Saleem, M., Khan, M. Y. & Zahir, Z. A. Synergistic Effect of Rhizobia and Biochar on Growth and Physiology of Maize. Agron. J. 107, 2327–2334 (2015).
Shaharoona, B., Arshad, M. & Zahir, Z. A. Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.). Lett. Appl. Microbiol. 42, 155–159 (2006).
Boutraa, T., Akhkha, A., Al-Shoaibi, A. A. & Alhejeli, A. M. Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. J. Taibah Univ. Sci. 3, 39–48 (2010).
Nazar, R., Khan, M. I. R., Iqbal, N., Masood, A. & Khan, N. A. Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulfur in mustard. Physiol. Plant. 152, 331–344 (2014).
Lutts, S., Kinet, J. M. & Bouharmont, J. NaCl-induced Senescence in Leaves of Rice (Oryza sativa L.) Cultivars Differing in Salinity Resistance. Ann. Bot. 78, 389–398 (1996).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207 (1973).
Arnon, D. I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15 (1949).
Steel, R. G., Torrie, J. H. & Dickey, D. A. Principles and Procedures of Statistics: A Biometrical Approach. (McGraw Hill Book International Co., 1997).
Acknowledgements
The first author is grateful to the Higher Education Commission (HEC) of Pakistan to provide Indigenous Scholarship (PIN: 315-8403-2AV3-049) for his Ph.D. study in Pakistan.
Author information
Authors and Affiliations
Contributions
S.D. conducted research, prepared biochar, collected data and wrote the manuscript; M.Z.H. designed the experiment, supervised and completed the manuscript writing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Danish, S., Zafar-ul-Hye, M. Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci Rep 9, 5999 (2019). https://doi.org/10.1038/s41598-019-42374-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42374-9
This article is cited by
-
Mitigation of cadmium-induced stress in maize via synergistic application of biochar and gibberellic acid to enhance morpho-physiological and biochemical traits
BMC Plant Biology (2024)
-
Fostering plant growth performance under drought stress using rhizospheric microbes, their gene editing, and biochar
Environmental Geochemistry and Health (2024)
-
Impact of Drought Stress on Plant Growth and Its Management Using Plant Growth Promoting Rhizobacteria
Indian Journal of Microbiology (2024)
-
Evaluation of potassium-enriched biochar and GA3 effectiveness for Improving wheat growth under drought stress
BMC Plant Biology (2023)
-
γ-Aminobutyric acid (GABA) and ectoine (ECT) impacts with and without AMF on antioxidants, gas exchange attributes and nutrients of cotton cultivated in salt affected soil
BMC Plant Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.