Abstract
To realize large lithium storage capacity and excellent rate capability lithium ion batteries, highly electrochemically active materials and rational design of structure are desirable. Here, we successfully synthesized CoO@N-doped carbon nanowire arrays derived from zeolitic imidazolate frameworks-67 (ZIF-67) on Ni foam (denoted as CoO@N-C/NF). Each CoO@N-C nanowire was built up of numerous ordered in-situ nitrogen-doped carbon coated CoO nanoparticles (around 20 nm) after annealing treatment. Benefited from the unique structural features, when served as anode for lithium ion batteries, the CoO@N-C/NF exhibit superior initial Coulombic efficiency of 78.04%, and excellent electrochemical cyclability (1884.1 mAh g−1 at 1 A g−1 after 100 cycles) and good rate capability (1169.2 mAh g−1 at the rate of 5000 mA g−1). To our knowledge, this is the highest capacity with similar electric current density that has been reported for CoO-based materials. Our results indicate that the CoO@N-C/NF electrode without any auxiliary materials are expected to open up new opportunities for CoO-based material to power electronic devices.
Similar content being viewed by others
Introduction
As a major energy storage device, lithium ion batteries (LIBs) are the most prevailing power sources for high-end and portable electronic products, such as laptops and mobile phones for quite a few years1,2,3,4,5. Nevertheless, the current commercial graphite anode, hosts a low specific capacity (372 mAh g−1), far from our expectations. Therefore, there is an urgent need for extensive exploration and special design of new electrode materials in order to improve the specific capacity6,7,8. In the various anode materials, transition metal oxides usually possess high theoretical capacity in view of the conversion mechanism (MOx + 2xLi + 2xe− ↔ M + xLi2O) by transmitting a number of electrons9. Since the above discovery, CoO has been regarded as a promising substitute to graphite due to its relatively high lithium storage capacity (716 mAh g−1). However, CoO LIBs anodes suffer from a large specific volume change during discharge-charge cycles and low conductivity, which often results in performance degradation. Thus, rational modification and design are needed for CoO-based electrodes material with high reversibility and excellent rate performances.
Great endeavors have been made to optimize the electrochemical properties of CoO-based electrodes. A general strategy is to design nanostructured materials with hierarchical architecture10. Among the previous works, one-dimensional(1D) nanomaterials with hierarchical structures have been extensively developed and proved to be the optimal structure of electrochemical electrodes due to their better electron transport capacity, higher surface-to-volume ratios, and relatively lower volume change during the lithiation/delithiation processes compared with other higher dimensional nanomaterials11,12,13. However, these 1D nanomaterials usually need to be mixed with polymeric binder and carbon black, and further pressed onto current collector. The addition of conductive additive and binder inevitably sacrifices overall energy storage capacity and decrease the electrical conductivity of the electrode materials, thus hindering their potential application in high-performance energy storage devices14,15,16. Alternatively, the fabrication of integrated adhesive-free electrodes by directly growing metal oxide nanowire arrays on self-supporting three-dimensional (3D) porous conductive substrates have received tremendous attention. The integrated electrode avoids the use of any adhesive or conductive additives, provides a larger electrode/electrolyte contact area, and provides a 3D interconnected network path for electrons and ions to produce effective reaction kinetics during charge/discharge. For example, Liu et al.17 synthesized mesoporous CoO nanowires on Ni foam through a hydrothermal reaction, which shows a reversible specific capacity of 1398 mAh g−1 after 80 cycles. Jiang et al.18 reported porous CoO nanowire arrays on Ti foil, which exhibits good high-rate capability. In spite of the above reports, the electrochemical performance of CoO has been improved to a certain extent, but its intrinsic poor electrochemical conductivity and dramatic volume variation in the cycle process still greatly inhibited its long cycling stability and superior rate performance.
Nitrogen-doped carbon coated metal oxide nanowires with a unique core-shell structure have proven to be an effective strategy for solving the above problems19,20. The N-C shell can not only accommodate a large volume expansion/contraction, but also effectively increase the conductivity of the metal oxide. Many reports have certified that 1D core-shell structured nanomaterials exhibit superior performance when used as anode materials for LIBs21,22. However, the process of forming a high quality N-C shell is relatively difficult and complicated. More recently, Gan et al.23 synthesized ZnO/NC-Z nanorods adopted a strategy for ZnO nanorods in-situ encapsulated by ZIF-8. This distinctive ZnO/NC-Z nanorods electrode has excellent electrochemical properties. Nevertheless, there has been no report on in-situ coated CoO nanowire arrays with nitrogen-doped carbon derived from MOFs.
Herein, we demonstrate a simple and extensible strategy to obtain integrated and binder-free CoO@N-doped carbon nanowire arrays derived from zeolitic imidazolate framework-67 (ZIF-67) on Ni foam (denoted as CoO@N-C/NF), in which each nanowire composed of ordered CoO nanoparticles encapsulated by nitrogen-doped carbon. With such a unique electrode design, the advantages can be listed as follows. Firstly, Ni foam with three-dimensional structure was adopted as the collector instead of traditionnal two-dimensional conductive collectors, due to its outstanding mechanical stability, higher electron conductivity, better corrosion resistance, and low cost. Secondly, the CoO@N-C nanowire arrays was grown directly on Ni foam, without requiring the addition of binders and thus avoiding the loss of lithium storage capacity. Thirdly, the unique 1D porous nanostructures provides a larger surface-to-volume ratio, shorten the distance of Li+ and charge diffusion and facile strain accommodation in the process of lithiation reaction, which is conducive to enhance the lithium storage properties. Fourthly, the outer N-doped carbon can not only promote the ion/electron transport kinetics, but also decrease the energy barrier of ion migration during charge/discharge processes. Benefited from the the advantages listed above for lithium storage, the designed CoO@N-C/NF exhibited excellent lithium-storage capacity and good rate capability as anode materials for LIBs.
Results and Discussion
The synthetic strategy of the integrated CoO@N-C/NF has been displayed in Fig. 1. Initially, a piece of cleaned Ni foam preprocessed was put into the homogeneous solution of Co2+ to conduct a hydrothermal reaction and then annealing to obtain the CoO/NF24. After the annealing, the Ni foam was uniformly covered with the vertical CoO nanowire arrays with diameter of 50 nm and length of 1.5–2.5 μm (Fig. 2a,b). CoO@ZIF-67/NF were prepared by using CoO/NF as sacrificial templates in the component solvent of absolute ethyl alcohol, H2O and Hmim. During the chemical transformation, with the help of solvent, CoO nanowires can dissolve itself into Co2+ ions, while Hmim acted as the ligand and etching reagent. As seen from the images of morphologies (Fig. 2c,d), it is obvious that the uniform CoO@ZIF-67 nanowire arrays were achieved after the ion exchange process, well adhering to the Ni foam substrate.
During the morphology evolution of CoO@ZIF-67/NF, ZIF-67 subunits are gradually grown on the surface of CoO/NF (Fig. S1). It is worth mentioning that the reaction time plays a significant role on the of the thickness of the ZIF-67 shell. At the beginning of the reaction (5 min), only some small ZIF-67 particles are formed on the surface of the CoO/NF (Fig. S1a,d). With the prolongation of reaction time(1 h), more nanoparticles were stacked up to form a continuous layer (Fig. S1b,e). The thickness of the ZIF-67 shell increased to ~50 nm (Fig. S1c,f) when the reaction time was 6 h. However, the excess of time will lead to the overgrowth and severely agglomerate of polyhedral subunits (Fig. S2).
Finally, the well-aligned CoO@ZIF-67/NF were subsequently annealed under N2 gas and air in turn. SEM images (Fig. 2e,f) show that the unique shape of the CoO@N-C nanowires is similar to that of an ear of wheat-like nanostructure. Compared with the pure CoO nanowire, each nanowire of target product with a hierarchical morphology, which assembled by many nanoparticles ≈20 nm in size. The introduction of carbon layer supress the agglomeration of the CoO nanoparticles, in addition, the direct connection between CoO@N-C nanowires and the growth substrate, avoiding the binder could greatly improve transmission rate of Li ions and electrons into/out of the CoO@N-C nanowires architecture.
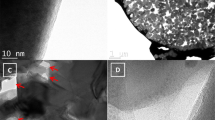
TEM results provide more details about the microstructure and morphology of CoO nanowire precursors and products. Typical TEM image (Fig. 3a) shows the single CoO nanowire with an average width of 70 nm. Obviously, there are many pore detects on the surface of CoO nanowires, due to the release and decomposition of gases(CO2, H2O) during annealing processes (Fig. 3b). Figure 3c shows the CoO@ZIF-67 nanowire with a typical core-shell configuration. According to the high magnification TEM (Fig. 3d), the shell thickness of ZIF-67 with light contrast was approximate 60 nm, while the core CoO nanowire with dark contrast was much thinner in diameter than the pure CoO nanowire without coating. In addition, it can be seen from Fig. 3d that ZIF-67 coating layer without obvious lattice fringe, indicating its low crystallinity, which is consistent with the characterization result of XRD. The TEM image in Fig. 3e shows that the original ordered 1D nanostructures of CoO@N-C remain well after annealing. However, in comparion with CoO@ZIF-67 nanowire, CoO@N-C exhibits rougher surface and nanopore composed of many nanoparticles, which may be due to gas release and further collapse of intermediate ZIF-67 product during the pyrolysis process. As shown in the high resolution TEM (HRTEM) image (Fig. 3f), the highly crystalline CoO nanoparticles and amorphous carbon substrate were produced after pyrolysis, and the CoO nanoparticles were encapsulated in several nanometers porous conductive carbon matrices, leaving massive mesopores for ion access and transportation (also see Fig. S3). Compared with other carbon coating methods, robust adhesion is generated between nitrogen-doped carbon and CoO nanoparticles, thus ensuring faster charge transfer on the electrode25. As a consequence, the nitrogen-doped carbon matrix could improve the electrical conductivity and accommodate a huge volume expansion/contraction of CoO nanoparticles, maintaining the integrity of the entire electrode. In addition, the nitrogen doped carbon produced by pyrolysis of MOFs is essentially a good conductive networks for both lithium-ion and electrons due to its interconnected nano-channels26.
The crystal structure and phase purity of the product are confirmed by the XRD pattern. As the Fig. 4a shows, all the diffraction peaks of CoO in the CoO/NF, CoO@ZIF-67/NF and CoO@N-C/NF samples could be well linked to a pure phase of CoO with a cubic structure (JCPDS No. 48–1719), apart from the peaks originating from the Ni foam (marked by*)27. The middle X-ray diffraction (XRD) pattern of the ZIF-67 is weak on account of the strong diffraction originating from the Ni foam. In order to further prove that ZIF-67 was prepared after the reaction between CoO and Hmim, the XRD pattern (Fig. S4a) of ZIF-67 was matched well with the typical crystalline structure of ZIF-67 powder synthesized according to the mature method. In addition, CoO@N-C/NF sample with an additional wide peak between 2θ = 15–28°, corresponding to the carbon diffraction peak28. The presence of carbon in CoO@N-C samples is also confirmed by the full XPS spectrum.
The complete XPS analysis in Fig. S4b validates the existence of Co, O, C, and N, and their atomic ratios are 69.51%, 23.69%, 5.42%, and 1.39%, respectively. In addition, the XPS spectrum Co 2p (Fig. 4b) exhibits two small satellite peaks located at 780.8 eV and 796.6 eV, corresponding to Co 2p3/2 and Co 2p1/2,respectively, which can prove the formation of the CoO29. As shown in Fig. 4c, the XPS spectrum of C 1 s contains four peaks. A strong peak of all at 284.8 eV that correspond to the nonoxygenated graphitic carbon, and the binding energy values of other three weaker peaks are 286.2 eV, 287.9 eV and 289.0 eV, corresponding to the oxygenated carbons, carbonyl C, and carboxylate C, respectively30. The N 1 s spectrum in Fig. 4d can be decomposed into three peaks located at 398.4, 399.8, and 400.7 eV, corresponding to pyridinic, pyrrolic and graphitic N, respectively, which are bonded to the C atoms in CoO@N-C nanoparticles31,32,33.
In this work, CoO@N-C/NF were studied as anode material in LIBs. Figure 5a shows the initial three cycles CV curves of the CoO@N-C/NF electrode in the potential range of 0.05–3.0 V at a scan rate of 0.1 mV s−1. During the first cathodic scan, one strong reduction peak between 1.0 and 0.37 V can be attributed to the reduction of Co2+ to metallic Co during the insertion of Li+, lithium ion insertion into the carbon and also formation of the solid electrolyte interface (SEI) film29. In the following cathode scanning, the cathodic peaks shift to higher voltages at 1.32 and 0.97 V, which is attributed to the reduction of CoO to metallic Co and the formation of SEI film resulting from the electrochemically driven electrolyte reduction30,34,35. In anodic scans, the peak at ≈1.4 V can be attributed to the partial decomposition of SEI film and the extraction of Li+ from carbon36,37. The anodic peak at ≈2.2 V is related to the electrochemical reaction between Li2O and Co (Li2O + Co → CoO + 2Li)37,38. In the following cycles, the good overlap of the CV curves indicates the highly reversible of the electrochemical reactions.
The core-shell CoO@N-C with a conductive carbon encapsulated CoO nanoparticles are expected to show excellent lithium storage properties which was indeed confirmed by the experimental results. Figure 5b shows the discharge/charge profiles of CoO@N-C/NF at a current density of 1 A g−1 in the 1st, 2nd, 3rd, 50th, 100th cycle. In the first discharge, the long stable voltage stage can be clearly observed at 0.75 V, which matched well with the above CV results (Fig. 5a). The first discharge and charge capacity reach up to 2015.2 and 1572.8 mAh g−1, respectively, corresponding to a initial Coulombic efficiency of 78.04%. The initial capacity loss is due to the incomplete conversion reaction and the formation of the SEI film. It should be emphasized that discharge-charge curves for the 50th and 100th almost no obvious changes are observed, indicating good cycling stability.
Figure 5c compares the cycle performance of the CoO@N-C/NF and CoO/NF electrodes at 1 A g−1. After 100 cycles, the CoO@N-C/NF electrode provides a high reversible capacity of 1884.1 mAh g−1 much higher than that (454.6 mAh g−1) of the pure CoO/NF anode and larger than the theoretical value (716 mAh g−1). The phenomenon of over-theoretical capacity is similar to the interesting results of other transition metal oxides9,39,40,41. The extra capacities could be affiliated with the reversible growth of polymeric gels originating from the electrolyte decomposition and the further lithium storage via interfacial charging at the metal/Li2O interface36,42,43. Moreover, there is a trend of the capacity of CoO@N-C/NF increase gradually, while the capacity of CoO/NF does not increase in Fig. 4c. For the CoO/NF electrode, just as most transition metal oxides, the pure CoO nanowire arrays without the nitrogen-doped carbon coating exhibit rapid capacity fading, which is due to their large volume change causing the pulverization of the electrode material during the charge/discharge process. In contrast, the CoO@N-C/NF electrode with an upward trend in capacity is attributed to the synergistic effect between CoO nanoparticles components and nitrogen-doped carbon skeleton. The nitrogen doped carbon coating plays a crucial role in improving the electronic conductivity, buffering the volume change, and enhancing the stability of the electrode. At the same time, the reversible growth of a polymeric gel-like film resulting from the kinetically activated electrolyte degradation have been suggested as one of the possible reasons for the capacity increase. Of course, in addition to the positive factors causing the capacity rise, the large volume change during the charge/discharge process results in the pulverization of the electrode and the capacity decreases correspondingly. These two factors competitively affect the trend of capacity retention. Similar phenomena have also been observed in other material systems44,45,46. For many energy storage devices, rate capability is one of the critical factor in practical application. Figure 5d futher shows the rate capabilities performance of CoO@N-C/NF electrode for current rates from 500 to 5000 mA g−1 for each 10 cycles, corresponding to the discharge of 1369.2, 1315.2, 1290, and 1169.2 mAh g−1, respectively. What is more important, when the rate is back to 0.5 A g−1, the reversible specific capacity of the samples recover to their initial values, implying a very stable cycling performance. In addition, when the current density is different, the charge-discharge curve does not have a large voltage hysteresis change. Their similarity means that only limited polarization exists in this system, even in the case of large current densities, thus indicating an excellent rate capability (Fig. S5)24. To our knowledge, this is the highest capacity with similar electric current density that has been reported for CoO-based materials, and the comparative results are listed in Supporting Information, Table S1.
The improvement of CoO@N-C/NF electrochemical performance might be ascribed to their unique structural characteristics as the following aspects. Firstly, the vertical core-shell architecture nanowires array was directly attached to the Ni foam, avoiding the addition of conductive carbon and binders, and greatly reducing the “dead volume” in the electrode, which will facilitate charge transport. Secondly, the continuous, complete and uniform nitrogen-doped carbon skeleton can effectively withstand the volume change in the process of alloying-dealloying, thus alleviating the pulverization problem to a certain extent and improving the cycling stability. To prove this, we compared the SEM of the CoO/NF and CoO@N-C/NF electrodes after 100 cycles of charge and discharge (see Fig. S6 in the Supporting Information). As expected, the nanoarrays morphology of CoO@N-C/NF electrode was basically retained after 100 cycles. In contrast, the pulverization of the CoO/NF electrode was observed due to the aforementioned volume excursion effects. Finally, there are many competitive advantages to the 1D robust nanowires arrays, such as more active sites, short ion diffusion distance, superior electron collection efficiency, and even attractive synergetic properties.
In order to further understand the reason why CoO@N-C/NF shows the best capacity than pure CoO/NF, the electrochemical impedance spectroscopy (EIS) measurements were carried out within the frequency range of 0.01–100 kHz. Figure 6 displays the impedance plots together with the equivalent circuit model of CoO@N-C/NF and pure CoO/NF electrodes after the 3th cycle at 1 A g−1. The Nyquist plots consists of one depressed semicircle in the high frequency and a sloped line at low frequency. In general, the semicircle is related to the internal resistance (Re) of the battery, the resistance (Rf) and constant phase element (CPEf) of SEI film, the charge transfer resistance (Rct) and constant phase element (CPEct) of the electrode/electrolyte interface. The sloped line indicates that the Warburg impedance (Zw) is attributed to the diffusion of Li+. The SEI film resistance Rf and charge-transfer resistance Rct of the CoO@N-C/NF electrode are 5.2 Ω and 9.8 Ω, which are much less than the corresponding value of the pristine CoO/NF electrode (7.4 Ω and 17.43 Ω). It is obvious that the special N-C matrix structure can significantly enhance the electronic conductivity of the materials surface, which is beneficial to the rapid transport of electrons within the hollow CoO nanoparticles during electrochemical lithium insertion/extraction process, thus greatly improving to the electrochemical performance of CoO@N-C/NF electrode.
Conclusions
In summary, we have successively synthesized the unique hierarchical CoO@N-C nanowires on 3D conductive Ni foam substrates based on a facile and extensible in-situ ion exchange process. Excitingly, CoO@N-C/NF electrode demonstrates excellent electrochemical performance compared to the precursor of CoO/NF, benefiting to the sufficient conductivity, unique hierarchical microstructure and good structure stability of the nanowires arrays. CoO@N-C/NF electrode exhibits a high reversible capacity of 1884.1 mAh g−1 at 1 A g−1 after 100 cycles and outstanding rate capability. Our results indicate that the CoO@N-C/NF electrodes without any auxiliary materials are expected to open up new opportunities for CoO-based material to power electronic devices.
Methods
Preparation of CoO/NF
Synthesis of CoO nanowire arrays on Ni foam (CoO/NF). All the chemicals are directly used after purchase without further purification. The hierarchical CoO nanowire arrays was synthesized through a modified two step of hydrothermal and annealing47. Typically, 2 mmol of Co(NO3)2.6H2O, 10 mmol of CO(NH2)2, and 8 mmol of NH4F were dissolved in 36 mL of deionized water under stirring. The resultant solution was added to a 100 mL Teflon-lined autoclave immersing a piece of cleaned Ni foam. The synthesis temperature was maintained at 120 °C for 10 h and after the equipment cooled down to room temperature naturally. The Ni foam with the precursor was taken out and rinsed with deionized water for several times. The CoO/NF is finally obtained by annealing the above precusor at 450 °C in Ar gas for 4 h at a ramping rate of 2 °C/min.
Preparation of CoO@ZIF-67/NF
In detail, 40 mmol of 2-methylimidazole (Hmim) was added into a beaker containing 10 mL each of deionized water and ethanol. Then, the rinsed intermediate of CoO/NF was immersed into the solution above and put it into the shaker to maintain a speed of 190 revolutions and maintain it at room temperature for 9 hours. The purple resultant product was fetched out and rinsed by ethanol for three times and then dried at 80 °C for 12 h to obtain CoO@ZIF-67/NF. For further investigate the formation process of CoO@ZIF-67/NF, the resultant products were fetched out at the reaction times of 5 min, 1 h, 6 h, and 12 h.
Preparation of CoO@N-C/NF
The obtained CoO@ZIF-67/NF was annealed at 550 °C for 2 h in argon atmosphere, and then annealed in air at 300 °C for 1 h with a heating rate of 2 °C/min to obtain the CoO@N-C/NF nanocomposite. The mass loading of CoO@N-C was around 1.34 mg cm−2, as calculated using the following formula48:
where m is the weight of CoO@N-C; m1 is the weight of fresh Ni foam and m2 is the weight of Ni foam covered with active material. SNi is the surface area of the electrode that was used in the coin cell and S is the total surface area of Ni foam covered with the active material.
Characterization techniques
The crystal structures and morphologies of the samples was characterized by X-ray diffractometer (XRD, Bruker AXS D8), scanning electron microscope (SEM, JEOL JSM-6490LV) and transmission electron microscope (TEM, FEI Tecnai G2). Surface analysis of the samples was examined using X-ray photoelectron spectroscopic (XPS, PHI1600 ESCA).
Electrochemical measurements
The electrochemical characterization were carried out using CR-2032 coin cells. The fabricated CoO@N-C/NF was used as working electrode and lithium metal as the counter electrode. The separator membrane was Celgard 2320. A electrolyte solution of 1 M LiPF6 consists of EC, DMC and EMC (v/v/v = 1: 1: 1). Galvanostatic charge–discharge was carried out on a LAND battery tester (LAND CT 2001A, China) in the fixed voltage window of 0.05–3.0 V. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were measured on a CHI604 C electrochemical workstation. The impedance spectra were carried out in the frequency range of 100 kHz to 0.01 Hz.
References
Bresser, D., Passerini, S. & Scrosati, B. Leveraging valuable synergies by combining alloying and conversion for lithium-ion anodes. Energy & Environmental Science 9, 3348–3367 (2016).
Scrosati, B. & Garche, J. Lithium batteries: Status, prospects and future. Journal of Power Sources 195, 2419–2430 (2010).
Zhao, Q., Wang, J., Chen, C., Ma, T. & Chen, J. Nanostructured organic electrode materials grown on graphene with covalent-bond interaction for high-rate and ultra-long-life lithium-ion batteries. Nano Research 10, 4245–4255, https://doi.org/10.1007/s12274-017-1580-9 (2017).
Deng, B., Lei, T., Zhu, W., Xiao, L. & Liu, J. In-Plane Assembled Orthorhombic Nb2O5 Nanorod Films with High-Rate Li+ Intercalation for High-Performance Flexible Li-Ion Capacitors. Advanced Functional Materials 28, 1704330, https://doi.org/10.1002/adfm.201704330 (2017).
Zheng, Z. et al. Robust erythrocyte-like Fe2O3@carbon with yolk-shell structures as high-performance anode for lithium ion batteries. Chemical Engineering Journal 347, 563–573, https://doi.org/10.1016/j.cej.2018.04.119 (2018).
Su, L., Hei, J., Wu, X., Wang, L. & Zhou, Z. Ultrathin Layered Hydroxide Cobalt Acetate Nanoplates Face‐to‐Face Anchored to Graphene Nanosheets for High‐Efficiency Lithium Storage. Advanced Functional Materials 27, 1605544, https://doi.org/10.1002/adfm.201605544 (2017).
Ji, J. et al. In Situ Activation of Nitrogen-Doped Graphene Anchored on Graphite Foam for a High-Capacity Anode. ACS Nano 9, 8609–8616, https://doi.org/10.1021/acsnano.5b03888 (2015).
Ji, H. et al. Ultrathin Graphite Foam: A Three-Dimensional Conductive Network for Battery Electrodes. Nano Letters 12, 2446–2451, https://doi.org/10.1021/nl300528p (2012).
Guan, H. et al. CoO octahedral nanocages for high-performance lithium ion batteries. Chemical Communications 48, 4878–4880 (2012).
Zhou, L. et al. Recent Developments on and Prospects for Electrode Materials with Hierarchical Structures for Lithium-Ion Batteries. Advanced Energy. Materials 8, 1701415, https://doi.org/10.1002/aenm.201701415 (2017).
Chan, C. K., Zhang, X. F. & Cui, Y. High Capacity Li Ion Battery Anodes Using Ge Nanowires. Nano Letters 8, 307–309, https://doi.org/10.1021/nl0727157 (2008).
Cui, L.-F., Ruffo, R., Chan, C. K., Peng, H. & Cui, Y. Crystalline-Amorphous Core−Shell Silicon Nanowires for High Capacity and High Current Battery Electrodes. Nano Letters 9, 491–495, https://doi.org/10.1021/nl8036323 (2009).
Taberna, P. L., Mitra, S., Poizot, P., Simon, P. & Tarascon, J. M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nature Materials 5, 567, https://doi.org/10.1038/nmat1672 (2006).
Zhang, S. et al. Nickel Nanocone-Array Supported Silicon Anode for High-Performance Lithium-Ion Batteries. Advanced Materials 22, 5378–5382, https://doi.org/10.1002/adma.201003017 (2010).
Miao, Z. et al. ZnO dense nanowire array on a film structure in a single crystal domain texture for optical and photoelectrochemical applications. Nanotechnology 23, 495602 (2012).
Chen, H. et al. Improved lithium ion battery performance by mesoporous Co3O4 nanosheets grown on self-standing NiSix nanowires on nickel foam. Journal of Materials Chemistry A 2, 8483–8490, https://doi.org/10.1039/C4TA00967C (2014).
Liu, M. et al. Solvent-controlled synthesis of mesoporous CoO with different morphologies as binder-free anodes for lithium-ion batteries. Ceramics International 44, 19631–19638, https://doi.org/10.1016/j.ceramint.2018.07.213 (2018).
Jiang, J. et al. Direct Synthesis of CoO Porous Nanowire Arrays on Ti Substrate and Their Application as Lithium-Ion Battery Electrodes. The Journal of Physical Chemistry C 114, 929–932, https://doi.org/10.1021/jp909785g (2010).
Sun, B., Chen, Z., Kim, H.-S., Ahn, H. & Wang, G. MnO/C core–shell nanorods as high capacity anode materials for lithium-ion batteries. Journal of Power Sources 196, 3346–3349, https://doi.org/10.1016/j.jpowsour.2010.11.090 (2011).
Kang, D. et al. Crosslinking-derived MnO/carbon hybrid with ultrasmall nanoparticles for increasing lithium storage capacity during cycling. Carbon 99, 138–147, https://doi.org/10.1016/j.carbon.2015.11.068 (2016).
Gu, X. et al. Hierarchical core–shell α-Fe2O3@C nanotubes as a high-rate and long-life anode for advanced lithium ion batteries. Journal of Materials Chemistry A 2, 3439–3444, https://doi.org/10.1039/C3TA14649A (2014).
Gao, L., Qu, F. & Wu, X. Hierarchical WO3@SnO2 core–shell nanowire arrays on carbon cloth: a new class of anode for high-performance lithium-ion batteries. Journal of Materials Chemistry A 2, 7367–7372, https://doi.org/10.1039/C4TA00206G (2014).
Gan, Q., Zhao, K., Liu, S. & He, Z. Solvent-free synthesis of N-doped carbon coated ZnO nanorods composite anode via a ZnO support-induced ZIF-8 in-situ growth strategy. Electrochimica Acta 250, 292–301, https://doi.org/10.1016/j.electacta.2017.08.075 (2017).
Cao, K. et al. Ultra-High Capacity Lithium-Ion Batteries with Hierarchical CoO Nanowire Clusters as Binder Free Electrodes. Advanced Functional Materials 25, 1082–1089, https://doi.org/10.1002/adfm.201403111 (2015).
Han, Y. et al. A novel anode material derived from organic-coated ZIF-8 nanocomposites with high performance in lithium ion batteries. Chemical Communications 50, 8057–8060, https://doi.org/10.1039/C4CC02691H (2014).
Zhang, G. et al. Tin quantum dots embedded in nitrogen-doped carbon nanofibers as excellent anode for lithium-ion batteries. Nano Energy 9, 61–70, https://doi.org/10.1016/j.nanoen.2014.06.030 (2014).
Chen, H. et al. Mesoporous ZnCo2O4 microspheres composed of ultrathin nanosheets cross-linked with metallic NiSix nanowires on Ni foam as anodes for lithium ion batteries. Nano Energy 10, 245–258 (2014).
Sun, Y., Huang, F., Li, S., Shen, Y. & Xie, A. Novel porous starfish-like Co3O4@nitrogen-doped carbon as an advanced anode for lithium-ion batteries. Nano. Research 10, 3457–3467 (2017).
Wang, F., Zhuo, H. Y., Han, X. G., Chen, W. & Sun, D. Foam-like CoO@N,S-codoped carbon composition derived from a well-designed N,S-rich Co-MOF for lithium-ion battery. Journal of Materials Chemistry A 5 (2017).
Sun, Y., Hu, X., Luo, W. & Huang, Y. Ultrathin CoO/Graphene Hybrid Nanosheets: A Highly Stable Anode Material for Lithium-Ion Batteries. Journal of Physical Chemistry C 116, 20794–20799 (2012).
Chen, L. F. et al. Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. Acs Nano 6, 7092–7102 (2012).
Zheng, F., Yang, Y. & Chen, Q. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework. Nature Communications 5, 5261 (2014).
Tang, X. et al. Carbon-coated cobalt oxide porous spheres with improved kinetics and good structural stability for long-life lithium-ion batteries. Journal of Colloid & Interface Science 510, 368–375 (2017).
Li, F., Zou, Q. Q. & Xia, Y. Y. CoO-loaded graphitable carbon hollow spheres as anode materials for lithium-ion battery. Journal of Power Sources 177, 546–552 (2008).
Mei, Z., Jia, M., Jin, Y. & Shi, X. Synthesis and electrochemical performance of CoO/graphene nanocomposite as anode for lithium ion batteries. Applied Surface Science 263, 573–578 (2012).
Liu, J. et al. Multifunctional CoO@C metasequoia arrays for enhanced lithium storage. Nano Energy 7, 52–62, https://doi.org/10.1016/j.nanoen.2014.04.013 (2014).
Wang, S. et al. Nanoparticle Cookies Derived from Metal-Organic Frameworks: Controlled Synthesis and Application in Anode Materials for Lithium-Ion Batteries. Small 12, 2365–2375, https://doi.org/10.1002/smll.201600106 (2016).
Xie, K. et al. Nitrogen-Doped Carbon-Wrapped Porous Single-Crystalline CoO Nanocubes for High-Performance Lithium Storage. Acs Appl Mater Interfaces 6, 10602–10607 (2014).
Xiong, S., Chen, J. S., Lou, X. W. & Zeng, H. C. Mesoporous Co3O4 and CoO@C Topotactically Transformed from Chrysanthemum-like Co(CO3)0.5(OH)·0.11H2O and Their Lithium-Storage Properties. Advanced Functional Materials 22, 861–871, https://doi.org/10.1002/adfm.201102192 (2011).
Wang, B. et al. Porous Co3O4 nanowires derived from long Co(CO3)0.5(OH)·0.11H2O nanowires with improved supercapacitive properties. Nanoscale 4, 2145–2149, https://doi.org/10.1039/C2NR11897A (2012).
Schiavi, P. G. et al. A versatile electrochemical method to synthesize Co-CoO core-shell nanowires anodes for lithium ion batteries with superior stability and rate capability. Electrochimica Acta 290, 347–355, https://doi.org/10.1016/j.electacta.2018.09.046 (2018).
Zhao, R., Li, Q., Wang, C. & Yin, L. Highly ordered mesoporous spinel ZnCo2O4 as a high-performance anode material for lithium-ion batteries. Electrochimica Acta 197, 58–67, https://doi.org/10.1016/j.electacta.2016.03.047 (2016).
Liu, X., Zhao, C., Zhang, H. & Shen, Q. Facile Synthesis of Porous ZnMnO3 Spherulites with a High Lithium Storage Capability. Electrochimica Acta 151, 56–62, https://doi.org/10.1016/j.electacta.2014.11.020 (2015).
Cheng, J. et al. CNT@Fe3O4@C Coaxial Nanocables: One-Pot, Additive-Free Synthesis and Remarkable Lithium Storage Behavior. Chemistry – A European Journal 19, 9866–9874, https://doi.org/10.1002/chem.201300037 (2013).
Guan, X. et al. CoO Hollow Cube/Reduced Graphene Oxide Composites with Enhanced Lithium Storage Capability. Chemistry of Materials 26, 5958–5964, https://doi.org/10.1021/cm502690u (2014).
Wu, Z.-S. et al. Graphene Anchored with Co3O4 Nanoparticles as Anode of Lithium Ion Batteries with Enhanced Reversible Capacity and Cyclic Performance. ACS Nano 4, 3187–3194, https://doi.org/10.1021/nn100740x (2010).
Cai, G., Zhang, W., Jiao, L., Yu, S.-H. & Jiang, H.-L. Template-Directed Growth of Well-Aligned MOF Arrays and Derived Self-Supporting Electrodes for Water Splitting. Chem 2, 791–802, https://doi.org/10.1016/j.chempr.2017.04.016 (2017).
Ma, Y. et al. MOF-Derived Vertically Aligned Mesoporous Co3O4 Nanowires for Ultrahigh Capacity Lithium-Ion Batteries Anodes. Advanced Materials Interfaces 5, 1800222, https://doi.org/10.1002/admi.201800222 (2018).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U1303291, 21865025).
Author information
Authors and Affiliations
Contributions
G.W. and D.W. designed the research, analysed data and wrote the paper. B.Y., Y.G., L.C. and F.Y. also contributed extensively to the work presented in this paper. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41598_2019_42371_MOESM1_ESM.docx
N-doped Carbon Coated CoO Nanowire Arrays Derived from Zeolitic Imidazolate Framework-67 as Binder-free Anodes for High-performance Lithium Storage
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, D., Yan, B., Guo, Y. et al. N-doped Carbon Coated CoO Nanowire Arrays Derived from Zeolitic Imidazolate Framework-67 as Binder-free Anodes for High-performance Lithium Storage. Sci Rep 9, 5934 (2019). https://doi.org/10.1038/s41598-019-42371-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42371-y
This article is cited by
-
Photoelectrochemical technology for solar fuel generation, from single photoelectrodes to unassisted cells: a review
Environmental Chemistry Letters (2022)
-
Templated interfacial synthesis of metal-organic framework (MOF) nano- and micro-structures with precisely controlled shapes and sizes
Communications Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.