Abstract
Mangrove forests are widespread along the subtropical and tropical coasts. They provide a habitat for a wide variety of plants, animals and microorganisms, and act as a buffer zone between the ocean and land. Along with other coastal environments, mangrove ecosystems are under increasing pressure from human activities, such as excessive input of nutrients and toxic pollutants. Despite efforts to understand the diversity of microbes in mangrove sediments, their metabolic capability in pristine and contaminated mangrove sediments remains largely unknown. By using metagenomic approach, we investigated the metabolic capacity of microorganisms in contaminated (CMS) and pristine (PMS) mangrove sediments at subtropical and tropical coastal sites. When comparing the CMS with PMS, we found that the former had a reduced diazotroph abundance and nitrogen fixing capability, but an enhanced metabolism that is related to the generation of microbial greenhouse gases via increased methanogenesis and sulfate reduction. In addition, a high concentration of heavy metals (mainly Zn, Cd, and Pb) and abundance of metal/antibiotic resistance encoding genes were found in CMS. Together, these data provide evidence that contamination in mangrove sediment can markedly change microbial community and metabolism; however, no significant differences in gene distribution were found between the subtropical and tropical mangrove sediments. In summary, contamination in mangrove sediments might weaken the microbial metabolisms that enable the mangrove ecosystems to act as a buffer zone for terrestrial nutrients deposition, and induce bioremediation processes accompanied with an increase in greenhouse gas emission.
Similar content being viewed by others
Introduction
Mangrove ecosystems are widely distributed in the transition between land and sea, particularly along the estuaries and coastlines in subtropical and tropical regions. The microorganisms that inhabit mangrove sediments play a critical role in the biogeochemical cycling of carbon, nitrogen, phosphorus, as well as the deposition of heavy metals from adjacent land and fluvial imports, through metabolic processes1,2. In recent years, the increasing level of contamination in mangrove sediments has started to pose a serious threat to mangrove forests, with plants disappearing at a rate estimated to be ~1–2% globally per year3. Although mangroves act as a natural sewage treatment plant, exhibiting a robust ecosystem restoration capacity4,5, the ever increasing levels of contaminants are now overloading mangrove sediments, and this influences their restoration and nutrient cycling capacity by affecting the sediment-inhabiting microorganisms6,7,8,9.
The effects of anthropogenic nutrients and pollutants on the microflora in mangrove sediments have been widely studied10,11,12. It has been demonstrated that contamination of the mangrove sediment can significantly promote the diversity of the microbial community as a whole, but at the same time it has a negative impact on some specific groups of microorganisms13,14. For example, acetoclastic/methylotrophic methanogens exhibited a decreased level of diversity in a contaminated mangrove in Singapore6. In addition, metals are retained in mangrove sediments rather than moving to adjacent environments, but this also markedly influences the autochthonic microbial community15. For example, both metals and metalloids have been shown to contribute to the abundance and distribution of ammonia-oxidizing archaea and beta-proteobacteria, both of which are crucial to the nitrogen cycle processes in mangrove sediments1,16,17. The fact that polycyclic aromatic hydrocarbon- (PAH-) and phenanthrene polluted mangrove sediments can also increase growth of specific microbial degraders, which illustrates the strong adaptation and bioremediation ability of mangrove sediment-residing microorganisms18,19,20,21. “To further confirm the distribution of microbial genes in mangrove sediment, researchers had applied metagenomic approach in many different regions recently. For instance, Andreote et al. had investigated the microbial participants in sulfur and nitrogen cycle in Brazilian mangrove sediment22; Imchen et al. studied the variation of microbial taxon and metabolic genes across diverse mangrove sediment system23; the prevalent distribution of heavy metals and antibiotic resistome in mangrove sediments at India, Brazil and Saudi Arabia was also reported24. However, the lack of a fundamental level of understanding of the microorganism-related metabolic capability in pristine and contaminated mangrove sediments hinders our determination of the carrying capacity of the ecosystem, as well as the capability of the microbiome in responding to a further increase in pollution in mangrove sediments.
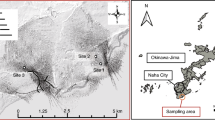
Mangroves located in the northern and southern parts of Hainan Island (China) are representative of subtropical and tropical habitats, respectively25,26. Located in the northern part of Hainan Island, Dongzhai harbor in Haikou city has one of the largest natural mangrove forests in China and it is the first to have been made into a nature reserve; however, the local aquaculture industry has resulted in severe eutrophication in the offshore part of reserve, while the inshore part is relatively pristine. In contrast, the mangroves in Sanya city, located at the southern tip of Hainan Island, have been partially contaminated by both pre-treated and untreated domestic sewage as well as by industrial wastewater in inshore part, while the offshore part is relatively pristine. Thus, the mangroves in Dongzhai and Sanya are ideal sites for us to investigate the metabolic capacity of microorganisms in both contaminated and uncontaminated mangrove sediments under different climatic conditions. By applying metagenomic approach, researchers had already investigated
In this study, we explored the community structure and metabolism capacity of the microorganisms in the heavily polluted and relatively pristine mangrove sediments in both tropical Sanya and subtropical Haikou using a metagenomic approach.
Results
Environmental conditions and methane flux
As shown in supplementary Table S1, the concentration of total phosphorus (TP) and total organic carbon (TOC) were higher in subtropical mangrove sediment than in the tropical sediments. On the other hand, the tropical mangrove sediment had a higher pH and a higher concentration of ammonia than the subtropical mangrove sediment. In both the subtropical and tropical mangroves, the contaminated sediments exhibited higher concentrations of nitrogen source (Supplementary Table S1).
With regards to the amounts of metals in the sediment, the concentration of Zn was higher in the CMS than in the PMS in both subtropical (i.e., with 3577.11 mg/kg in contaminated mangrove sediment of Haikou (HKC) and 2687.74 mg/kg in pristine mangrove sediment of Haikou (HKP)) and tropical (i.e., with 371.14 mg/kg in contaminated mangrove sediment of Sanya (SYC) and 205.53 mg/kg in pristine mangrove sediment of Sanya (SYP)) samples. In addition, the concentration of Zn in the subtropical sites was an order magnitude higher than that in the tropical sites. Similarly, the amounts of Cd (0.37 mg/kg in HKC, 0.27 mg/kg in HKP, 0.16 mg/kg in SYC, and 0.11 mg/kg in SYP) and Pb (18.57 mg/kg in HKC, 16.11 mg/kg in HKP, 22.94 mg/kg in SYC, and 7.26 mg/kg in SYP) were also all higher in the CMS than in the PMS (Table S1). In addition, the methane flux across the mangrove sediment-atmosphere interface was higher in the CSM (0.286~0.578 mg·m−2 h−1) than in the PMS (−0.143~−0.017 mg·m−2 h−1), and a higher methane flux was measured in the subtropical mangrove sediment than in the tropical sites (Table S1).
Microbial community composition
After assembly of the quality controlled short reads, a total of 810,027 contigs (>400 bp) were obtained, ranging from 172,238 to 231,903 contigs in the four samples (Supplementary Table S2). The detail information of sequence reads before and after QC, and annotation was summarized into Table S3. As the average coverage of assembled contigs in each sample was >18, this suggests that the sequencing well covered the genomic information in samples. The coverage number of all identified functional genes and SSU rRNAs were normalized to the sequencing size of each sample to achieve comparison across samples.
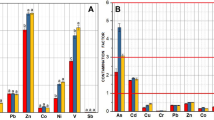
Information of microbial community was identified by screening SSU rRNAs from the assembled contigs in each sample. Twenty prokaryotic phyla and major subclasses within Proteobacteria and Archaea were found in the samples (Fig. 1). In general, three subclasses of Proteobacteria (i.e., Deltaproteobacteria, Alphaproteobacteria and Gammaproteobacteria), were widely distributed among the samples. The most abundant, i.e., Gammaproteobacteria, accounted for more than 25% of all the phyla across the four samples, but they showed a marked decrease in the CMS (Fig. 1A,C). The highest and lowest relative abundance of Deltaproteobacteria was found in the HKC (14.4%) and SYP (12.3%). In addition, the relative abundance of Alphaproteobacteria ranged from 4.5% to 8.3% across all the samples, with the highest and lowest values found in the SYC and HKP, respectively, and this class was markedly enriched in the tropical mangrove sediment with a log10 odds ratio of −0.1 (Fig. 1B). Microbial taxa that were significantly enriched (i.e., with a log10 odds ratio >0.1 or <−0.1) were also found in the samples. For example, Chloroflexi, Euryarchaeota, Deltaproteobacteria and Crenarchaeota were highly enriched in the subtropical samples; whereas Epsilonproteobacteria, Alphaproteobacteria, and Thaumarchaeota were more abundant in the tropical samples (Fig. 1B). Furthermore, Epsilonproteobacteria, Chloroflexi, and Euryarchaeota were enriched in the CMS; whereas Crenarchaeota were more abundant in the PMS (Fig. 1C).
(A) The composition of the microbial communities in the various sampling sites at the phylum level, (B) and a differential analysis between the tropical and subtropical regions; (C) and between the pristine (PMS) and contaminated (CMS) regions. For the differential analysis, values are the base-10 logarithm of the odds ratio. The positive and negative values indicate taxa that are more in (B) and (C), respectively.
At the family level, the microbial taxa also showed distinct distribution patterns in the different regions (i.e., subtropical vs tropical (Fig. 2B)) and at the different levels of contamination (CMS vs PMS (Fig. 2C)). For example, Acetobacteraceae, Bradyrhizobiaceae, Sphingobacteriaceae, Lanchnospiraceae, and Chitinophahaceae were significantly enriched (i.e., with an odds ratio <−0.6) in the tropical mangrove sediment, whereas Peptococcaceae, Halomonadaceae, Desulfurococcaceae, Methanobacteriaceae, Methanococcaceae, Methanomicrobiaceae were all more abundant (with an odds ratio >0.6) in the subtropical mangrove sediment (Fig. 2B). A comparison of the microbial taxa found in the contaminated and pristine mangrove sediments revealed that Bradyrhizobiaceae, Erythrobacteraceae, Sphingomonadaceae, Rhodocyclaceae, Cystobacteraceae, Enterobacteriaceae, Xanthomonadaceae, Methanosarcinaceae, and Methanobacteriaceae were all enriched (with an odds ratio >0.4) in the CMS, while Desulfohalobiaceae, Oceanospirillaceae, and Puniceicoccaceae were more abundant (with odds ratios of −0.42, −0.58 and −0.57, respectively) in the PMS (Fig. 2C).
(A) The composition of the microbial community at the family level, (B) and a differential analysis between the tropical and subtropical regions; (C) and between the pristine (PMS) and contaminated (CMS) regions. For the differential analysis, values are the base-10 logarithm of the odds ratio. The positive and negative values indicate taxa that are more in (B) and (C), respectively.
Nutrient metabolism and metal resistance encoding genes
The nutrient metabolism-related genes were distributed differently among the various samples. A higher abundance of the nitrogen fixation gene (nifH) was found in the subtropical region (HKC and HKP), whereas key genes in ammonia oxidation (amoA), assimilatory nitrate reduction (nirA), dissimilatory nitrate reduction (nirD), and denitrification (nosZ) were more abundant in the tropical region (SYC and SYP) (Fig. 3A). In addition, nifH, amoA, and the dissimilatory nitrate reduction genes (nirB and nirD) exhibited a higher abundance in the pristine samples; whereas nirA and nosZ were more abundant in the contaminated samples (Fig. 3A,B). The organophosphate solubilizing genes were more abundant in the PMS (HKP and SYP), whereas genes encoding the zinc-lead efflux system, cobalt-zinc-cadmium resistance, and dissimilatory sulfate reduction were more abundant in the CMS (HKC and SYC) (Fig. 4). In addition, although our results indicated that there was no difference in the distribution of carbon fixation genes in across the samples, the preferred carbon fixation pathway (i.e., Carbon fixation in reductive tricarboxylic acid cycle) was still being found (Fig. 4).
(A) Pie charts and schematic to show the contribution of the various samples (HKC, HKP, SYP and SYC) to different aspects of nitrogen metabolism. The different colored arrows represent different parts of the metabolic pathway (as shown in the key in the upper right), whereas the black dashed arrows represent the pathways and genes that were omitted. The pie charts represent the relative abundance of key nitrogen metabolism-related genes, which was calculated by dividing the sum of a gene’s coverage in one sample by the sum of the gene’s coverage in all the samples. (B) The absolute abundance of each gene was double square rooted. (C) The microbial taxa of the genes and its relative abundance in each sample.
To distinguish between the affiliated microbial taxa of each nitrogen-related metabolism gene, the microbial taxa were extracted using the MEGAN software, and the percentage they accounted in each gene was plotted (Fig. 3C). A higher abundance of Gammaproteobacteria (affiliated with nirD and nosZ), and Alphaproteobacteria (affiliated with nirB and nirD) were found in the subtropical and tropical sediments, respectively. In addition, we also found that within the nosZ-affiliated microbial taxa, Pseudomonadales, which is a typical heterotrophic denitrifying bacteria, was abundant in the CMS (Fig. S1). However, diazotrophs were more diverse in the PMS than in the CMS; for example, Spirochaetia, Methanomicrobia, and Dehalococcoidia all uniquely inhabited the PMS (Fig. 3C). The most prevalent diazotroph, Deltaproteobacteria, contributed >25% of all of the nifH genes in each sample; this was followed by Gammaproteobacteria, which exhibited a much higher relative abundance of nifH in the contaminated samples than in the uncontaminated samples, with relative abundances ranging from 24.2% to 25.3% in the CMS. The nirA affiliated microbial taxa showed a higher level of diversity in the CMS than the PMS. However, the amoA affiliated taxa exhibited a lower diversity of microbial affiliation across all the samples. For example, unknown Crenarchaeota were the only contributor of amoA gene in the subtropical samples, and it was the major contributor in the tropical samples. In addition, Betaproteobacteria contributed 15.4% and 18.2% in the SYC and SYP, respectively, whereas a small proportion of amoA gene in the SYP was affiliated with Thaumarchaeota.
Methane metabolism encoding genes
The abundance of essential microbial methane generation and utilization genes was investigated (Fig. 5). A comparison of the methanogenic bacteria in the CMS and PMS showed that key genes in heterotrophic methanogens, such as those generating methane with acetate (acetyl-CoA decarbonylase/synthase; cdhC) or methanol (methyltransferase; mtaA), were more abundant in the CMS (HKC and SYC). In contrast, formylmethanofuran dehydrogenase (fwdA), which is a key gene in autotrophic methanogens, was more abundant in the PMS (HKP and SYP). Essential genes within the metabolic pathway of methanotrophic bacteria (for example pmoA and mdh), were more abundant in the CMS (HKC and SYC). It was also noticeable that within the methanotrophic pathway, the gene functioning to generate carbon dioxide was more abundant in the CMS.
(A) Pie charts and schematic to show the contribution of the various samples (HKC, HKP, SYP and SYC) to different aspects of methane metabolism. The pie charts show the relative abundance of key methane metabolism related genes, which was calculated by dividing the sum of one gene’s coverage in an individual sample by the sum of the same gene’s coverage in all the samples. The dashed lines indicate where other genes involved in the pathway were omitted. (B) The absolute abundance of each gene was double square rooted.
Discussion
Effect of contamination on the microbial community
Our results showed that contamination of mangrove sediment did shape the microbial community. It is known that diazotrophs are frequently influenced by the level of nutrients, the type of organic and inorganic compounds, and the presence of metals27,28,29. This might therefore explain why less abundant diazotrophs, such as Rhodospirillacene within the Alphaproteobacteria class30, and Clostridiaceae within the Firmicutes phylum31 (Fig. 2), were both found in the CMS when comparing with that in PMS. Although the usual deficit of oxygen together with an abundant amount of organic matter in mangrove sediments naturally selects for a number of anaerobic organisms, including methanogens, sulphate-reducing bacteria (SRB) and denitrifiers32, the marked enrichment of methanogens (Methanomicrobiaceae, Methanosarcinaceae, Methanococcaceae, and Methanobacteriaceae) and SRB bacteria (Desulfovibrionaceae and Desulfobacteraceae) in the CMS, reflects the impact of contamination on the microbial communities (Fig. 2). As methanogenic or sulfate-reducing conditions stimulate the degradation of organic contamination33,34,35, the enriched levels of methanogens and SRB in the CMS might be an effect of bioremediation. As mentioned above, the different contaminants in the CMS not only affects the distribution of diazotrophs, but also stimulates bioremediation-related microbial taxa. In previous study, researchers had found significant differences in microbial community and functions between ocean and terrestrial mangrove sediment23. Our study further identified the difference of microbial taxa in the sediments of the subtropical and tropical mangrove sediments, and the similar gene enrichment response to anthropogenic inputs of nutrients and pollutants.
Nitrogen cycling within mangrove sediments
One of the main pollutants was inorganic nitrogen (Supplementary Table S1); thus, in contaminated mangrove sediments, the nitrogen source is more available for microbe to be assimilated into biomass when comparing with that in pristine mangrove sediment. The high abundance of denitrification and nitrogen assimilation encoding genes, in the CMS, together with the low abundance of nitrogen-fixing genes in the CMS (Fig. 3) suggests that nitrogen is not limited in the contaminated ecosystem, and thus nitrogen acquisition through nitrogen fixation is not critical. Similarly, an enrichment of denitrification encoding genes was also found in Brazilian contaminated mangrove sediments, which further supports the contamination could induce the microbial denitrification process22. The more abundant nitrogen fixation encoding genes in the pristine mangroves indicates that the microbes are struggling to achieve the same result, to assimilate nitrogen source into biomass, in the pristine sediments but more rely on atmospheric N2. This also indicates a faster resource recycle metabolism, since the energy for N2 fixation is mainly derived from the decomposition of dead leaves and roots in mangrove sediments36,37. The massive input of nitrate/nitrite and the high levels of heavy metals in the CMS might contribute to the observed decrease in the ammonia oxidation encoding genes, as these types of contamination are known to have a negative effect on the activity and abundance of ammonia oxidizers38,39.
In addition, the low level of oxygen in the CMS, which is shown by the increase both in methane flux and anaerobic methanogens (Fig. 2)40,41, might also lead to a decrease in the abundance of aerobic ammonia oxidizing microorganisms. This is because anaerobic conditions are known to stimulate heterotrophic denitrifiers42,43 but inhibit aerobic ammonia oxidation microorganisms44. Consistent with the previous reports42,43, we found a typical heterotrophic denitrifying bacteria Pseudomonadales to be enriched in the CMS (Fig. S1). As discussed previously, excessive contamination of the mangrove sediment might reduce the function of mangrove to absorb atmospheric nitrogen but stimulate the ability to buffer anthropogenic nitrogen inputs by discharging them to atmosphere as inert N2 gas.
Contamination promotes microbial capability in generating greenhouse gases
The microbial capacity to generate greenhouse gases was stimulated in contaminated mangrove sediments. It has previously been reported that contamination in mangrove sediments can significantly change the structure of methanogenic microbial communities6,45,46. In this new study, we found that contamination in the mangrove sediment also promoted the emission of methane (Supplementary Table S1), and altered methanogenic strategy of methanogens (Fig. 5). There are two types of methanogenic strategy utilized by methanogens; autotrophic and heterotrophic synthesis. Heterotrophic methanogens can metabolize simple substrates (e.g., methanol and acetyl-CoA) into methane via methyltransferase (mtaA) and acetyl-CoA decarbonylase/synthase (cdhc), whereas autotrophic methanogens can process the reduction of CO2 to methane via formylmethanofuran dehydrogenase (fwdA)47,48. Within the PMS, the consumption of atmospheric methane (Supplementary Table S1) and the abundance of the fwdA gene (Fig. 5), indicated that the sediment was relatively oligotrophic and that both methanogens and methanotrophs were functioning to reduce the amount of greenhouse gas in the total budget. In contrast, in the CMS, the abundant mtaA gene (Fig. 5), together with the emission of methane gas (Supplementary Table S1), indicated that the excessive amount of contamination not only promoted methane emission but also provided an adequate substrate for heterotrophic methanogens.
As sulfate reduction accounts for almost 100% of the total emission of CO2 from mangrove sediments49, the enriched levels of sulfate reduction bacteria (Fig. 2) and the sulfate reduction gene (Fig. 4) suggest an increase of CO2 emission in the CMS. The enrichment of sulfate reduction related genes in contaminated mangroves were also found in previous metagenomic study, which reported a higher gene abundance of sulfate reduction in Brazilian contaminated mangrove sediments22. The lack of difference in the distribution of carbon fixation genes between the various samples might be due to the consistent anaerobic conditions in the mangrove sediment. For example, genes encoding the reductive tricarboxylic acid cycle (Fig. 4), which is an anaerobic carbon fixation metabolism, are the most frequently encoded across samples50,51,52,53. Therefore, with the increase in CO2 generation and the similar carbon fixation capability of microorganisms, the carbon balance in the CMS was bias to releasing instead of fixing. Overall, PMS residing microbes exhibited a strong metabolic ability in absorption of aerial greenhouse gas, while contaminate in mangrove sediments might stimulate bioremediation with an increase of greenhouse gas emission.
Resistance to anthropogenic pollution
In addition to the input of nitrogen, heavy metals are another major source of pollution in our study areas. Consistent with the previous global investigation24, we found that genes associated with the Zn-Pb efflux system and with Co-Zn-Cd resistance were ubiquitous across our terrestrial samples with higher abundant in the contaminated regions; this is in line with the markedly high concentrations of Zn and Pb detected (Fig. 4). Metal contamination is a common phenomenon in polluted mangrove sediments54,55. However, the heavy metal resistance genes we detected (Fig. 4) suggest that microbes have developed the capability to minimize the threat of metal pollution.
In our study, organophosphate solubilization-related genes were abundant in the pristine regions. As soluble phosphate is an essential element for the healthy and rapid growth of plants, mangrove plants benefit from sediments that have strong organophosphate solubilization ability56,57,58. The differences we observed in the occurrence of metal resistance and organophosphate solubilizing genes in the contaminated and pristine mangrove sediments, respectively, are likely to be a result of microbial adaptation in battle with heavy metal toxicity in the CMS, and phosphorus limitation in the PMS.
Conclusions
Through a combination of metagenomic profiling and measurements of sediment quality parameters and methane flux, we have constructed a more comprehensive network analysis for the microbial metabolism in mangrove sediments. Microorganisms exhibit different metabolic capability in pristine and contaminated mangrove sediments as an effect of bioremediation. We found that microorganisms residing in the PMS were more proficient at depositing nitrogen, carbon dioxide, and methane, as well as stimulating organophosphate solubilization; together these could benefit the growth of mangrove plants and reduce the emission of greenhouse gases. In contrast, the microorganisms in the CMS were more proficient in denitrification, greenhouse gas generation, and metal resistance. Overall, contamination in mangrove sediments might have a negative effect on the nutrient deposition ability of microbes, and at the same time it might stimulate bioremediation with an increase of greenhouse gas emission. Determining whether contamination in mangrove sediments might also affect gene expression requires further physiological and transcriptomics investigation.
Materials and Methods
Sample collection, genomic dna extraction and sequencing
The triplicate mud samples were collected from mangroves located in Haikou (19°194′N, 110°35.214′E) and Sanya (18°15.242′N, 109°30.585′E) in Hainan Island, China, in August 2016 (Fig. 6). Contaminated and pristine mangrove sediment from Haikou were collected from offshore and inshore part of the mangrove forest respectively; whereas, contaminated and pristine sediment from Sanya were sampled from inshore and offshore part of the mangrove forest respectively. At each location, approximately 50 g surface sediment (i.e., from a depth of ~0 cm to 3 cm) was collected and immediately transferred to a 15 ml Falcon tube. The samples were kept on ice while in the field, and then (when back in the laboratory) stored at −80 °C prior to further procedures. Contaminated mangrove sediment (CMS) from Haikou and Sanya was called HKC and SYC, respectively, whereas, pristine mangrove sediment (PMS) from Haikou and Sanya was called HKP and SYP, respectively. DNA extractions of triplicates were performed with the Fast DNA® Spin Kit for Soil (Qbio gene INC), after which the parallel extracts were pooled and shotgun sequencing was performed using an Illumina HiSeq2500 PE 150 bp platform and the HiSeq Cluster Kit v4 kit (San Diego, CA, USA).
Metagenome assembly
Raw reads were treated using the FASTX-Toolkit package59. Reads containing 3 or more ambiguous bases (Ns) and an average quality score <20, or a length <140 bp were removed. The resulting clear paired-end reads were assembled using four different assemblers: IDBA_UD60; Velvet61; MEGAHIT62; and SPAdes63, each using their default setting. After comparing the assembly results obtained from the different assemblers, those from the SPAdes software were selected for further analyses.
Prokaryotic taxonomic assignment and analysis
Screening of SSU rRNAs from the assembled contigs was conducted using Metaxa264. Following the analysis pipe-line previously described65, the extracted SSU rRNAs were analyzed using QIIME 1.9.1 against the Silva database (version 123). A filtered OTUs table at 0.1% abundance of each sample was generated with QIIME 1.9.1, and the community structure was visualized with the R software using the ‘vegan’ package66.
Functional annotation
The open reading frames (ORFs) of assembled contigs were predicted using the Prodigal software67, with a minimum length of 200 bp. The extracted ORFs were then annotated with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database68, the SEED database, and the non-redundant protein (NR) database via DIAMOND69 using the following parameters, blastp, k = 1, and an e-value = 10−7 to select the best annotation result. For each sample, clean short reads were mapped back to ORFs using Bowtie 2.2.970 to reveal the coverage information for individual functional genes. The web-based analysis tool, KAAS (KEGG Automatic Annotation Server), was used to validate the annotation results from a BLAST (Basic Local Alignment Search Tool) search. In addition, KEGGMAPPER (http://www.genome.jp/kegg/mapper.html) was used to reconstruct the metabolic pathways.
Chemical analysis of mangrove sediment
For the measurement of nutrients and metals, mangrove sediment samples were collected in acrylic tubes and analyzed in the laboratory on land. To measure the total nitrogen (TN), total phosphate (TP) and total organic carbon (TOC), 1 g dried chips was taken out from the 150 μm mesh filtered mangrove sediment, and 2 gram of catalyst (1 (cupric sulfate, purity >99%): 10 (sodium sulfate, purity >99%)) was then added into the powder grinded from the chips. Then, 2 ml distilled water and 5 ml concentrated sulfuric acid were added to the powder before microwave digestion of the mixture (30 seconds). After digestion, the sample was filtered through a 0.45 μm elutriation membrane, after which the filtrate was analyzed with a FUTURA-II continuously flowing analyzer (Alliance Instruments, France). To determine the salinity, NO3−, NH4+, and NO2−, 30 ml KCl (2 mol/L) was added to the wet sediment, after which the liquid was vibrated for 1 h and then allowed to settle for 30 min before being filtered through a 0.2 μm elutriation membrane. This filtrate was then also analyzed with the FUTURA-II analyzer71.
The pH was measured by mixing 5 g sediment with 5 ml water, as reported previously72. The sediment was then oven dried for 2 days at 100 °C, after which it was ground up, homogenized and sieved through a 2 mm mesh. Then 7 mL HNO3 (72% v/v) and 1 mL H2O2 (30% v/v) (Fisher Scientific) were added to 0.5 g of each dried sediment sample, and the samples were heated using a closed microwave digestion system (Start D, Milestone) for 90 min until the solutions were clear. The solutions were filtered and then analyzed in triplicate for total concentrations of Zn, Fe, Mn, Cd, and Pb using a 7500 series Inductively Coupled Plasma Mass Spectrometry (ICP-MS) system (Agilent Technologies)73. All the measurements for major elements were carried out in triplicates and the standard error was <1%.
Measurement of sediment methane flux
The flux of methane across the mangrove sediment-atmosphere interface was measured using the Closed Chamber method; the operation and construction of the Perspex chamber (length 50 cm, volume 10 L, enclosing 1000 cm2) was as previously reported74. In brief, at each location, a Perspex chamber was inserted 5 cm into the sediment for gas collection. The gas in the chamber was extracted with a 10 ml gas-tight syringe every 30 min over a period of 120 min (i.e., at 0, 30, 60, 90, 120 min). The sampled gas was then transferred to 12 ml pre-evacuated exetainers (Labco Limited, USA), and preserved in a small, cold and dry refrigerator (Amoi, china). Within 24 h, the samples were analyzed with gas chromatography (Auto-system, Perkin-Elmer (PE), USA; 3800, Varian, Netherlands), and the methane flux of sampled mangrove sediment was then calculated based on mathematic equations75. Measurements were conducted in duplicate for each location, and the final value was averaged.
Data Availability
The sequencing data supporting the results of this article are available in NCBI Genbank under the accession numbers: PRJNA505165. The predicted open reading frame (ORF) of the assembled contigs were also uploaded to the MG-RAST and annotated with it. The public MG-RAST ID for samples are: mgm4837260.3; mgm4837250.3; mgm4837252.3; mgm4837251.3.
References
Cao, H., Li, M., Hong, Y. & Gu, J.-D. Diversity and abundance of ammonia-oxidizing archaea and bacteria in polluted mangrove sediment. Systematic and applied microbiology 34(7), 513–523 (2011).
Li, M., Hong, Y.-G., Cao, H.-L. & Gu, J.-D. Mangrove trees affect the community structure and distribution of anammox bacteria at an anthropogenic-polluted mangrove in the Pearl River Delta reflected by 16S rRNA and hydrazine oxidoreductase (HZO) encoding gene analyses. Ecotoxicology 20(8), 1780 (2011).
Duke, N. C. et al. A world without mangroves? Science 317(5834), 41–42 (2007).
Li, C.-H., Zhou, H.-W., Wong, Y.-S. & Tam, N. F.-Y. Vertical distribution and anaerobic biodegradation of polycyclic aromatic hydrocarbons in mangrove sediments in Hong Kong, South China. Science of the Total Environment 407(21), 5772–5779 (2009).
Neumann, D., Heuer, A., Hemkemeyer, M., Martens, R. & Tebbe, C. C. Importance of soil organic matter for the diversity of microorganisms involved in the degradation of organic pollutants. The ISME journal 8(6), 1289 (2014).
Jing, H. et al. Spatial variations of the methanogenic communities in the sediments of tropical mangroves. PloS one 11(9), e0161065 (2016).
Pan, Y., Chen, J., Zhou, H., Farzana, S. & Tam, N. F. Vertical distribution of dehalogenating bacteria in mangrove sediment and their potential to remove polybrominated diphenyl ether contamination. Marine pollution bulletin 124(2), 1055–1062 (2017).
Tam, N. F. et al. Nutrients and heavy metal contamination of plants and sediments in Futian mangrove forest. Hydrobiologia 295(1-3), 149–158 (1995).
Brito, E. M. et al. A case study of in situ oil contamination in a mangrove swamp (Rio De Janeiro, Brazil). Marine Pollution Bulletin 58(3), 418–423 (2009).
Bayen, S. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: a review. Environment international 48, 84–101 (2012).
Barbier, E. B. The protective service of mangrove ecosystems: A review of valuation methods. Marine pollution bulletin 109(2), 676–681 (2016).
Maiti, S. K. & Chowdhury, A. Effects of anthropogenic pollution on mangrove biodiversity: a review. Journal of Environmental Protection 4(12), 1428 (2013).
Dos Santos, H. F. et al. Mangrove bacterial diversity and the impact of oil contamination revealed by pyrosequencing: bacterial proxies for oil pollution. PloS one 6(3), e16943 (2011).
El-Tarabily, K. A. Total microbial activity and microbial composition of a mangrove sediment are reduced by oil pollution at a site in the Arabian Gulf. Canadian journal of microbiology 48(2), 176–182 (2002).
Holguin, G., Vazquez, P. & Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biology and fertility of soils 33(4), 265–278 (2001).
Giblin, A. E. et al. The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26(3), 124–131 (2013).
Meyer, R. L., Risgaard-Petersen, N. & Allen, D. E. Correlation between anammox activity and microscale distribution of nitrite in a subtropical mangrove sediment. Applied and environmental microbiology 71(10), 6142–6149 (2005).
Ramsay, M. A., Swannell, R. P., Shipton, W. A., Duke, N. C. & Hill, R. T. Effect of bioremediation on the microbial community in oiled mangrove sediments. Marine Pollution Bulletin 41(7-12), 413–419 (2000).
Guo, C., Zhou, H., Wong, Y. & Tam, N. Isolation of PAH-degrading bacteria from mangrove sediments and their biodegradation potential. Marine Pollution Bulletin 51(8-12), 1054–1061 (2005).
Tian, Y. et al. Contamination and potential biodegradation of polycyclic aromatic hydrocarbons in mangrove sediments of Xiamen, China. Marine Pollution Bulletin 56(6), 1184–1191 (2008).
Stroud, J., Paton, G. & Semple, K. T. Microbe‐aliphatic hydrocarbon interactions in soil: implications for biodegradation and bioremediation. Journal of Applied Microbiology 102(5), 1239–1253 (2007).
Andreote, F. D. et al. The microbiome of Brazilian mangrove sediments as revealed by metagenomics. PloS one 7(6), e38600 (2012).
Imchen, M. et al. Searching for signatures across microbial communities: Metagenomic analysis of soil samples from mangrove and other ecosystems. Scientific reports 7(1), 8859 (2017).
Imchen, M. et al. Comparative mangrove metagenome reveals global prevalence of heavy metals and antibiotic resistome across different ecosystems. Scientific reports 8(1), 11187 (2018).
Peng, L. Ecological notes on mangroves in southeast coast of china including taiwan province and hainan island [J]. Acta Ecologica Sinica 3, 011 (1981).
Liao, Q. et al. An ecological analysis of soil sarcodina at Dongzhaigang mangrove in Hainan Island, China. European Journal of Soil Biology 45(3), 214–219 (2009).
Ghosh, A. et al. Culture independent molecular analysis of bacterial communities in the mangrove sediment of Sundarban, India. Saline systems 6(1), 1 (2010).
Lorenz, S. E., Mcgrath, S. P. & Giller, K. E. Assessment of free-living nitrogen fixation activity as a biological indicator of heavy metal toxicity in soil. Soil Biology and Biochemistry 24(6), 601–606 (1992).
Jing, H. et al. Anthropogenic impact on diazotrophic diversity in the mangrove rhizosphere revealed by nifH pyrosequencing. Frontiers in microbiology 6, 1172 (2015).
Ramírez-Bahena, M. et al. Nitrogen fixing endosymbiotic bacteria: old chaps and new findings. Beneficial plant-microbial interactions: ecology and applications. CRC Press, London, 1–19 (2016).
Pepe-Ranney, C. et al. Non-cyanobacterial diazotrophs mediate dinitrogen fixation in biological soil crusts during early crust formation. The ISME journal 10(2), 287 (2016).
Taketani, R. G., Yoshiura, C. A., Dias, A. C. F., Andreote, F. D. & Tsai, S. M. Diversity and identification of methanogenic archaea and sulphate-reducing bacteria in sediments from a pristine tropical mangrove. Antonie van Leeuwenhoek 97(4), 401–411 (2010).
Qiao, W. et al. In Establishment of a Methanogenic Benzene-Degrading Culture and its Implication in Bioremediation, AGU Fall Meeting Abstracts, 2017 (2017).
Cavaleiro, A. J. et al. Facts and challenges on hydrocarbons bioremediation (2017).
Anderson, R. T. & Lovley, D. R. Anaerobic bioremediation of benzene under sulfate-reducing conditions in a petroleum-contaminated aquifer. Environmental science & technology 34(11), 2261–2266 (2000).
Zuberer, D. A. & Silver, W. Biological dinitrogen fixation (acetylene reduction) associated with Florida mangroves. Applied and environmental Microbiology 35(3), 567–575 (1978).
Zuberer, D. & Silver, W. N2‐Fixation (acetylene reduction) and the microbial colonization of mangrove roots. New Phytologist 82(2), 467–471 (1979).
Chen, J., Zhou, H. C., Pan, Y., Shyla, F. S. & Tam, N. F.-Y. Effects of polybrominated diphenyl ethers and plant species on nitrification, denitrification and anammox in mangrove soils. Science of the Total Environment 553, 60–70 (2016).
Li, N. et al. Response of ammonia-oxidizing archaea to heavy metal contamination in freshwater sediment. Journal of Environmental Sciences (2018).
Peters, V. & Conrad, R. Methanogenic and other strictly anaerobic bacteria in desert soil and other oxic soils. Applied and environmental microbiology 61(4), 1673–1676 (1995).
Adekunle, K. F. & Okolie, J. A. A review of biochemical process of anaerobic digestion. Advances in Bioscience and Biotechnology 6(03), 205 (2015).
Shapleigh, J. P. Denitrifying prokaryotes. In The prokaryotes, Springer:, pp 405–425 (2013).
Carlson, C. A. & Ingraham, J. L. Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Applied and Environmental Microbiology 45(4), 1247–1253 (1983).
Zhu, X., Burger, M., Doane, T. A. & Horwath, W. R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proceedings of the National Academy of Sciences, 201219993 (2013).
Jiang, X.-T. et al. Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microbial ecology 66(1), 96–104 (2013).
Taketani, R. G., Franco, N. O., Rosado, A. S. & van Elsas, J. D. Microbial community response to a simulated hydrocarbon spill in mangrove sediments. The Journal of Microbiology 48(1), 7–15 (2010).
Serrano-Silva, N., Sarria-Guzmán, Y., Dendooven, L. & Luna-Guido, M. Methanogenesis and methanotrophy in soil: a review. Pedosphere 24(3), 291–307 (2014).
Thatoi, H., Behera, B. C., Mishra, R. R. & Dutta, S. K. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: a review. Annals of Microbiology 63(1), 1–19 (2013).
Kristensen, E., Holmer, M. & Bussarawit, N. Benthic metabolism and sulfate reduction in a southeast Asian mangrove swamp. Marine Ecology Progress Series, 93–103 (1991).
Wang, J. & Shen, X. Citric Acid Cycle and Its Metabolic Engineering Applications. Engineering Microbial Metabolism For Chemical Synthesis: Reviews And Perspectives 35 (2017).
Shiba, H., Kawasumi, T., Igarashi, Y., Kodama, T. & Minoda, Y. The CO2 assimilation via the reductive tricarboxylic acid cycle in an obligately autotrophic, aerobic hydrogen-oxidizing bacterium, Hydrogenobacter thermophilus. Archives of Microbiology 141(3), 198–203 (1985).
Fuchs, G., Stupperich, E. & Eden, G. Autotrophic CO2 fixation in Chlorobium limicola. Evidence for the operation of a reductive tricarboxylic acid cycle in growing cells. Archives of Microbiology 128(1), 64–71 (1980).
Hügler, M., Wirsen, C. O., Fuchs, G., Taylor, C. D. & Sievert, S. M. Evidence for autotrophic CO2 fixation via the reductive tricarboxylic acid cycle by members of the ε subdivision of proteobacteria. Journal of bacteriology 187(9), 3020–3027 (2005).
Tam, N. & Yao, M. Normalisation and heavy metal contamination in mangrove sediments. Science of the Total Environment 216(1-2), 33–39 (1998).
Defew, L. H., Mair, J. M. & Guzman, H. M. An assessment of metal contamination in mangrove sediments and leaves from Punta Mala Bay, Pacific Panama. Marine Pollution Bulletin 50(5), 547–552 (2005).
Sahoo, K. & Dhal, N. Potential microbial diversity in mangrove ecosystems: a review (2009).
Behera, B. C. et al. Phosphate solubilising bacteria from mangrove soils of Mahanadi river delta, Odisha, India. World journal of agricultural research 4(1), 18–23 (2016).
Akbar, S. & Sultan, S. Soil bacteria showing a potential of chlorpyrifos degradation and plant growth enhancement. brazilian journal of microbiology 47(3), 563–570 (2016).
Pearson, W. R., Wood, T., Zhang, Z. & Miller, W. Comparison of DNA sequences with protein sequences. Genomics 46(1), 24–36 (1997).
Peng, Y., Leung, H. C., Yiu, S.-M. & Chin, F. Y. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 28(11), 1420–1428 (2012).
Zerbino, D. R. & Birney, E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome research 18(5), 821–829 (2008).
Li, D., Liu, C.-M., Luo, R., Sadakane, K. & Lam, T.-W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31(10), 1674–1676 (2015).
Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of computational biology 19(5), 455–477 (2012).
Bengtsson‐Palme, J. et al. METAXA2: improved identification and taxonomic classification of small and large subunit rRNA in metagenomic data. Molecular Ecology Resources 15(6), 1403–1414 (2015).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic acids research 41(D1), D590–D596 (2012).
Oksanen, J. et al. Package ‘vegan’. Community ecology package, version, 2(9) (2013).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC bioinformatics 11(1), 119 (2010).
Kanehisa, M. et al. KEGG for linking genomes to life and the environment. Nucleic acids research 36(suppl_1), D480–D484 (2007).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nature methods 12(1), 59–60 (2015).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nature methods 9(4), 357–359 (2012).
Wang, J., Wu, Y., Zhou, J., Bing, H. & Sun, H. Carbon demand drives microbial mineralization of organic phosphorus during the early stage of soil development. Biology and fertility of soils 52(6), 825–839 (2016).
Rastogi, G. et al. Microbial diversity in uranium mining-impacted soils as revealed by high-density 16S microarray and clone library. Microbial ecology 59(1), 94–108 (2010).
M’kandawire, E. et al. Sediment metal contamination in the Kafue River of Zambia and ecological risk assessment. Bulletin of environmental contamination and toxicology 99(1), 108–116 (2017).
Allen, D. E. et al. Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biology and Biochemistry 39(2), 622–631 (2007).
Lu, C. Y., Wong, Y. S., Tam, N. F., Ye, Y. & Lin, P. Methane flux and production from sediments of a mangrove wetland on Hainan Island, China. Mangroves and Salt Marshes 3(1), 41–49 (1999).
Acknowledgements
This work was supported by the following grants awarded to H. Jing: Training Program of the Major Research Plan of the National Natural Science Foundation of China (91751116), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB06010202), the Academy-Locality Science and Technology Cooperation of Sanya City, China (Grant No. 2014YD05, and the National Natural Science Foundation of China (41776147). H. Liu acknowledges the support of the Hong Kong Research Grants Council (16128416, N_HKUST609/15, and T21/602/16).
Author information
Authors and Affiliations
Contributions
H.J. and H.L. conceived and designed the experiments. L.Z. and Y.Z. performed the experiments. Y.L. analyzed the data. H.J. and H.L. contributed reagents, materials and analysis tools. Y.L. wrote the paper. H.J. and H.L. contributed writing and analysis guidance.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Zheng, L., Zhang, Y. et al. Comparative metagenomics study reveals pollution induced changes of microbial genes in mangrove sediments. Sci Rep 9, 5739 (2019). https://doi.org/10.1038/s41598-019-42260-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42260-4
This article is cited by
-
Finding microbial composition and biological processes as predictive signature to access the ongoing status of mangrove preservation
International Microbiology (2024)
-
Traditional and new proposals for environmental microbial indicators—a review
Environmental Monitoring and Assessment (2023)
-
Microbial diversity and ecological interactions of microorganisms in the mangrove ecosystem: Threats, vulnerability, and adaptations
Environmental Science and Pollution Research (2022)
-
Development of Rhizophora mangle (Rhizophoraceae) and Avicennia schaueriana (Avicenniaceae) in the presence of a hydrocarbon-degrading bacterial consortium and marine diesel oil
3 Biotech (2022)
-
Mangrove roots model suggest an optimal porosity to prevent erosion
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.